Identification and Characterization of the Delta-12 Fatty Acid Desaturase from Euglena gracilis
Abstract
1. Introduction
2. Methods
- Organisms and growth conditions
- 2.
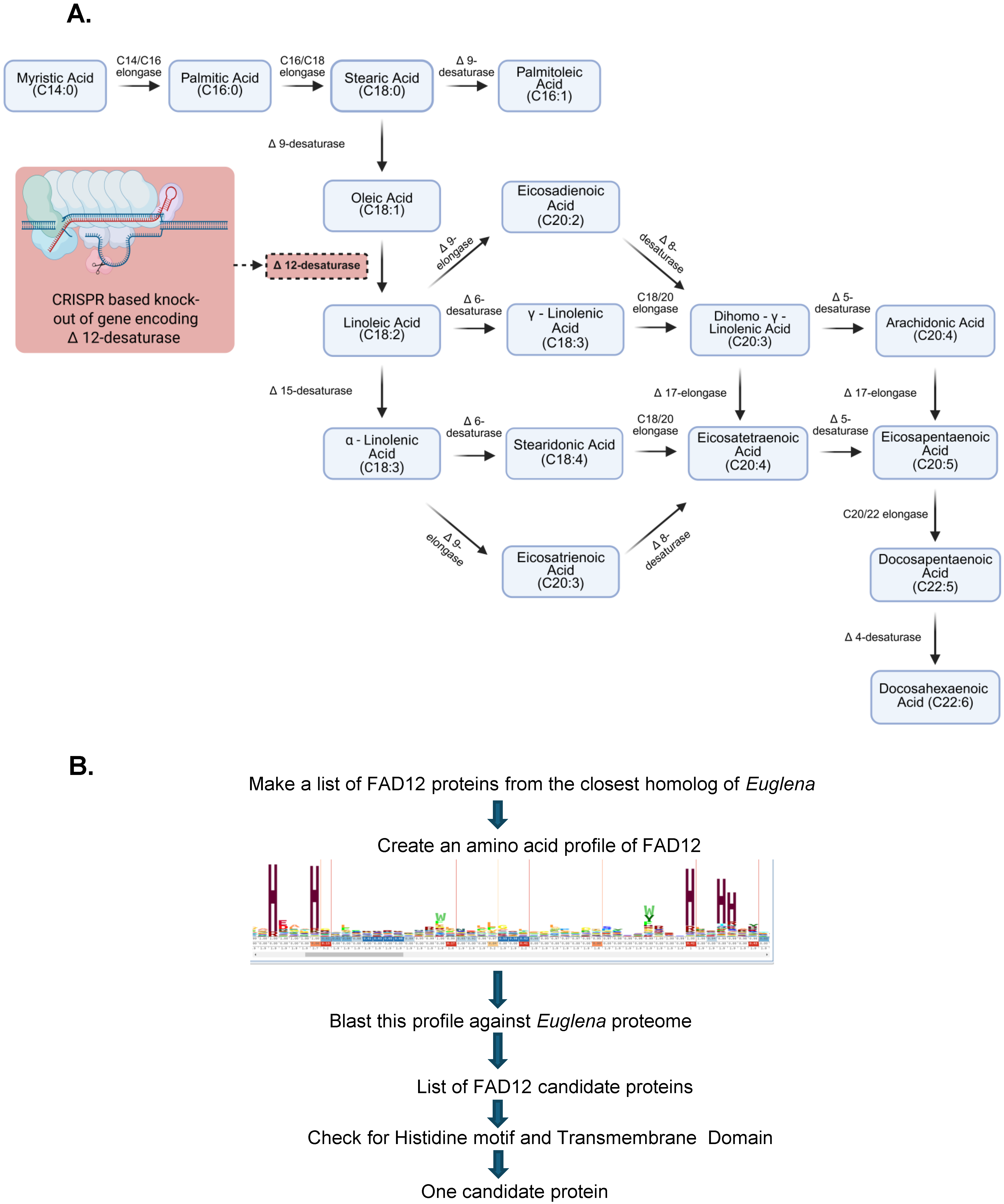
- Bioinformatics Identification of the FAD12 gene in Euglena gracilis
- 3.
- FAD12 gene synthesis and plasmid construction for bacterial transformation
- 4.
- Heterologous expression of E.G FAD12 in Tobacco
- 5.
