Ammonia Losses, Wheat Biomass, and N Dynamics as Influenced by Organo-Mineral Fertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Soil Characterization
2.2. Treatments and N-NH3 Volatilization Experiment
2.3. Determination of N-NH3 Losses
2.4. Wheat Cultivation and Harvest
2.5. Total N Concentration in Wheat Plants
2.6. Statistical Analyses
3. Results
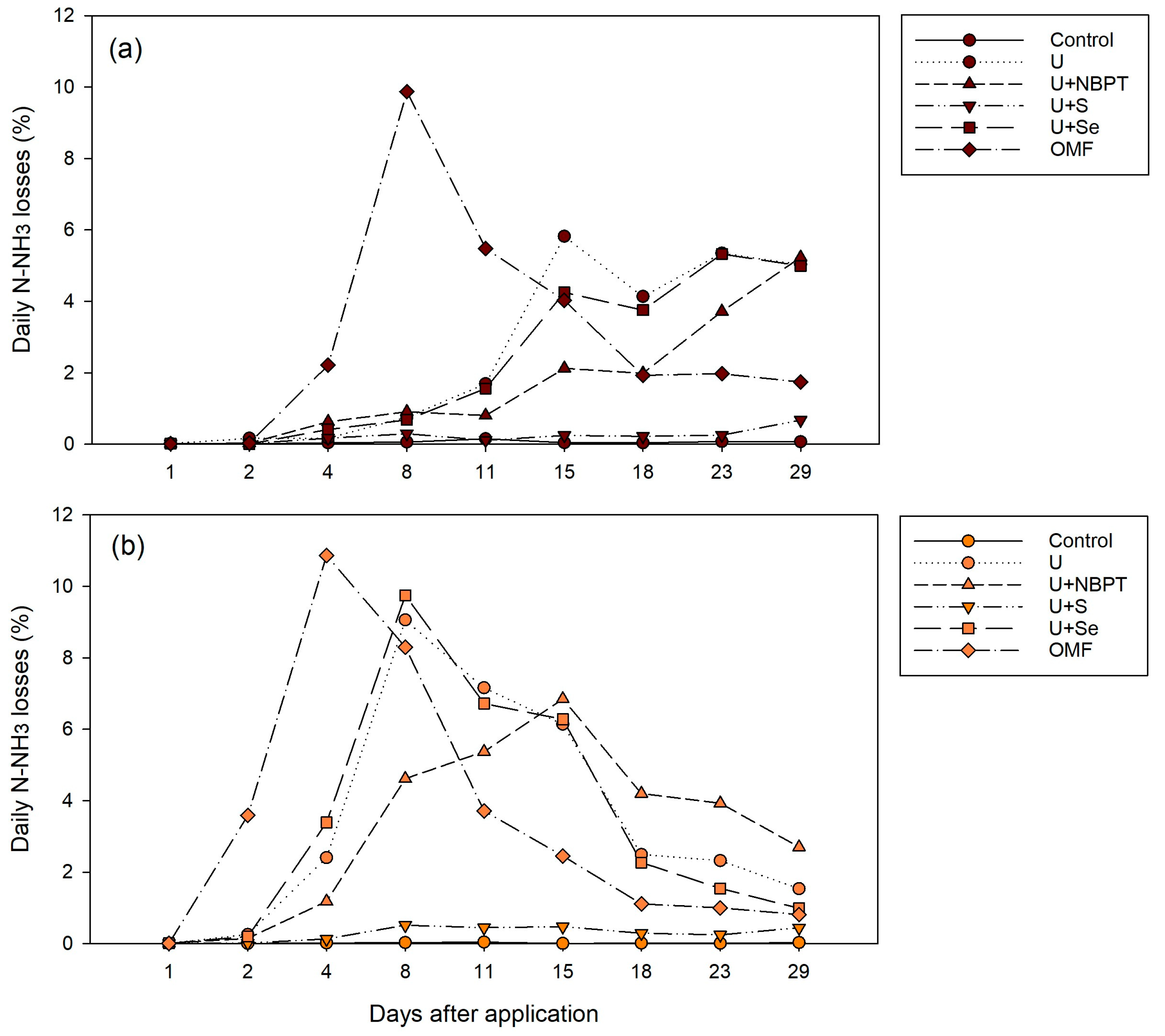
3.1. Daily N-NH3 Losses by Volatilization
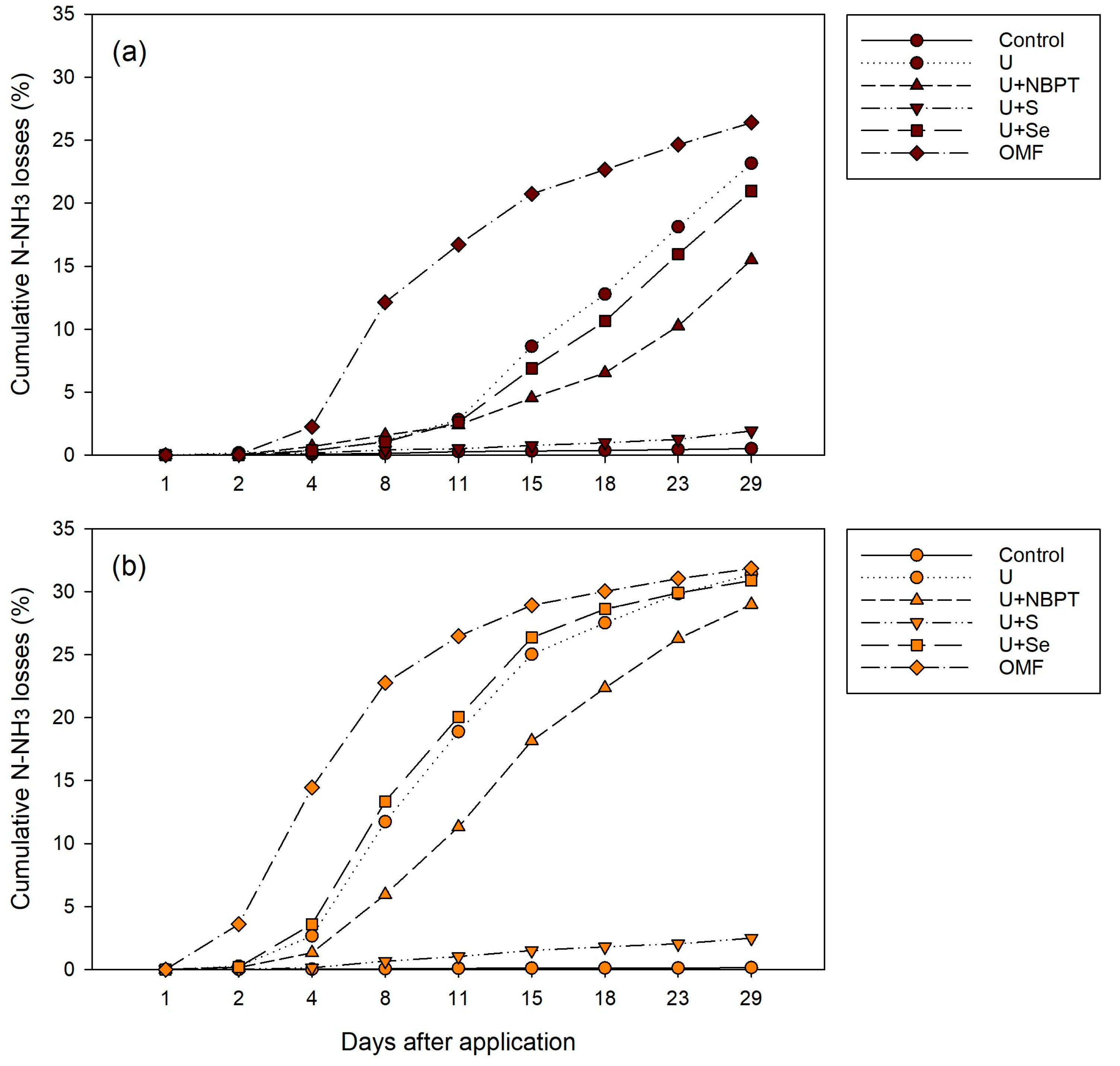
3.2. Cumulative N-NH3 Losses as Affected by N-Fertilizers
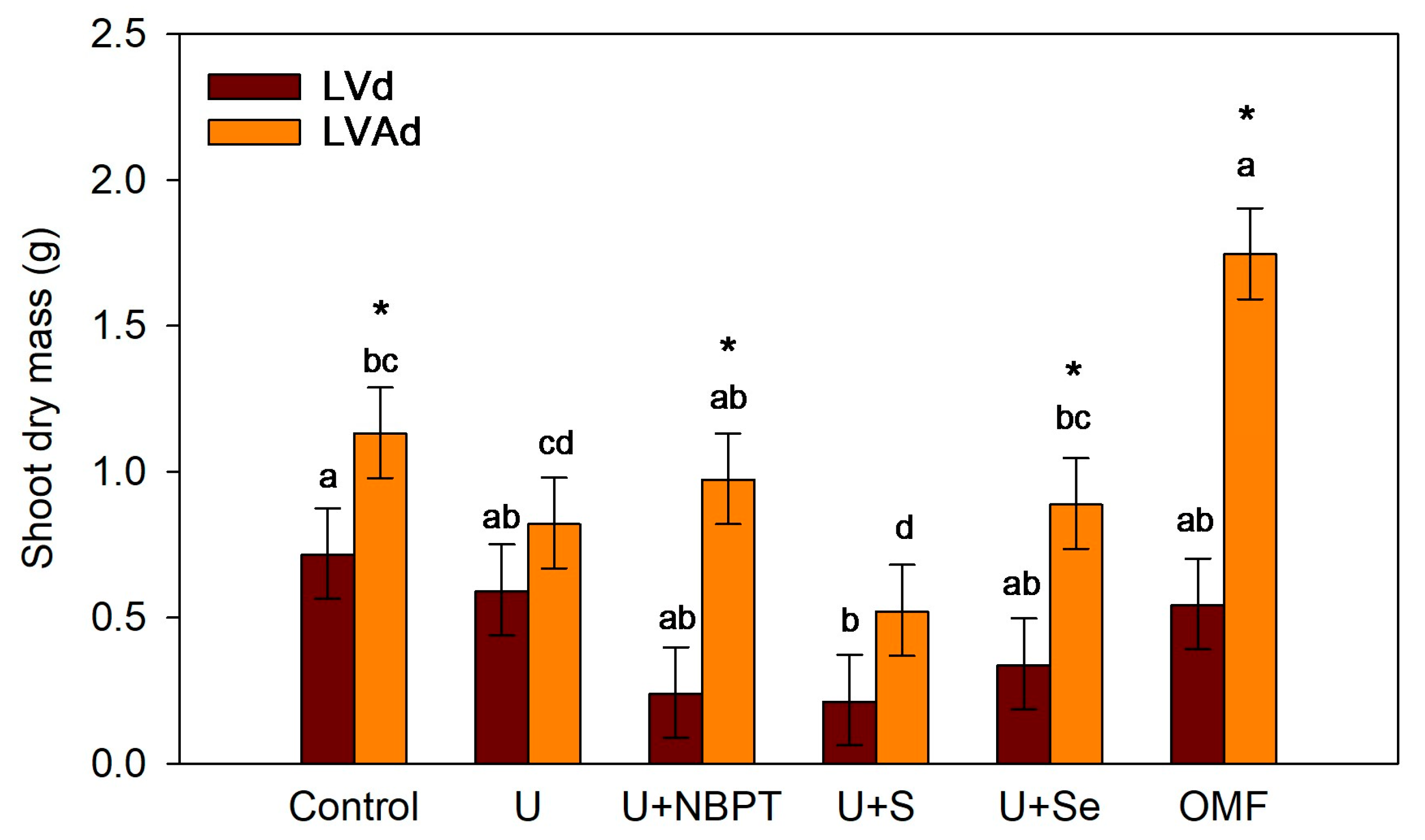
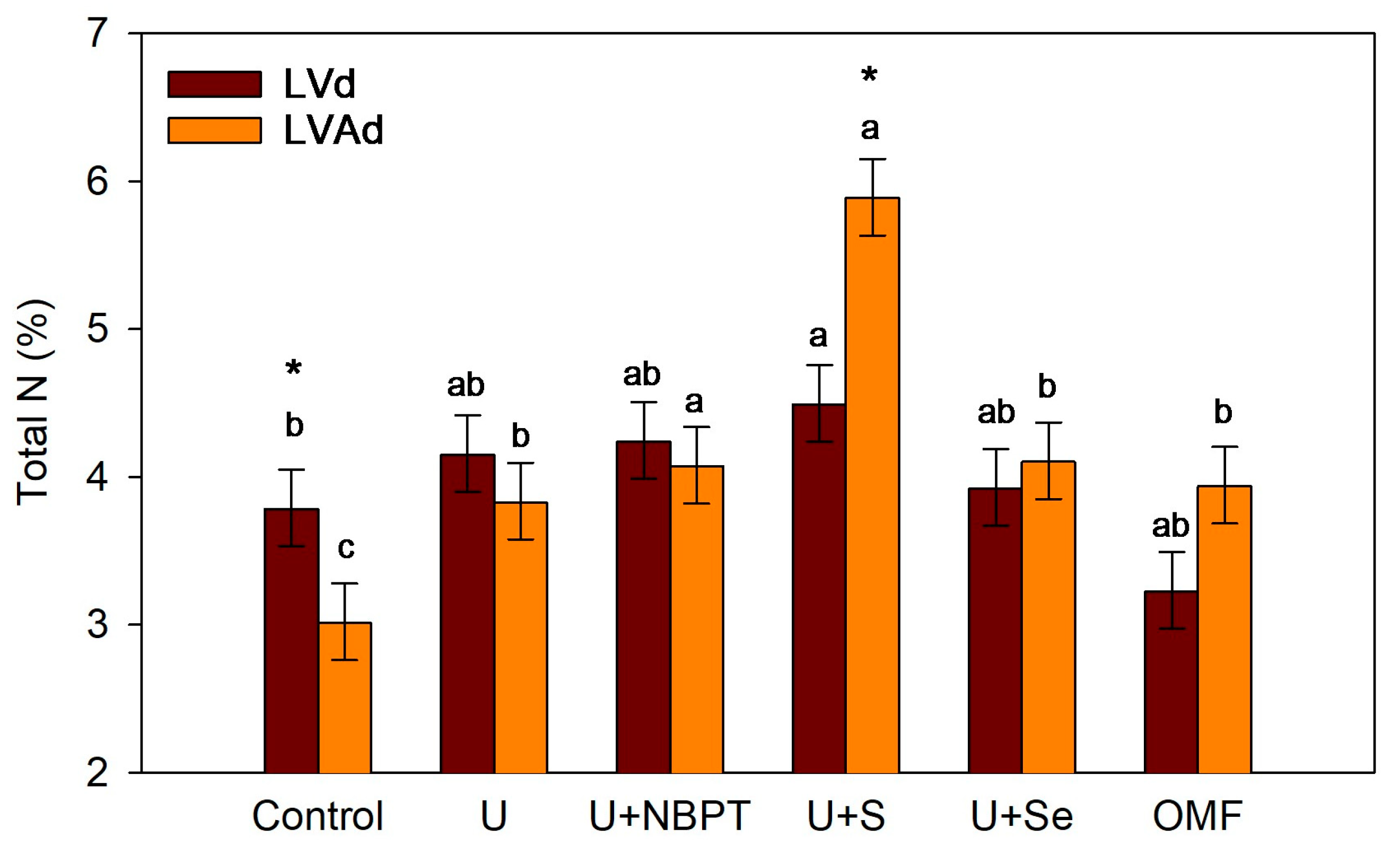
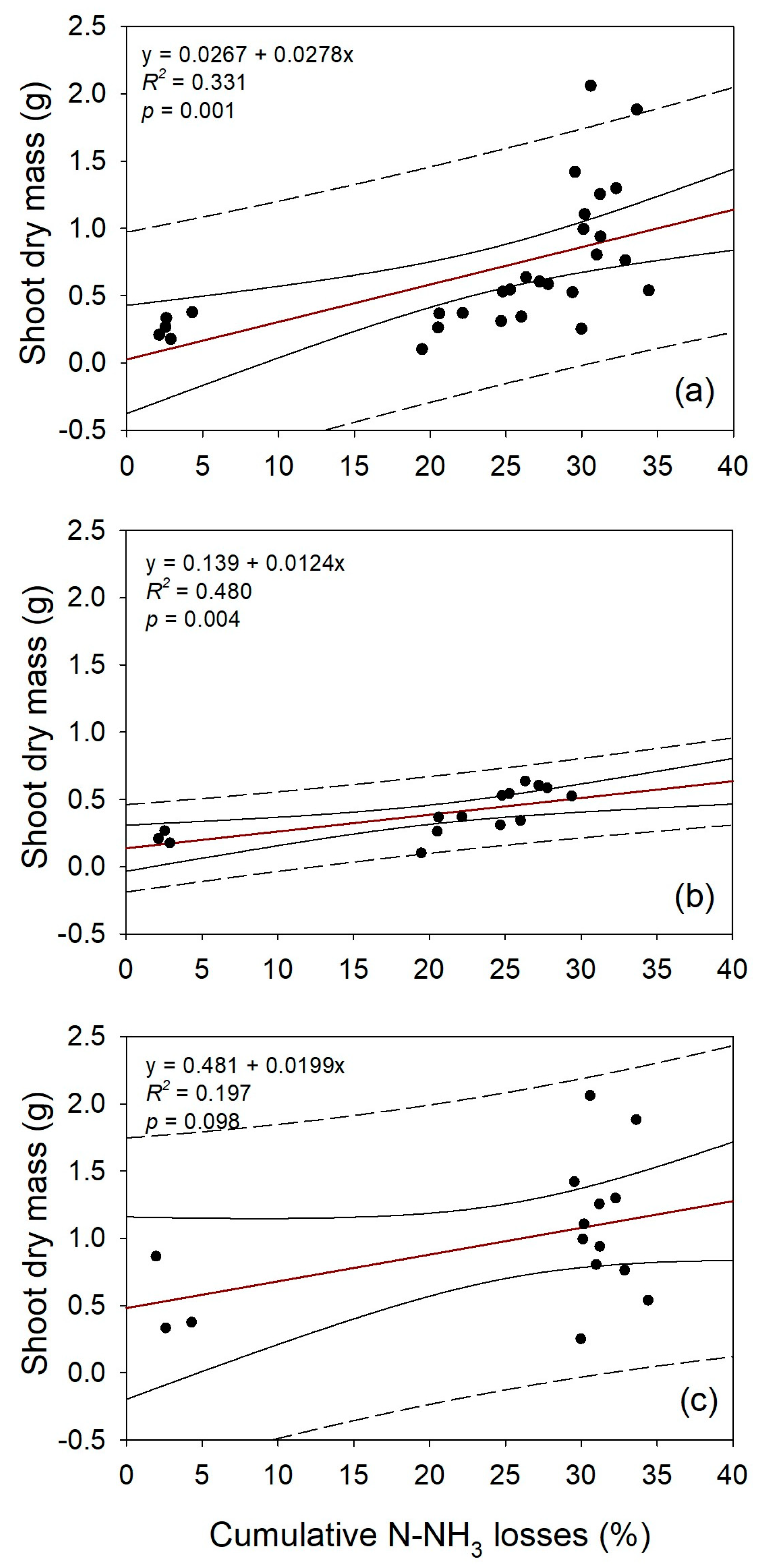
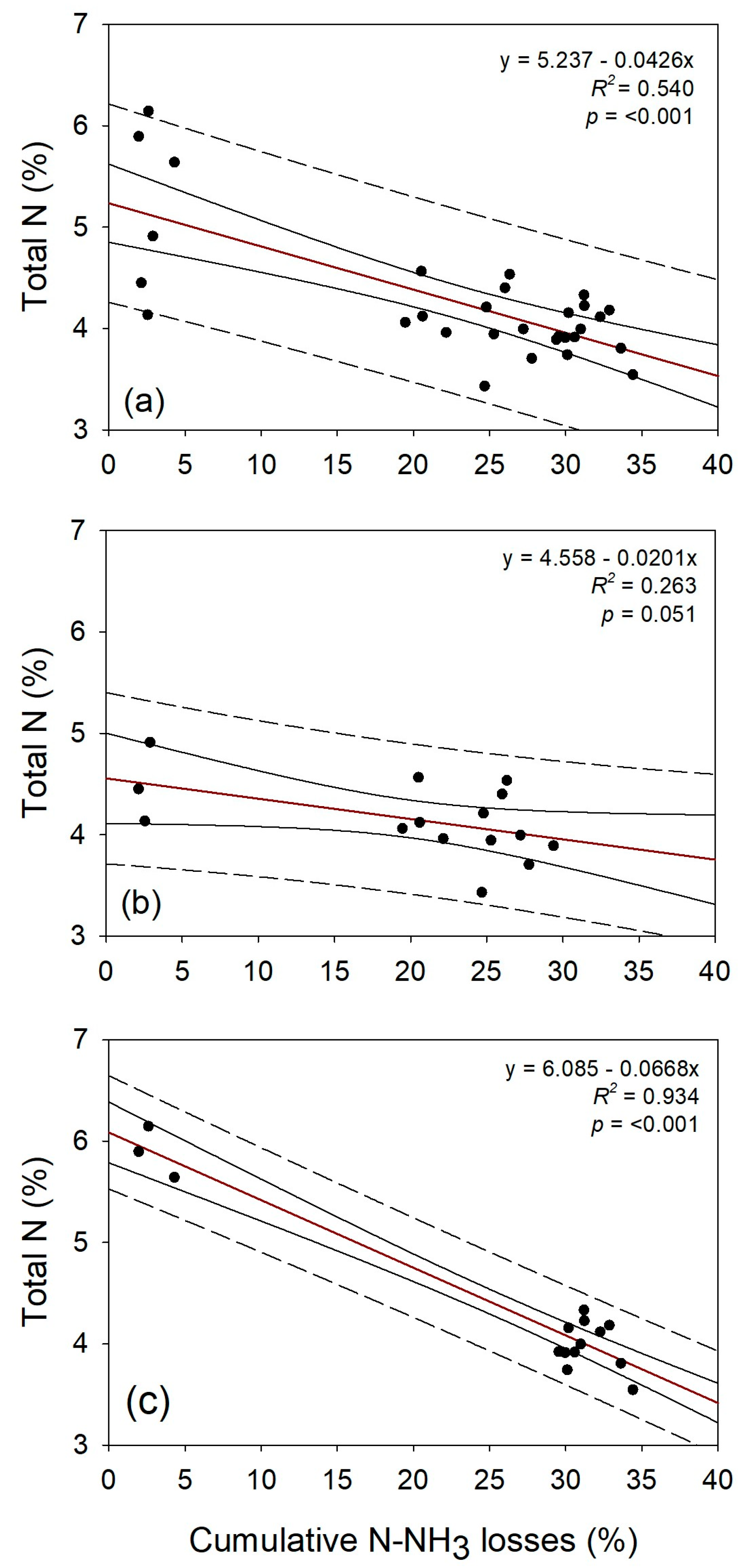
