Influence of Quercetin and tt-Farnesol Enrichment on Physicochemical Properties of a Universal Adhesive System
Abstract
:1. Introduction
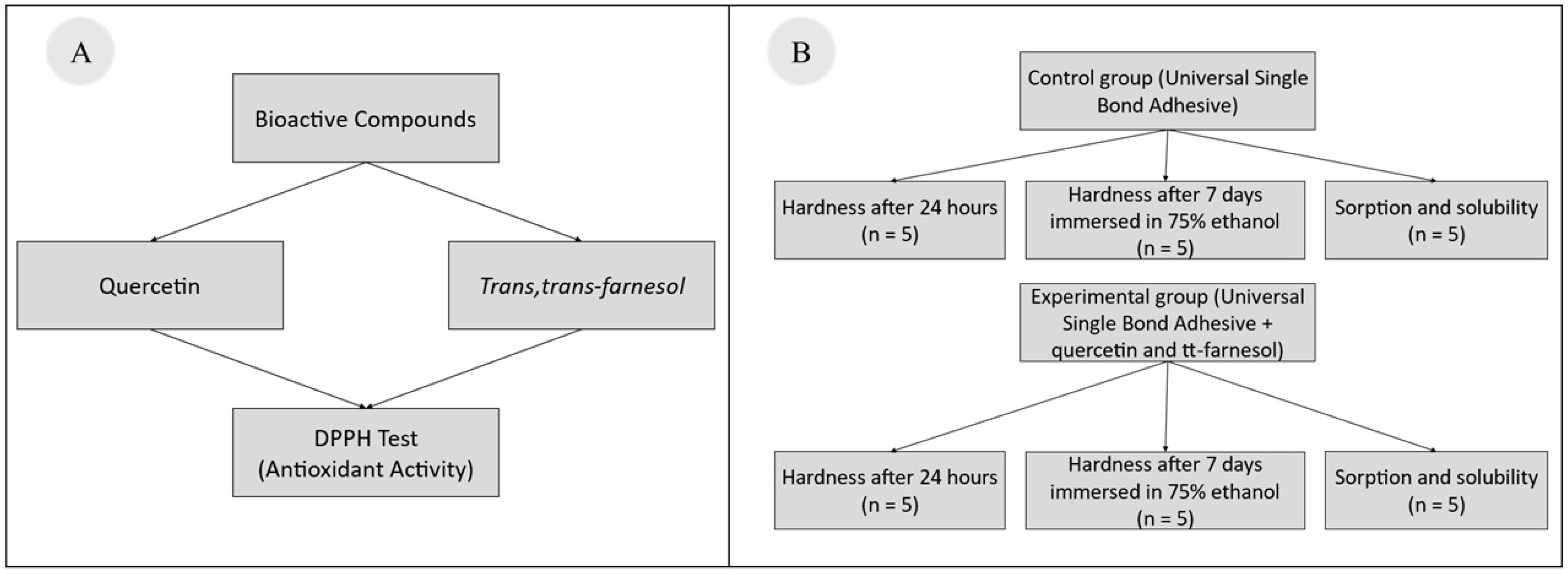
2. Materials and Methods
2.1. DPPH Radical Scavenging Assay
2.2. Specimen Preparation
2.3. Water Sorption and Solubility
2.4. Hardness and Softening Percentage
2.5. Statistical Analysis
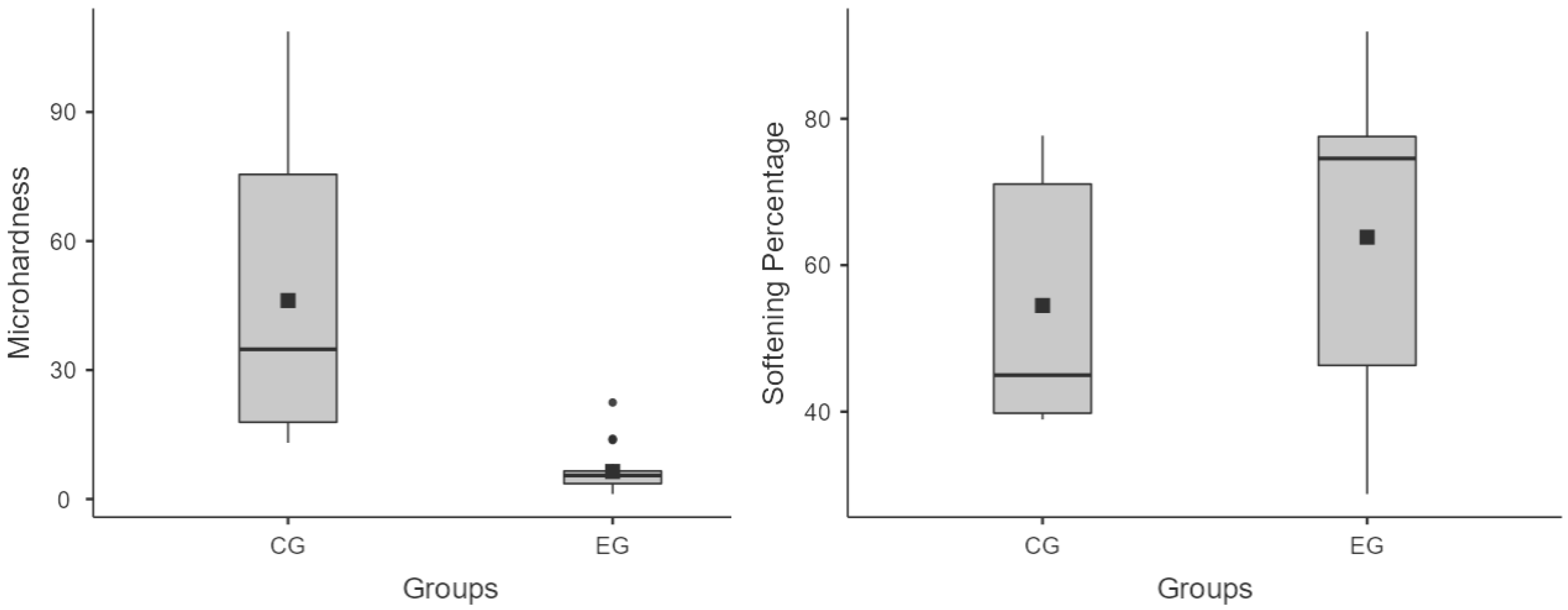
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying adhesive materials to improve the longevity of resinous restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef]
- Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion–not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.; Carvalho, R.M.; Tay, F.R.; et al. Strategies to prevent hydrolytic degradation of the hybrid layer—A review. Dent. Mater. 2013, 29, 999–1011. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Hardan, L.; Daood, U.; Bourgi, R.; Cuevas-Suárez, C.E.; Devoto, W.; Zarow, M.; Jakubowicz, N.; Zamarripa-Calderón, J.E.; Radwanski, M.; Orsini, G.; et al. Effect of collagen crosslinkers on dentin bond strength of adhesive systems: A systematic review and meta-analysis. Cells 2022, 11, 2417. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef]
- Beckman, C.K.d.C.; Costa, T.d.L.; Puppin-Rontani, R.M.; de Castilho, A.R.F. Exploring the role of flavonoids in caries-affected dentin adhesion: A comprehensive scoping review. Arch. Oral Biol. 2024, 162, 105942. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.M.; Barbosa, C.d.B.; de Cena, J.A.; Ribeiro, E.; Garcia, F.C.P.; Stefani, C.M.; Dame-Teixeira, N. Effects of cross-linking agents on hydroxyproline release and root caries lesion size: Systematic review and network meta-analysis of in vitro studies. Eur. J. Oral Sci. 2024, 132, e13028. [Google Scholar] [CrossRef] [PubMed]
- Pawłowski, W.; Caban, M.; Lewandowska, U. Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms. Cancers 2024, 16, 3193. [Google Scholar] [CrossRef] [PubMed]
- del Rio, D.L.; Sartori, N.; Tomblin, N.B.; Phark, J.-H.; Pardi, V.; Murata, R.M.; Duarte, S. Bioactive dental adhesive system with tt-farnesol: Effects on dental biofilm and bonding properties. Front. Bioeng. Biotechnol. 2020, 8, 865. [Google Scholar] [CrossRef]