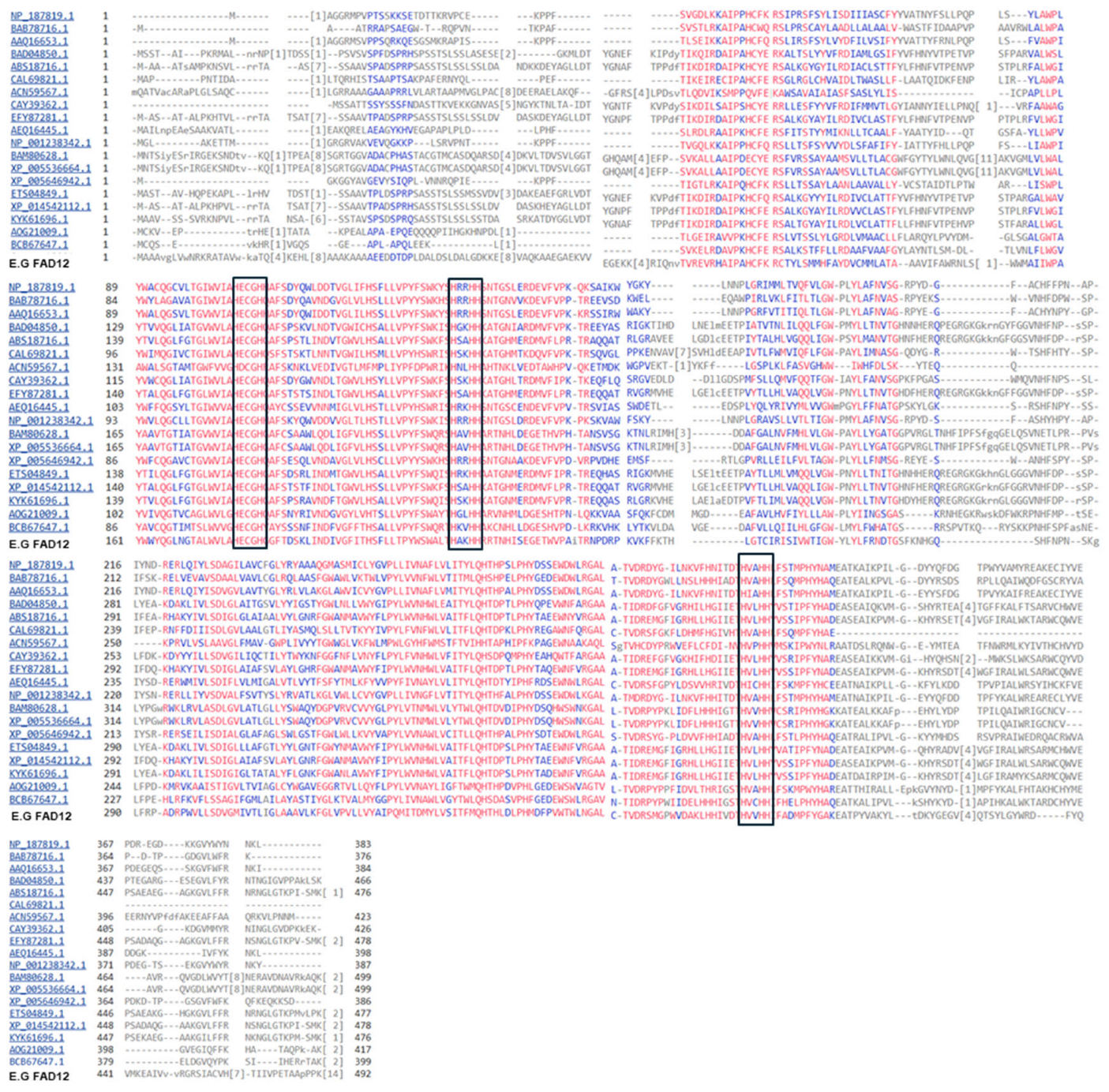
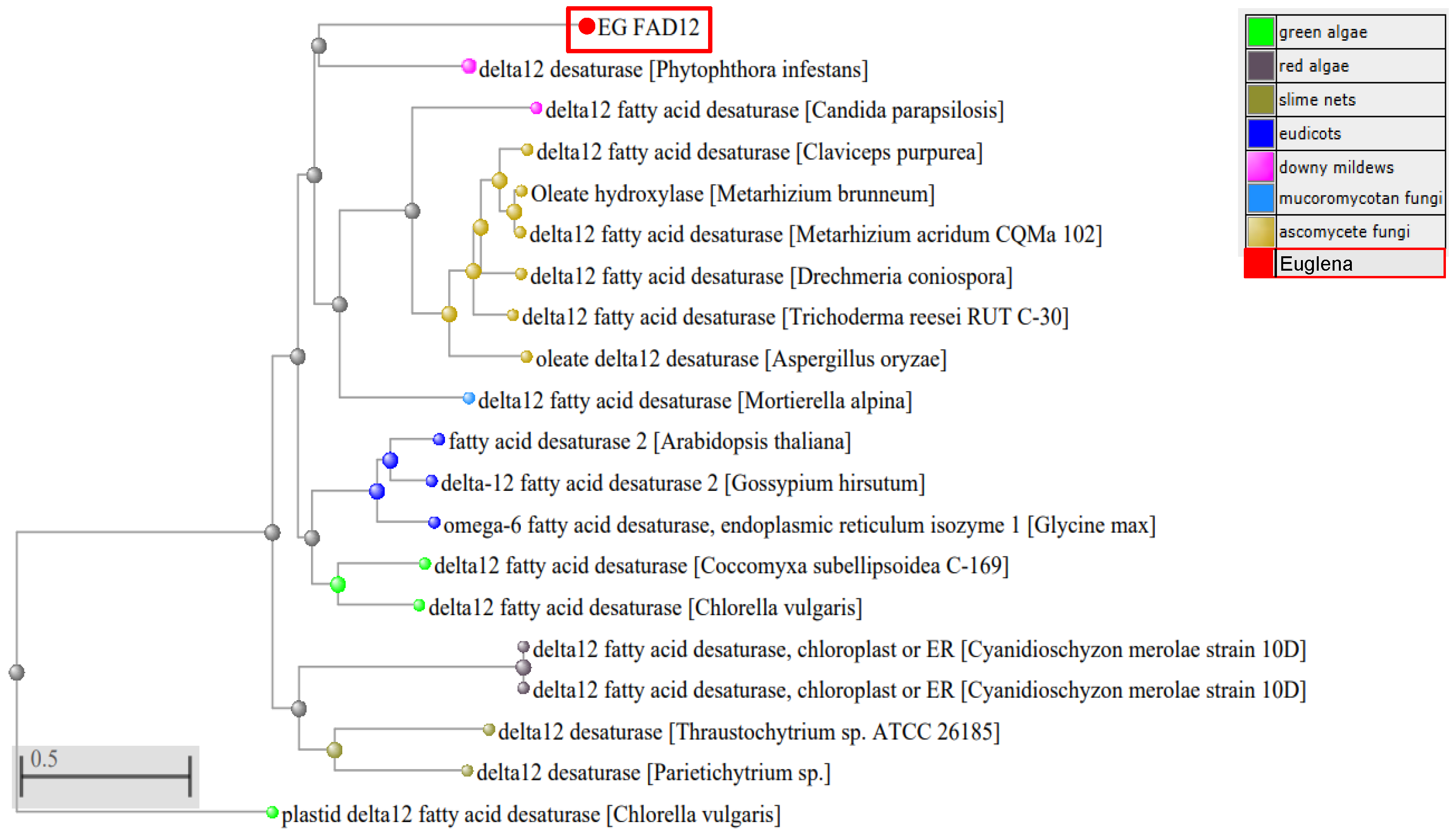
- Amino acid alignment and Phylogenetic Tree Analysis
- 6.
- Biomass and Lipid Quantification
- 7.
- Fatty acid analysis by Gas Chromatography Flame Ionization Detector (GC-FID)
- 8.
