1. Introduction
West Nile virus (WNV) is a neurotropic, mosquito-borne virus belonging to the Flavivirus genus [
1]. In nature, it is sustained through a mosquito–bird–mosquito transmission cycle, with
Culex species widely recognized as the primary vectors. Wild birds are the predominant reservoir hosts of the virus [
2]. Humans are incidental hosts, while vulnerable population groups at risk are the elderly and people with an underlying disease or with immunosuppression. Still no vaccine is available for humans [
3,
4].
Several studies across Europe and beyond have identified a range of environmental factors associated with the prevalence of WNV-infected mosquitoes and human cases [
5,
6,
7]. Temperature has consistently emerged as a key driver, with higher temperatures linked to earlier onset and prolonged mosquito seasons, increased infection rates, and, in some cases, reduced adult mosquito survival during peak summer periods [
8,
9,
10,
11,
12,
13]. Precipitation has shown a more complex relationship: reduced rainfall has been associated with higher mosquito infection rates and increased WNV circulation in some areas, while early-season precipitation has been linked to delayed mosquito emergence but greater overall abundance later in the season [
8,
9,
10,
11,
14]. Ecological and landscape variables, such as proximity to breeding habitats, elevation, vegetation indices, land use, and habitat fragmentation, have also been found to influence mosquito abundance and infection dynamics [
11,
15]. Additional climatic variables, including wind speed and overwintering conditions, have been shown to affect mosquito survival and WNV transmission [
16,
17,
18]. These findings reflect the multifactorial nature of WNV transmission dynamics and highlight the increasing influence of climate change. Ongoing shifts in temperature, precipitation, and the frequency of extreme weather events are altering the ecological balance in many regions, creating more favorable conditions for mosquito proliferation and virus amplification. As a result, the interplay between changing climate conditions and the geographic expansion of WNV has become a growing concern in public health research [
19,
20].
In this study, we investigated the short-term effects of 2 m air temperature and total precipitation on the occurrence of WNV human cases in Greece from 2010 to 2024. Employing a space-time-stratified case-crossover design and distributed lag nonlinear models, we aimed to capture the delayed and nonlinear associations between environmental conditions and disease incidence at the provincial (NUTS3) level. By quantifying these associations through Odds Ratios (OR), our goal was to understand the spatiotemporal dynamics of WNV transmission and to identify critical environmental thresholds that may support the development of targeted public health interventions and early warning systems.
2. Materials and Methods
2.1. Investigation Area and Data
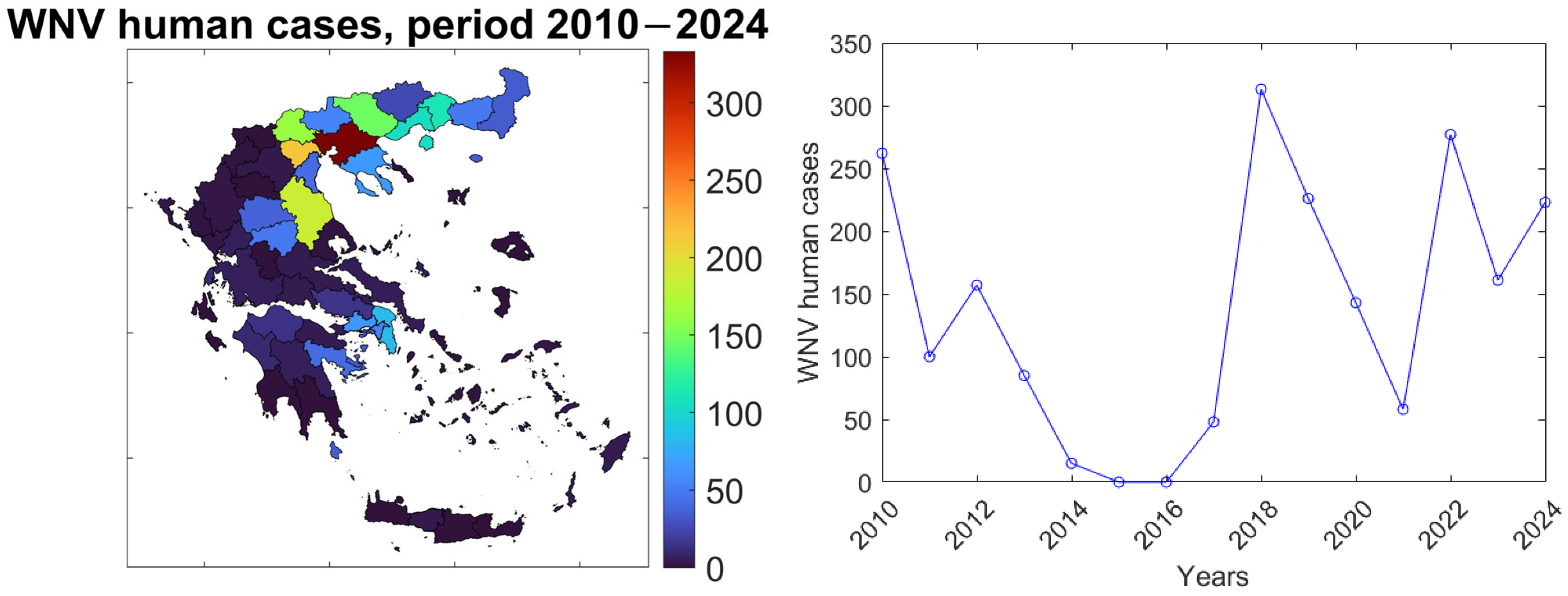