- Vinholes, J.; Gonçalves, P.; Martel, F.; Coimbra, M.A.; Rocha, S.M. Assessment of the antioxidant and antiproliferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models. Food Chem. 2014, 156, 204–211. [Google Scholar] [CrossRef]
- Balakin, K.V.; Ivanenkov, Y.A.; Skorenko, A.V.; Nikolsky, Y.V.; Savchuk, N.P.; Ivashchenko, A.A. In silico estimation of DMSO solubility of organic compounds for bioscreening. J. Biomol. Screen. 2004, 9, 22–31. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- ISO 4049:2019; Dentistry: Polymer-Based Restorative Materials. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- Wambier, L.; Malaquias, T.; Wambier, D.S.; Patzlaff, R.T.; Bauer, J.; Loguercio, A.D.; Reis, A. Effects of prolonged light exposure times on water sorption, solubility and cross-linking density of simplified etch-and-rinse adhesives. J. Adhes. Dent. 2014, 16, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, R.; Song, L.; Yuca, E.; Xie, S.-X.; Boone, K.; Misra, A.; Spencer, P.; Tamerler, C. Bioinspired multifunctional adhesive system for next generation bio-additively designed dental restorations. J. Mech. Behav. Biomed. Mater. 2021, 113, 104135. [Google Scholar] [CrossRef]
- Gotti, V.B.; Feitosa, V.P.; Sauro, S.; Correr-Sobrinho, L.; Leal, F.B.; Stansbury, J.W.; Correr, A.B. Effect of antioxidants on the dentin interface bond stability of adhesives exposed to hydrolytic degradation. J. Adhes. Dent. 2015, 17, 35–44. [Google Scholar] [PubMed]
- Hong, D.-W.; Chen, L.-B.; Lin, X.-J.; Attin, T.; Yu, H. Dual function of quercetin as an MMP inhibitor and crosslinker in preventing dentin erosion and abrasion: An in situ/in vivo study. Dent. Mater. 2022, 38, e297–e307. [Google Scholar] [CrossRef]
- Lopes, A.P.; Branco, R.R.d.O.C.; Oliveira, F.A.d.A.; Campos, M.A.S.; Sousa, B.d.C.; Agostinho, R.C.; Gonzalez, A.G.M.; Rocha, J.A.; Pinheiro, R.E.E.; Araújo, A.R.; et al. Antimicrobial, modulatory, and antibiofilm activity of tt-farnesol on bacterial and fungal strains of importance to human health. Bioorganic Med. Chem. Lett. 2021, 47, 128192. [Google Scholar] [CrossRef]