- Guide RNA (gRNA) design and CRISPR knockout of FAD12
3. Results
3.1. Bioinformatic Identification of FAD12 in Euglena gracilis
3.2. Amino Acid Alignment and Phylogenetic Analysis of FAD12
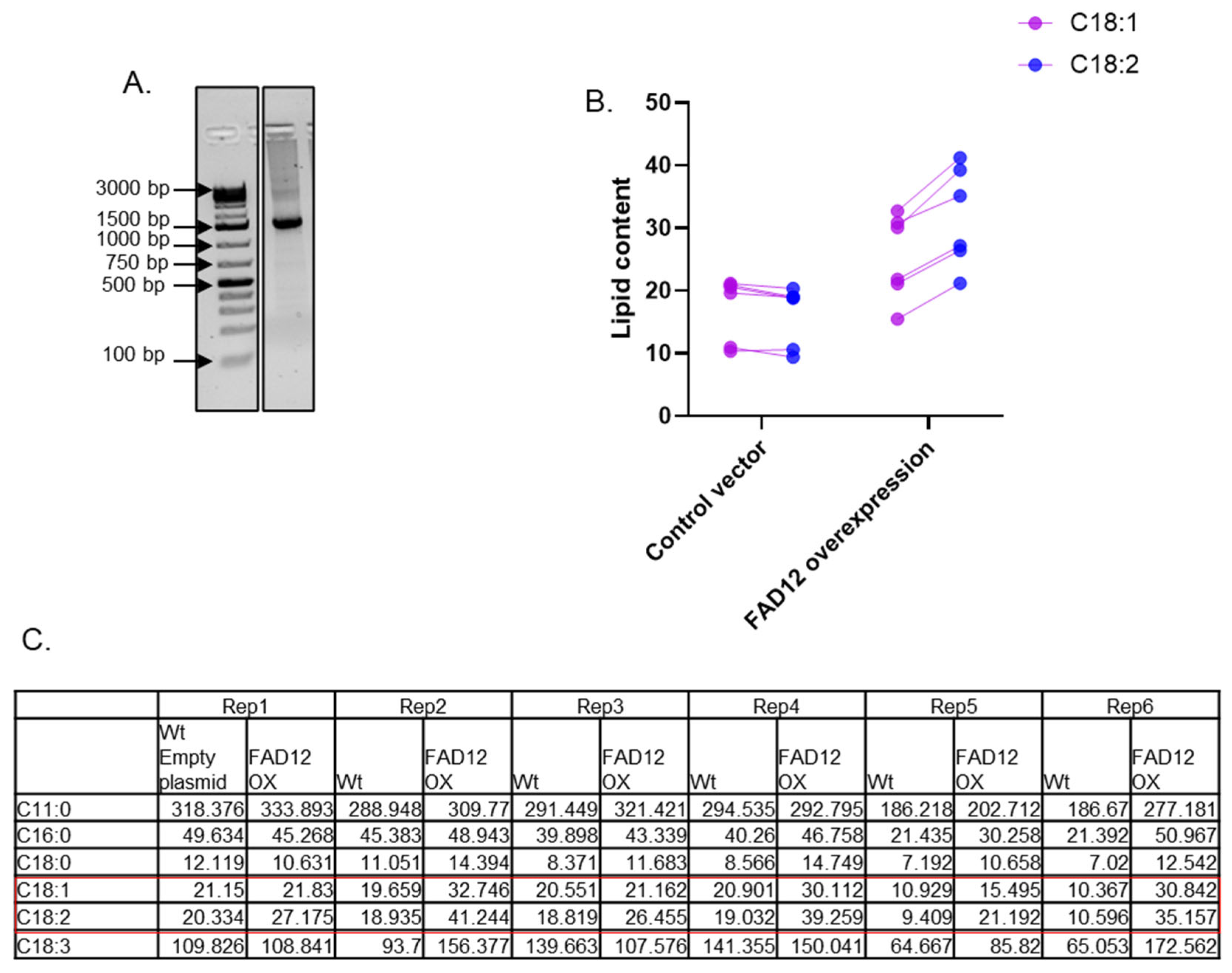
3.3. Cloning of EG FAD12 and Heterologous Expression in Tobacco
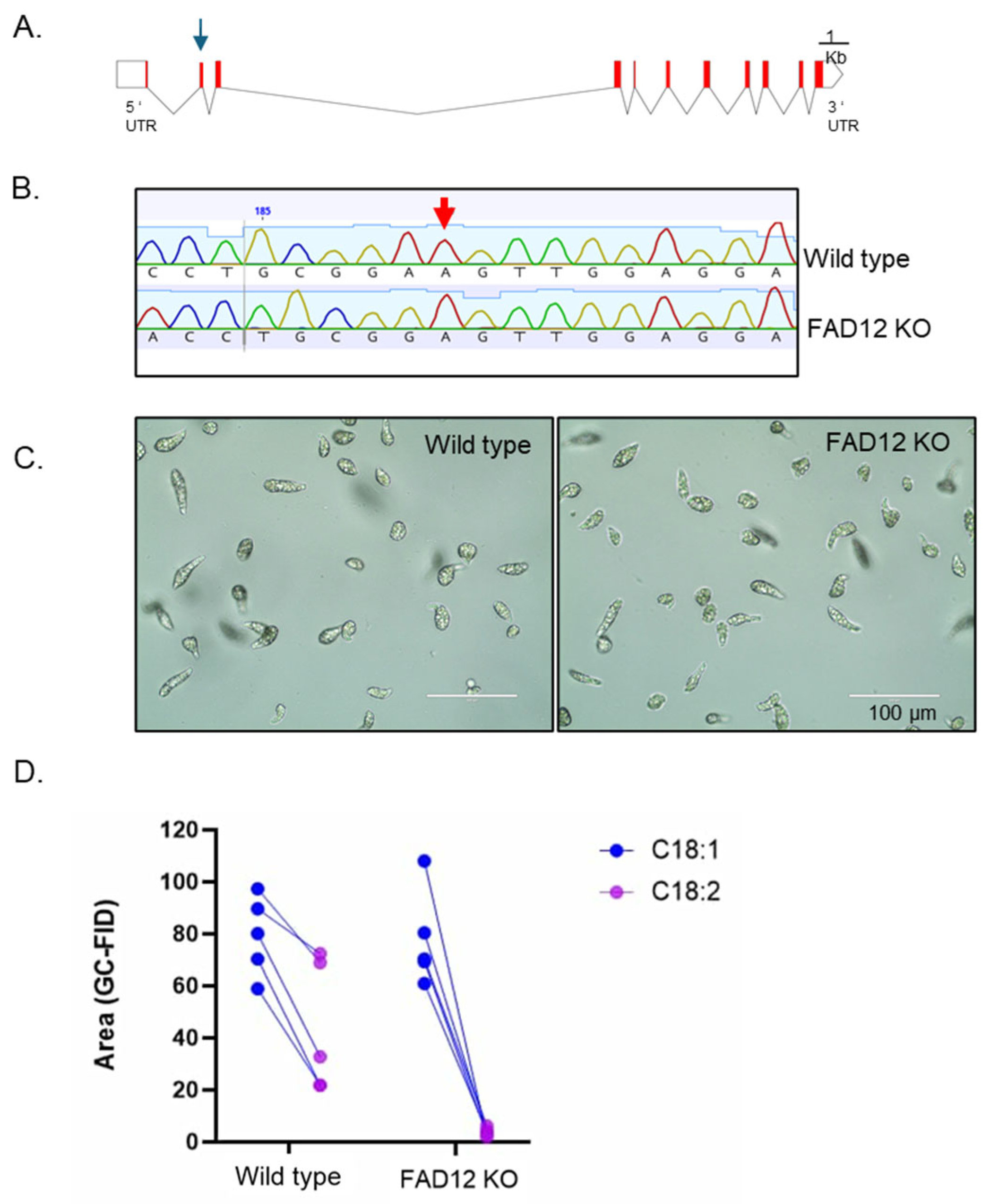
3.4. CRISPR-Cas9-Mediated Knockout of FAD12
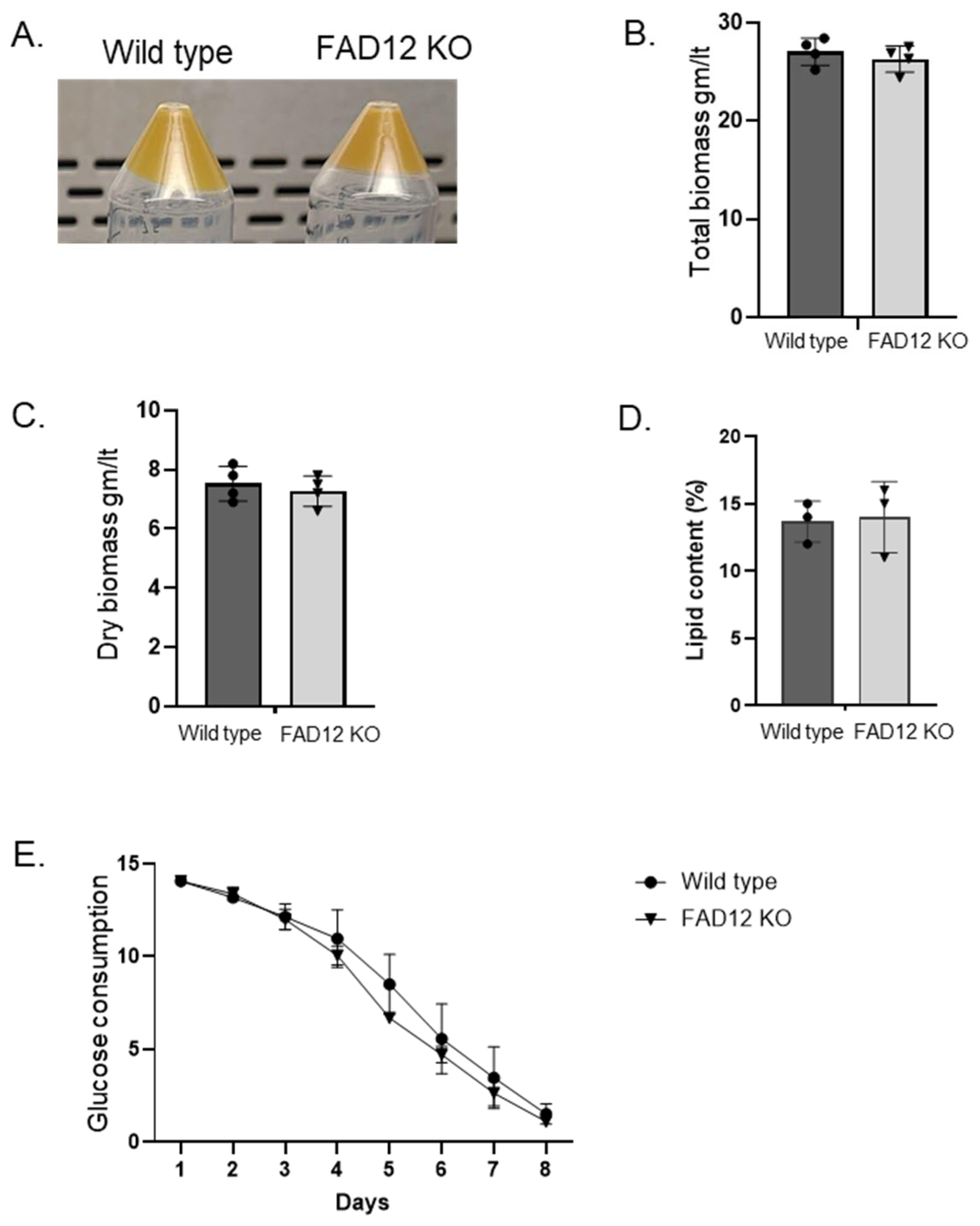
3.5. Characterization of the FAD12 kd Cell Line for Commercial Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uprety, B.K.; Morrison, E.N.; Emery, R.J.N.; Farrow, S.C. Customizing lipids from oleaginous microbes: Leveraging exogenous and endogenous approaches. Trends Biotechnol. 2022, 40, 482–508. [Google Scholar] [CrossRef] [PubMed]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production Strategies and Applications of Microbial Single Cell Oils. Front. Microbiol. 2016, 7, 2016. [Google Scholar] [CrossRef]
- Farjallah; Fillion, M.; Guéguen, C. Metabolic responses of Euglena gracilis under photoheterotrophic and heterotrophic conditions. Protist 2024, 175, 126035. [Google Scholar] [CrossRef]
- Kuhne, A.M.; Morrison, E.N.; Sultana, T.; Kisiala, A.B.; Horlock-Roberts, K.; Noble, A. Cultivation of heterotrophic Euglena gracilis: The effects of recycled media on culture growth and associations with growth-regulating phytohormone profiles. J. Appl. Phycol. 2023, 35, 2161–2175. [Google Scholar] [CrossRef]
- Lewis; Guéguen, C. How growth conditions of Euglena gracilis cells influence cellular composition as evidenced by Fourier transform infrared spectroscopy and direct infusion high-resolution mass spectrometry. J. Appl. Phycol. 2020, 32, 153–163. [Google Scholar] [CrossRef]
- Rodríguez-Zavala, J.S.; Ortiz-Cruz, M.A.; Mendoza-Hernández, G.; Moreno-Sánchez, R. Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J. Appl. Microbiol. 2010, 109, 2160–2172. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Chanakya, H.N.; Ramachandra, T.V. Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J. Appl. Phycol. 2013, 25, 855–865. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Nguyen, Q.T.; Dang, D.H.; Emery, R.J.N. Phytohormones enhance heavy metal responses in Euglena gracilis: Evidence from uptake of Ni, Pb and Cd and linkages to hormonomic and metabolomic dynamics. Environ. Pollut. 2023, 320, 121094. [Google Scholar] [CrossRef]