Our study focused on the provinces of Greece (NUTS3 level). Reported WNV human case data spanning the period 2010–2024 were obtained from the National Public Health Organization (EODY) and included essential information such as symptom onset and clinical classification (e.g., asymptomatic, WNF (West Nile Fever), or WNND (West Nile Neuroinvasive Disease)), all georeferenced at the NUTS3 level. This study included 41 out of the 52 provinces of Greece (
Figure 1 (left)), selected based on the presence of a non-zero number of reported human cases through the study period. The multiannual variability of the total number of observed human cases each year is presented in
Figure 1 (right).
Meteorological variables, specifically daily mean 2 m temperature and total daily precipitation, were retrieved from the ERA5 dataset provided by the European Centre for Medium-Range Weather Forecasts (ECMWF).
2.2. Methodology
Using a space-time-stratified case–crossover design, we investigated the short-term effects of meteorological variables on WNV human cases in Greece. Based on the methodology of [
6], distributed lag nonlinear models were applied within a conditional logistic regression framework to capture delayed and nonlinear associations. The analysis covered human cases reported between 2010 and 2024. We examined the influence of several environmental factors—2 m air temperature and total precipitation—on the occurrence of human infections. In this study, we used OR to quantify the strength and direction of the association between environmental variables and the occurrence of WNV human cases. The OR is defined as the ratio of the odds of the event occurring in the exposed group to the odds in the non-exposed group: OR = (odds of the event in the exposed group)/(odds of the event in the non-exposed group). Specifically, we compared the odds of WNV occurrence under specific environmental conditions—defined as values above the 75th percentile of 2 m temperature and precipitation distributions—across progressively higher levels. An OR greater than 1 indicates increased odds of WNV cases with exposure to the corresponding elevated environmental conditions.
3. Results
We identified a significant, positive, and delayed effect of 2 m temperature and total precipitation on the risk of WNV human cases in Greece. Heatmaps (
Figure 2 (left)) present the overall trends in the association between weekly average temperature and total precipitation with WNV incidence across different lag periods. These visualizations highlight general positive associations suggesting that warmer and wetter conditions may promote environments conducive to virus transmission. In addition, we simulated an increase in temperature of 1, 2 and 3 °C and in total precipitation of 10, 20 and 30 mm, both relative to the 75th percentile of their respective distributions (
Figure 2 (right)). This approach allowed us to identify the time windows during which these environmental changes had the greatest impact on disease risk. The analysis revealed that increased temperature was associated with higher incidence at a lag of 1.5 weeks, while elevated total precipitation showed a significant effect at a lag of around 3.75 weeks. These findings underscore the relevance of environmental factors in shaping the spatiotemporal dynamics of WNV transmission and may inform the design of early warning systems based on environmental monitoring.