3.3. Shoot Drymass and N Concentration in Wheat
3.4. Relationship Between N-NH3 Losses, Aboveground Biomass, and N Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture—Alternative Pathways to 2050, 1st ed.; FAO: Rome, Italy, 2018; Available online: https://openknowledge.fao.org/handle/20.500.14283/i8429en (accessed on 26 March 2025).
- United Nations. The 17 Goals; United Nations: New York, NY, USA, 2025; Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 26 March 2025).
- Velenturf, A.P.M.; Purnell, P. Principles for a sustainable circular economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Lima, D.Q.; Oliveira, L.C.A.; Bastos, A.R.R.; Carvalho, G.S.; Marques, J.J.G.S.M.; Carvalho, J.G.; De Souza, G.A. Leather industry solid waste as nitrogen source for growth of common bean plants. Appl. Environ. Soil Sci. 2010, 2010, 703842. [Google Scholar] [CrossRef]
- Coelho, L.C.; Ferreira, M.M.; Bastos, A.R.R.; Oliveira, L.C.A.D.; Ferreira, E.D. Resíduo de curtumes como fonte de nitrogênio para trigo e arroz em sucessão. Rev. Bras. Ciênc. Solo 2015, 39, 1445–1455. [Google Scholar] [CrossRef]
- Majee, S.; Halder, G.; Mandal, D.D.; Tiwari, O.N.; Mandal, T. Transforming wet blue leather and potato peel into an eco-friendly bio-organic NPK fertilizer for intensifying crop productivity and retrieving value-added recyclable chromium salts. J. Hazard. Mater. 