- Ko, Y.; Baek, H.; Hwang, J.-H.; Kim, Y.; Lim, K.-M.; Kim, J.; Kim, J.W. Nonanimal Euglena gracilis-Derived Extracellular Vesicles Enhance Skin-Regenerative Wound Healing. Adv. Mater. Interfaces 2023, 10, 2202255. [Google Scholar] [CrossRef]
- Dai, J.; He, J.; Chen, Z.; Qin, H.; Du, M.; Lei, A.; Zhao, L.; Wang, J. Euglena gracilis Promotes Lactobacillus Growth and Antioxidants Accumulation as a Potential Next-Generation Prebiotic. Front. Nutr. 2022, 9, 864565. [Google Scholar] [CrossRef]
- Park, S.-y.; Kim, K.J.; Jo, S.M.; Jeon, J.-Y.; Kim, B.-R.; Hwang, J.E.; Kim, J.Y. Euglena gracilis (Euglena) powder supplementation enhanced immune function through natural killer cell activity in apparently healthy participants: A randomized, double-blind, placebo-controlled trial. Nutr. Res. 2023, 119, 90–97. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Zhang, H.; Qin, H.; He, J.; Zheng, Z.; Zhao, L.; Lei, A.; Wang, J. Evaluation of Euglena gracilis 815 as a New Candidate for Biodiesel Production. Front. Bioeng. Biotechnol. 2022, 10, 827513. [Google Scholar] [CrossRef]
- Kim, S.; Im, H.; Yu, J.; Kim, K.; Kim, M.; Lee, T. Biofuel production from Euglena: Current status and techno-economic perspectives. Bioresour. Technol. 2023, 371, 128582. [Google Scholar] [CrossRef]
- Shibata, S.; Arimura, S.I.; Ishikawa, T.; Awai, K. Alterations of Membrane Lipid Content Correlated With Chloroplast and Mitochondria Development in Euglena gracilis. Front. Plant Sci. 2018, 9, 370. [Google Scholar] [CrossRef]
- Wang, Y.; Seppänen-Laakso, T.; Rischer, H.; Wiebe, M.G. Euglena gracilis growth and cell composition under different temperature, light and trophic conditions. PLoS ONE 2018, 13, e0195329. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Cordeiro, N. Microalgae as Sustainable Biofactories to Produce High-Value Lipids: Biodiversity, Exploitation, and Biotechnological Applications. Mar. Drugs 2021, 19, 573. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: A review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Bedard, S.; Roxborough, E.; O’Neill, E.; Mangal, V. The biomolecules of Euglena gracilis: Harnessing biology for natural solutions to future problems. Protist 2024, 175, 126044. [Google Scholar] [CrossRef]