Table 1 presents the OR at different temperature and total precipitation levels, relative to the reference values (26 °C and 30 mm, respectively). For temperature, compared to the reference value of 26 °C, the OR increases progressively with higher temperatures. Specifically, at a lag of 1.5 weeks, at 27 and 28 °C, the OR is 0.11 and 0.23 higher, respectively, while at 29 °C the OR is 0.34 higher, indicating the probability of occurrence is 34% higher at 29 °C than at 26 °C. For total precipitation, at 40, 50, and 60 mm of precipitation, the OR is 0.03, 0.07, and 0.1 higher at a lag of 3.75 weeks. These findings confirm that both higher temperatures and increased precipitation are associated with an elevated risk of the health outcome, with the effect being more pronounced for higher temperature values.
4. Discussion
Understanding the environmental drivers of WNV transmission is crucial for anticipating outbreaks and ensuring public health. In Greece, where WNV has become endemic, identifying the climatic conditions that favor transmission can support the development of early warning systems and targeted interventions. This study aimed to clarify the short-term associations between temperature, precipitation, and the incidence of human WNV cases by applying advanced statistical modeling capable of capturing delayed and nonlinear effects and using high-resolution epidemiological and meteorological data spanning a 15-year period.
Our findings reveal a significant, positive, and temporally lagged association between 2 m air temperature, total precipitation, and the occurrence of human WNV cases. Specifically, higher temperatures were linked to increased WNV risk with a lag of 1.5 weeks, while precipitation showed the strongest effect about 3.75 weeks later. These results suggest that warmer and wetter conditions may foster mosquito activity and enhance viral transmission.
Previous studies highlighted that seasonal climatic anomalies could elevate WNV transmission risk across different years [
21,
22]. Furthermore, numerous studies exploring short-term meteorological influences have underscored that increases in weekly mean temperatures are often followed by delayed increases in WNV cases, a pattern observed across diverse countries and climatic zones [
6,
16,
23,
24,
25,
26]. Our findings align with these results, reinforcing the role of temperature in shaping transmission dynamics. Similarly, several studies have reported positive associations between cumulative precipitation and WNV incidence [
6,
24,
26], which is consistent with our observations and further highlights the dual impact of temperature and rainfall on disease risk.
Our research contributes to an understanding of how short-term weather variability influences WNV transmission risk, offering valuable evidence to support the development of early warning systems and targeted public health interventions in Greece. Limitations of this study include the reliance on reported human cases, which may underrepresent true incidence due to asymptomatic infections or underreporting. Moreover, other environmental or ecological factors—such as vector abundance, avian host dynamics, or land use changes—not considered in this study. Future research that integrates entomological and ecological data could further enhance predictive models and inform more comprehensive surveillance strategies.
Author Contributions
Conceptualization, A.A., N.I.S. and I.K.; methodology, A.A. and I.K.; software, A.A.; validation, A.A. and I.K.; formal analysis, A.A.; investigation, A.A.; writing—original draft preparation, A.A., I.K. and N.I.S.; writing—review and editing, A.A., I.K. and N.I.S.; visualization, A.A.; supervision, I.K.; project administration, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the project “Support for upgrading the operation of the National Network for Climate Change (CLIMPACT)”, which is financed by the National Section of the PDE National Development Program 2021–2025 (General Secretariat of Research and Innovation, Ministry of Development). This work was also funded by the project “Modeling health risk assessment of West Nile Virus in Europe under different climate scenarios”, which is financed by the European Commission.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated or analyzed during the current study are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, transmission, and human infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef]
- Valiakos, G.; Touloudi, A.; Iacovakis, C.; Athanasiou, L.; Birtsas, P.; Spyrou, V.; Billinis, C. Molecular detection and phylogenetic analysis of West Nile virus lineage 2 in sedentary wild birds (Eurasian magpie), Greece, 2010. Euro Surveill. 2011, 16, 19862. [Google Scholar] [CrossRef]
- Maxmen, A. The hidden threat of West Nile virus. Nature 2012, 489, 349–350. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. West Nile Virus. Available online: https://www.who.int/en/news-room/fact-sheets/detail/west-nile-virus (accessed on 10 May 2025).