- Breschi, L.; Cadenaro, M.; Antoniolli, F.; Sauro, S.; Biasotto, M.; Prati, C.; Tay, F.R.; Di Lenarda, R. Polymerization kinetics of dental adhesives cured with LED: Correlation between extent of conversion and permeability. Dent. Mater. 2007, 23, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, N.; Nagpal, R.; Singh, U.; Agarwal, M. Effect of dentin biomodification techniques on the stability of the bonded interface. J. Conserv. Dent. 2021, 24, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Münchow, E.A.; da Silva, A.F.; Piva, E.; Cuevas-Suárez, C.E.M.; de Albuquerque, T.P.; Pinal, R.; Gregory, R.L.; Breschi, M.C.; Bottino, M.A. Development of an antibacterial and anti-metalloproteinase dental adhesive for long-lasting resin composite restorations. J. Mater. Chem. B 2021, 176, 139–148. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Lanza, M.D.S.; Dourado, L.G.; Carvalho, E.M.; Bauer, J. Impact of solvent evaporation and curing protocol on degree of conversion of etch-and-rinse and multimode adhesives systems. Int. J. Dent. 2019, 2019, 5496784. [Google Scholar] [CrossRef] [PubMed]
- Salim Al-Ani, A.A.S.; Salim, I.A.; Seseogullari-Dirihan, R.; Mutluay, M.; Tjäderhane, L.; Tezvergil-Mutluay, A. Incorporation of dimethyl sulfoxide into experimental hydrophilic and hydrophobic adhesive resins: Evaluation of cytotoxic activities. Eur. J. Oral Sci. 2021, 129, e12756. [Google Scholar] [CrossRef]
- Mehtälä, P.; Pashley, D.; Tjäderhane, L. Effect of dimethyl sulfoxide on dentin collagen. Dent. Mater. 2017, 33, 915–922. [Google Scholar] [CrossRef]
- Salim Al-Ani, A.A.S.; Scarabello Stape, T.H.; Mutluay, M.; Tjäderhane, L.; Tezvergil-Mutluay, A. Incorporation of dimethyl sulfoxide to model adhesive resins with different hydrophilicities: Physico/mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 93, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Salim Al-Ani, A.A.; Mutluay, M.; Stape, T.H.S.; Tjäderhane, L.; Tezvergil-Mutluay, A. Effect of various dimethyl sulfoxide concentrations on the durability of dentin bonding and hybrid layer quality. Dent. Mater. J. 2018, 37, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Ezazi, M.; Ye, Q.; Misra, A.; Tamerler, C.; Spencer, P. Autonomous-Strengthening Adhesive Provides Hydrolysis-Resistance and Enhanced Mechanical Properties in Wet Conditions. Molecules 2022, 27, 5505. [Google Scholar] [CrossRef]
| Product Name | Composition | CAS Number | Molecular Weight | Molecular Formula | Brand | Batch |
|---|---|---|---|---|---|---|
| Quercetin * | - | 849061-97-8 | 302.24 | C15H10O7 | Sigma- Aldrich | LRAB7760 |
| Trans,trans-farnesol * | - | 106-28-5 | 222.37 | C15H26O | Sigma- Aldrich | BCCC6806 |