- Pollak, D.W.; Bostick, M.W.; Yoon, H.; Wang, J.; Hollerbach, D.H.; He, H.; Damude, H.G.; Zhang, H.; Yadav, N.S.; Hong, S.-P.; et al. Isolation of a Δ5 Desaturase Gene from Euglena gracilis and Functional Dissection of Its HPGG and HDASH Motifs. Lipids 2012, 47, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.G.; Browse, J. The Δ8-Desaturase of Euglena gracilis: An Alternate Pathway for Synthesis of 20-Carbon Polyunsaturated Fatty Acids. Arch. Biochem. Biophys. 1999, 365, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Morrison, E.N.; Noble, A.J.; Farrow, S.C. A simple and effective cryopreservation protocol for the industrially important and model organism, Euglena gracilis. STAR Protoc. 2022, 3, 101043. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Mangal, V.; Donaldson, M.E.; Lewis, A.; Saville, B.J.; Guéguen, C. Identifying Euglena gracilis Metabolic and Transcriptomic Adaptations in Response to Mercury Stress. Front. Environ. Sci. 2022, 10, 836732. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Thapa, R.K.; Tian, G.; Lu, Q.S.M.; Yu, Y.; Shu, J.; Chen, C.; Song, J.; Xie, X.; Shan, B.; Nguyen, V.; et al. NUCLEOPORIN1 mediates proteasome-based degradation of ABI5 to regulate Arabidopsisseed germination. bioRxiv 2023. [Google Scholar] [CrossRef]
- Papadopoulos, J.S.; Agarwala, R. COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef]
- Axelsson, M.; Gentili, F. A Single-Step Method for Rapid Extraction of Total Lipids from Green Microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.B.; Taylor, M.C.; Zhou, X.-R.; Vanhercke, T.; Wood, C.C.; Blanchard, C.L.; Singh, S.P.; Petrie, J.R. Metabolic engineering of medium-chain fatty acid biosynthesis in Nicotiana benthamiana plant leaf lipids. Front. Plant Sci. 2015, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.; Nguyen, T.-T.; Frøyland, L.; Wang, J.; Kang, J.X. Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J. Chromatogr. A 2008, 1212, 106–113. [Google Scholar] [CrossRef]