2021, 411, 125046. [Google Scholar] [CrossRef] [PubMed]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- Tasca, F.A.; Ernani, P.R.; Rogeri, D.A.; Gatiboni, L.C.; Cassol, P.C. Volatilização de amônia do solo após a aplicação de ureia convencional ou com inibidor de urease. Rev. Bras. Ciênc. Solo 2011, 35, 493–509. [Google Scholar] [CrossRef]
- Santos, C.F.; Aragão, O.O.S.; Silva, D.R.G.; Jesus, E.C.; Chagas, W.F.T.; Correia, P.S.; Moreira, F.M.S. Environmentally friendly urea produced from the association of N-(n-butyl) thiophosphoric triamide with biodegradable polymer coating obtained from a soybean processing byproduct. J. Clean. Prod. 2020, 276, 123014. [Google Scholar] [CrossRef]
- Santos, C.F.; Nunes, A.P.P.; Aragão, O.O.S.; Guelfi, D.; Souza, A.A.; Abreu, L.B.; Lima, A.D.C. Dual functional coatings for urea to reduce ammonia volatilization and improve nutrients use efficiency in a Brazilian corn crop system. J. Soil Sci. Plant Nutr. 2021, 21, 1591–1609. [Google Scholar] [CrossRef]
- Cancellier, E.L.; Silva, D.R.G.; Faquin, V.; Gonçalves, B.A.; Cancellier, L.L.; Spehar, C.R. Ammonia volatilization from enhanced-efficiency urea on no-till maize in Brazilian cerrado with improved soil fertility. Ciênc. Agrotec. 2016, 40, 133–144. [Google Scholar] [CrossRef]
- Pereira, H.S.; Leão, A.F.; Verginassi, A.; Carneiro, M.A.C. Ammonia volatilization of urea in the out-of-season corn. Rev. Bras. Ciênc. Solo 2009, 33, 1685–1694. [Google Scholar] [CrossRef]
- Prando, A.M.; Zucareli, C.; Fronza, V.; Bassoi, M.C.; Oliveira, F.Á. Formas de ureia e doses de nitrogênio em cobertura no desempenho agronômico de genótipos de trigo. Semin. Agrar. 2012, 33, 621–632. [Google Scholar] [CrossRef]
- Zavaschi, E.; Faria, L.A.; Vitti, G.C.; Nascimento, C.A.C.; Moura, T.A.; Vale, D.W.; Mendes, F.L.; Kamogawa, M.Y. Ammonia volatilization and yield components after application of polymer-coated urea to maize. Rev. Bras. Ciênc. Solo 2014, 38, 1200–1206. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.B. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA-Natural Resources Conservation Service (NRCS): Washington, DC, USA, 2022.