- Brugueras, S.; Fernández-Martínez, B.; Martínez-de la Puente, J.; Figuerola, J.; Porro, T.M.; Rius, C.; Larrauri, A.; Gómez-Barroso, D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: A systematic review. Environ. Res. 2020, 191, 110038. [Google Scholar] [CrossRef] [PubMed]
- Moirano, G.; Fletcher, C.; Semenza, J.C.; Lowe, R. Short-term effect of temperature and precipitation on the incidence of West Nile Neuroinvasive Disease in Europe: A multi-country case-crossover analysis. Lancet Reg. Health Eur. 2024, 48, 101149. [Google Scholar] [CrossRef]
- Angelou, A.; Schuh, L.; Stilianakis, N.I.; Mourelatos, S.; Kioutsioukis, I. Unveiling spatial patterns of West Nile virus emergence in northern Greece, 2010–2023. One Health 2024, 19, 100888. [Google Scholar] [CrossRef]
- Ruiz, M.O.; Chaves, L.F.; Hamer, G.L.; Sun, T.; Brown, W.M.; Walker, E.D.; Haramis, L.; Goldberg, T.L.; Kitron, U.D. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasites Vectors 2010, 3, 19. [Google Scholar] [CrossRef]
- Rosa, R.; Marini, G.; Bolzoni, L.; Neteler, M.; Metz, M.; Delucchi, L.; Chadwick, E.A.; Balbo, L.; Mosca, A.; Giacobini, M.; et al. Early warning of West Nile virus mosquito vector: Climate and land use models successfully explain phenology and abundance of Culex pipiens mosquitoes in north-western Italy. Parasit. Vectors 2014, 7, 269. [Google Scholar] [CrossRef]
- Calzolari, M.; Pautasso, A.; Montarsi, F.; Albieri, A.; Bellini, R.; Bonilauri, P.; Defilippo, F.; Lelli, D.; Moreno, A.; Chiari, M.; et al. West Nile virus surveillance in 2013 via mosquito screening in northern Italy and the influence of weather on virus circulation. PLoS ONE 2015, 10, e0140915. [Google Scholar] [CrossRef]
- Marcantonio, M.; Rizzoli, A.; Metz, M.; Rosa, R.; Marini, G.; Chadwick, E.; Neteler, M. Identifying the environmental conditions favouring West Nile virus outbreaks in Europe. PLoS ONE 2015, 10, e012118. [Google Scholar] [CrossRef]
- Cotar, A.I.; Falcuta, E.; Prioteasa, L.F.; Dinu, S.; Ceianu, C.S.; Paz, S. Transmission Dynamics of the West Nile Virus in Mosquito Vector Populations under the Influence of Weather Factors in the Danube Delta, Romania. EcoHealth 2016, 13, 796–807. [Google Scholar] [CrossRef]
- Mori, H.; Wu, J.; Ibaraki, M.; Schwartz, F.W. Key Factors Influencing the Incidence of West Nile Virus in Burleigh County, North Dakota. Int. J. Environ. Res. Public Health 2018, 15, 1928. [Google Scholar] [CrossRef]
- Roiz, D.; Vazquez, A.; Rosa, R.; Munoz, J.; Arnoldi, D.; Rosso, F.; Figuerola, J.; Tenorio, A.; Rizzoli, A. Blood meal analysis, flavivirus screening, and influence of meteorological variables on the dynamics of potential mosquito vectors of West Nile virus in northern Italy. J. Vector Ecol. 2012, 37, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Bisanzio, D.; Giacobini, M.; Bertolotti, L.; Mosca, A.; Balbo, L.; Kitron, U.; Vazquez-Prokopec, G.M. Spatio-temporal patterns of distribution of West Nile virus vectors in eastern Piedmont Region, Italy. Parasit. Vectors 2011, 4, 230. [Google Scholar] [CrossRef] [PubMed]