| Single Bond Universal ** | 2-hydroxyethyl methacrylate, bisphenol A dimethacrylate dimethacrylate dimethyl (BisGMA), 2-propenoic acid, 2-methyl-, reaction products with 1,10- decanediol and phosphorus oxide (P2O5), ethanol, water, silica treated from silane, acrylic copolymer and itaconic acid, caphorquinone, dimethylaminobenzoat (-4), 2-dimethylaminoethyl methacrylate, 2,6-di-terc-butyl-p-cresol | - | - | - | 3M™ ESPE | 4126641269 4127841279 41282 |
| Compounds | Concentration | % of DPPH Absorption | |
|---|---|---|---|
| Mean | Quercetin | 0.988 | 85.8 |
| tt-farnesol | 0.988 | 36.3 | |
| Median | Quercetin | 0.388 | 88.0 |
| tt-farnesol | 0.388 | 20.6 | |
| Standard deviation | Quercetin | 1.04 | 3.51 |
| tt-farnesol | 1.04 | 44.3 | |
| Minimum | Quercetin | 0.244 | 80.2 |
| tt-farnesol | 0.244 | −5.30 | |
| Maximum | Quercetin | 2.62 | 88.3 |
| tt-farnesol | 2.62 | 85.2 | |
| 25th percentile | Quercetin | 0.258 | 84.7 |
| tt-farnesol | 0.258 | −0.920 | |
| 50th percentile | Quercetin | 0.388 | 88.0 |
| tt-farnesol | 0.388 | 20.6 | |
| 75th percentile | Quercetin | 1.43 | 88.1 |
| tt-farnesol | 1.43 | 82.0 |
| Group | Mean (SD) | |
|---|---|---|
| Sorption | CG | 132.3 (18.04) a |
| EG | 173 (12.7) a | |
| Solubility | CG | 34.4 (9.02) b |
| EG | 163 (11.8) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, R.J.S.; Puppin-Rontani, R.M.; de Castilho, A.R.F. Influence of Quercetin and tt-Farnesol Enrichment on Physicochemical Properties of a Universal Adhesive System. Adhesives 2025, 1, 3. https://doi.org/10.3390/adhesives1010003
Mendes RJS, Puppin-Rontani RM, de Castilho ARF. Influence of Quercetin and tt-Farnesol Enrichment on Physicochemical Properties of a Universal Adhesive System. Adhesives. 2025; 1(1):3. https://doi.org/10.3390/adhesives1010003
Chicago/Turabian StyleMendes, Roberta Janaína Soares, Regina Maria Puppin-Rontani, and Aline Rogéria Freire de Castilho. 2025. "Influence of Quercetin and tt-Farnesol Enrichment on Physicochemical Properties of a Universal Adhesive System" Adhesives 1, no. 1: 3. https://doi.org/10.3390/adhesives1010003
APA StyleMendes, R. J. S., Puppin-Rontani, R. M., & de Castilho, A. R. F. (2025). Influence of Quercetin and tt-Farnesol Enrichment on Physicochemical Properties of a Universal Adhesive System. Adhesives, 1(1), 3. https://doi.org/10.3390/adhesives1010003