- Lima, F.R.D.D.; Silva, A.O.; Amorim, H.C.S.; Figueredo, E.S.S.; Carneiro, R.M.C.; Jara, M.S.; Carneiro, M.A.C.; Guilherme, L.R.G. Agronomic and environmental aspects of organo-mineral fertilizers produced with a by-product of the intermediate process of tanning. Front. Agron. 2023, 5, 1215448. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. (Eds.) Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1085209 (accessed on 26 March 2025).
- Malavolta, E. Elementos da Nutrição Mineral de Plantas; Agronômica Ceres: Piracicaba, Brazil, 1980. [Google Scholar]
- Araújo, E.D.S.; Marsola, T.; Miyazawa, M.; Soares, L.H.B.; Urquiaga, S.; Boddey, R.M.; Alves, B.J.R. Calibration of a semi-opened static chamber for the quantification of volatilized ammonia from soil. Pesqui. Agropecu. Bras. 2009, 44, 769–776. [Google Scholar] [CrossRef]
- Martins, M.R.; Sarkis, L.F.; Sant’Anna, S.A.C.; Santos, C.A.; Araujo, K.E.; Santos, R.C.; Araújo, E.S.; Alves, B.J.R.; Jantalia, C.P.; Boddey, R.M.; et al. Optimizing the use of open chambers to measure ammonia volatilization in field plots amended with urea. Pedosphere 2021, 31, 243–254. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Fonseca, A.B.; Santos, C.; Nunes, A.P.P.; Oliveira, D.P.; Melo, M.E.A.; Takayama, T.; Mansur, B.L.; Fernandes, T.J.; Alexandrino, G.C.; Dias, M.A.N.; et al. Urease inhibitors technologies as strategy to mitigate agricultural ammonia emissions and enhance the use efficiency of urea-based fertilizers. Sci. Rep. 2023, 13, 22739. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Zantua, M.I.; Dumenil, L.C.; Bremner, J.M. Relationships between soil urease activity and other soil properties. Soil Sci. Soc. Am. J. 1977, 41, 350–352. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Roscoe, R.; Vasconcellos, C.A.; Furtini Neto, A.E.; Guedes, G.A.A.; Fernandes, L.A. Urease activity and its relation to soil organic matter, microbial biomass nitrogen and urea-nitrogen assimilation by maize in a Brazilian Oxisol under no-tillage and tillage systems. Biol. Fertil. Soils 2000, 32, 52–59. [Google Scholar] [CrossRef]
- Sousa, R.D.M.; Cantarella, H.; Soares, J.R.; Abreu, F.D.; Moraes, J.G.D. Volatilização de NH3 de ureia recoberta por polímero não hidrossolúvel aplicada na superfície do solo. In Proceedings of the Congresso Brasileiro de Ciência do Solo, Florianópolis, SC, Brazil, 28 July–2 August 2013; Volume 1, pp. 1–4. [Google Scholar]
- Faria, L.; Karp, F.H.S.; Machado, M.C.; Abdalla, A.L. Ammonia volatilization losses from urea coated with copper, boron, and selenium. Semin. Ciênc. Agrar. 2020, 41, 1415–1420. [Google Scholar] [CrossRef]
- Cantarella, H.; Trivelin, P.C.O.; Contin, T.L.M.; Dias, F.L.F.; Rossetto, R.; Marcelino, R.; Coimbra, R.B.; Quaggio, J.A. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci. Agric. 2008, 65, 397–401. [Google Scholar] [CrossRef]
- Prasad, M. Gaseous Loss of Ammonia from Sulfur-Coated Urea, Ammonium Sulfate, and Urea Applied to Calcareous Soil (pH 7.3). Soil Sci. Soc. Am. J. 1976, 40, 131–134. [Google Scholar] [CrossRef]