- Stilianakis, N.I.; Syrris, V.; Petroliagkis, T.; Part, P.; Gewehr, S.; Kalaitzopoulou, S.; Mourelatos, S.; Baka, A.; Pervanidou, D.; Vontas, J.; et al. Identification of climatic factors affecting the epidemiology of human West Nile virus infections in northern Greece. PLoS ONE 2016, 11, e0161510. [Google Scholar] [CrossRef] [PubMed]
- Koenraadt, C.J.M.; Möhlmann, T.W.R.; Verhulst, N.O.; Spitzen, J.; Vogels, C.B.F. Effect of overwintering on survival and vector competence of the West Nile virus vector Culex pipiens. Parasit. Vectors 2019, 12, 147. [Google Scholar] [CrossRef]
- Ducrocq, J.; Forest-Bérard, K.; Ouhoummane, N.; Laouan Sidi, E.; Ludwig, A.; Irace-Cima, A. A meteorological-based forecasting model for predicting minimal infection rates in Culex pipiens-restuans complex using Québec’s West Nile virus integrated surveillance system. Can. Commun. Dis. Rep. 2022, 48, 196–207. [Google Scholar] [CrossRef]
- Wang, H.R.; Liu, T.; Gao, X.; Wu, T.; Lu, J.; Sun, Y. Impact of climate change on the global circulation of West Nile virus and adaptation responses: A scoping review. Infect. Dis. Poverty 2024, 13, 38. [Google Scholar] [CrossRef]
- Ebi, K.L.; Nealon, J. Dengue in a changing climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef]
- Farooq, Z.; Rocklöv, J.; Wallin, J.; Abiri, N.; Sewe, M.O.; Sjödin, H.; Semenza, J.C. Artificial intelligence to predict West Nile virus outbreaks with eco-climatic drivers. Lancet Reg. Health Eur. 2022, 17, 100370. [Google Scholar] [CrossRef]
- Tran, A.; Sudre, B.; Paz, S.; Rossi, M.; Desbrosse, A.; Chevalier, V.; Semenza, J.C. Environmental predictors of West Nile fever risk in Europe. Int. J. Health Geogr. 2014, 13, 26. [Google Scholar] [CrossRef]
- Paz, S.; Malkinson, D.; Green, M.S.; Tsioni, G.; Papa, A.; Danis, K.; Sirbu, A.; Ceianu, C.; Katalin, K.; Ferenczi, E.; et al. Permissive summer temperatures of the 2010 European West Nile fever upsurge. PLoS ONE 2013, 8, e56398. [Google Scholar] [CrossRef]
- Moirano, G.; Gasparrini, A.; Acquaotta, F.; Fratianni, S.; Merletti, F.; Maule, M.; Richiardi, L. West Nile Virus infection in Northern Italy: Case-crossover study on the short-term effect of climatic parameters. Environ. Res. 2018, 167, 544–549. [Google Scholar] [CrossRef]
- Paz, S. The West Nile Virus outbreak in Israel (2000) from a new perspective: The regional impact of climate change. Int. J. Environ. Health Res. 2006, 16, 1–13. [Google Scholar] [CrossRef]
- Soverow, J.E.; Wellenius, G.A.; Fisman, D.N.; Mittleman, M.A. Infectious disease in a warming world: How weather influenced West Nile virus in the United States (2001–2005). Environ. Health Perspect. 2009, 117, 1049–1052. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).