- Nomura, T.; Yoshikawa, M.; Suzuki, K.; Mochida, K. Highly Efficient CRISPR-Associated Protein 9 Ribonucleoprotein-Based Genome Editing in Euglena gracilis. STAR Protoc. 2020, 1, 100023. [Google Scholar] [CrossRef]
- Ebenezer, T.E.; Low, R.S.; O’Neill, E.C.; Huang, I.; DeSimone, A.; Farrow, S.C.; Field, R.A.; Ginger, M.L.; Guerrero, S.A.; Hammond, M.; et al. Euglena International Network (EIN): Driving euglenoid biotechnology for the benefit of a challenged world. Biol. Open 2022, 11, bio059561. [Google Scholar] [CrossRef]
- Ebenezer, T.E.; Zoltner, M.; Burrell, A.; Nenarokova, A.; Novák Vanclová, A.M.G.; Prasad, B.; Soukal, P.; Santana-Molina, C.; O’Neill, E.; Nankissoor, N.N.; et al. Transcriptome, proteome and draft genome of Euglena gracilis. BMC Biol. 2019, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dong, Y.; Duan, S.; He, J.; Qin, H.; Bian, C.; Chen, Z.; Liu, C.; Zheng, C.; Du, M.; et al. A chromosome-level genome assembly for the paramylon-producing microalga Euglena gracilis. Sci. Data 2024, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Matouk, A.M.; Abu-Elreesh, G.M.; Abdel-Rahman, M.A.; Desouky, S.E.; Hashem, A.H. Response surface methodology and repeated-batch fermentation strategies for enhancing lipid production from marine oleaginous Candida parapsilosis Y19 using orange peel waste. Microb. Cell Factories 2025, 24, 16. [Google Scholar] [CrossRef]
- Uprety, B.K.; Rakshit, S.K. Use of Essential Oils From Various Plants to Change the Fatty Acids Profiles of Lipids Obtained From Oleaginous Yeasts. J. Am. Oil Chem. Soc. 2018, 95, 135–148. [Google Scholar] [CrossRef]
- Toyama, T.; Hanaoka, T.; Yamada, K.; Suzuki, K.; Tanaka, Y.; Morikawa, M.; Mori, K. Enhanced production of biomass and lipids by Euglena gracilis via co-culturing with a microalga growth-promoting bacterium, Emticicia sp. EG3. Biotechnol. Biofuels 2019, 12, 205. [Google Scholar] [CrossRef]
- Du, F.; Zhang, F.; Hang, Y.; Jing, H.; Zheng, Y.; Ma, W.; Sun, X.; Huang, H. Advances in production of customized functional unsaturated fatty acids in Yarrowia lipolytica. Agric. Prod. Process. Storage 2025, 1, 14. [Google Scholar] [CrossRef]