- Oliveira-Longatti, S.M.; Cannata, M.G.; Costa Ferrazani, T.S.C.C.C.; Guilherme, L.R.G. Agronomic and environmental implications of using a by-product of the intermediate process. Sci. Agric. 2017, 74, 250–257. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Pooniya, V.; Pal, M.; Ghasal, P.C.; Bana, R.; Jat, S.L. Coated Urea Materials for Improving Yields, Profitability, and Nutrient Use Efficiencies of Aromatic Rice. Glob. Chall. 2019, 3, 1900013. [Google Scholar] [CrossRef]
- Santos, W.M.; Alves, B.J.R.; Urquiaga, S.; Pacheco, E.P.; Barros, I.; Fernandes, M.F.; Batista, J.N.; Bender, E.P.; Souza, H.N.; Jantalia, C.P. Ammonia volatilization and yield of corn fertilized with different nitrogen sources in the Brazilian semiarid. Pesqui. Agropecu. Bras. 2020, 55, e01036. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Ashworth, A.J.; Arsi, K.; Rojas, M.G.; Morales-Ramos, J.A.; Donoghue, A.; Robinson, K. Insect frass composition and potential use as an organic fertilizer in circular economies. J. Econ. Entomol. 2024, 117, 1261–1268. [Google Scholar] [CrossRef]

| Soil † | Chemical Characterization ‡ | ||||||
|---|---|---|---|---|---|---|---|
| pH | SOM | P | K | Ca | Mg | S | |
| 1:2.5 (H2O) | % | mg dm−3 | |||||
| LVd | 5.85 | <0.2 | 2.51 | 19.81 | 0.46 | <0.1 | 15.27 |
| LVAd | 4.81 | <0.2 | 3.15 | 52.68 | 0.14 | <0.1 | 2.70 |
| SB | t | T | V | m | Al | H + Al | |
| cmolc dm−3 | % | cmolc dm−3 | |||||
| LVd | 0.61 | 0.71 | 1.19 | 51.00 | 0 | <0.1 | 0.58 |
| LVAd | 0.37 | 0.47 | 2.35 | 16.00 | 0 | <0.1 | 1.98 |
| Treatments | Soils | |
|---|---|---|
| LVd | LVAd | |
| Control | 27.0 ± 5.7 b † | 32.2 ± 5.7 b |
| U | 24.8 ± 5.7 bc | 32.0 ± 5.7 b |
| U + NBPT | 10.4 ± 5.7 d | 39.9 ± 5.7 b |
| U + S | 9.7 ± 5.7 d | 20.8 ± 7.1 bc |
| U + Se | 13.5 ± 5.7 cd | 36.7 ± 5.7 ab |
| OMF | 21.5 ± 5.7 bc | 68.6 ± 5.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana Amorim, H.C.; Dias de Lima, F.R.; Duarte, M.H.; Carbone Carneiro, R.M.; Van Opbergen, G.A.Z.; Corrêa, R.F.R.; Guilherme, L.R.G. Ammonia Losses, Wheat Biomass, and N Dynamics as Influenced by Organo-Mineral Fertilizer. Biosphere 2025, 1, 4. https://doi.org/10.3390/biosphere1010004
Santana Amorim HC, Dias de Lima FR, Duarte MH, Carbone Carneiro RM, Van Opbergen GAZ, Corrêa RFR, Guilherme LRG. Ammonia Losses, Wheat Biomass, and N Dynamics as Influenced by Organo-Mineral Fertilizer. Biosphere. 2025; 1(1):4. https://doi.org/10.3390/biosphere1010004
Chicago/Turabian StyleSantana Amorim, Helen Carla, Francielle Roberta Dias de Lima, Mariene Helena Duarte, Rafael Marta Carbone Carneiro, Gustavo Avelar Zorgdrager Van Opbergen, Raphael Felipe Rodrigues Corrêa, and Luiz Roberto Guimarães Guilherme. 2025. "Ammonia Losses, Wheat Biomass, and N Dynamics as Influenced by Organo-Mineral Fertilizer" Biosphere 1, no. 1: 4. https://doi.org/10.3390/biosphere1010004
APA StyleSantana Amorim, H. C., Dias de Lima, F. R., Duarte, M. H., Carbone Carneiro, R. M., Van Opbergen, G. A. Z., Corrêa, R. F. R., & Guilherme, L. R. G. (2025). Ammonia Losses, Wheat Biomass, and N Dynamics as Influenced by Organo-Mineral Fertilizer. Biosphere, 1(1), 4. https://doi.org/10.3390/biosphere1010004