- Duman-Özdamar, Z.E.; Martins dos Santos, V.A.P.; Hugenholtz, J.; Suarez-Diez, M. Tailoring and optimizing fatty acid production by oleaginous yeasts through the systematic exploration of their physiological fitness. Microb. Cell Factories 2022, 21, 228. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Wang, K.; Lin, L.; Liu, H.-H.; Ledesma-Amaro, R.; Ji, X.-J. Reprogramming the fatty acid metabolism of Yarrowia lipolytica to produce the customized omega-6 polyunsaturated fatty acids. Bioresour. Technol. 2023, 383, 129231. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.G.; Rousseau, D.L. Hydrogen bonding of iron-coordinated histidine in heme proteins. J. Struct. Biol. 1992, 109, 13–17. [Google Scholar] [CrossRef]
- Thumuluri, V.; Almagro Armenteros, J.J.; Johansen, A.R.; Nielsen, H.; Winther, O. DeepLoc 2.0: Multi-label subcellular localization prediction using protein language models. Nucleic Acids Res. 2022, 50, W228–W234. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K.; Arumugam, N. The FAD2 Gene in Plants: Occurrence, Regulation, and Role. Front. Plant Sci. 2017, 8, 1789. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, R.K.; Uprety, B.K.; Emery, R.J.N.; Farrow, S.C. Identification and Characterization of the Delta-12 Fatty Acid Desaturase from Euglena gracilis. Bioresour. Bioprod. 2025, 1, 8. https://doi.org/10.3390/bioresourbioprod1020008
Thapa RK, Uprety BK, Emery RJN, Farrow SC. Identification and Characterization of the Delta-12 Fatty Acid Desaturase from Euglena gracilis. Bioresources and Bioproducts. 2025; 1(2):8. https://doi.org/10.3390/bioresourbioprod1020008
Chicago/Turabian StyleThapa, Raj Kumar, Bijaya Kumar Uprety, R. J. Neil Emery, and Scott C. Farrow. 2025. "Identification and Characterization of the Delta-12 Fatty Acid Desaturase from Euglena gracilis" Bioresources and Bioproducts 1, no. 2: 8. https://doi.org/10.3390/bioresourbioprod1020008
APA StyleThapa, R. K., Uprety, B. K., Emery, R. J. N., & Farrow, S. C. (2025). Identification and Characterization of the Delta-12 Fatty Acid Desaturase from Euglena gracilis. Bioresources and Bioproducts, 1(2), 8. https://doi.org/10.3390/bioresourbioprod1020008






