Abstract
This study investigated the minimization of the N2O emissions related to brewery wastewater treatment by malt-sprout-derived biochar. The biochar was derived using slow pyrolysis (300 (B1), 400 (B2), and 550 °C (B3)). The correspondence between the N2O emission and wastewater treatment quality was investigated by Monte Carlo simulation. An average of 25.6% of minimization in N2O emissions was reported by malt-sprout-derived biochar. The simulation results showed that the Total Kjeldahl Nitrogen (TKN) and ammonium (NH4-N) had the highest correspondence with N2O emissions.
1. Introduction
Industrial wastewater treatment is regarded as one of the significant greenhouse gases (GHG) resources [1,2,3,4,5]. Nitrous oxide (N2O) is a major GHG which could be released from agro-industrial wastewater treatment plants. Biochar adsorption which decreases the GHG emission achieves higher wastewater quality. Biochar is an up-and-coming application which could adsorb the GHG emissions from wastewater [6,7]. Biochars could adsorb the greenhouse gases owing to their stable texture, larger carbonaceous and porous structure [8,9,10]. The major aim of the study was the decrease in N2O emissions from brewery wastewater treatment applying the biochar adsorption process. The hypothesis of this study was that biochar can highly remove the N2O from wastewater due to the higher adsorption capacity. In this context, this study investigated the minimization of the N2O emissions related to brewery wastewater treatment by malt-sprout-derived biochar. This paper was unique in that malt-sprout-derived biochar was the N2O adsorbent for brewery wastewater. There is a limitation and assumption in this study. The indirect GHG emission related to sludge treatment was ignored. The biochar application was performed as a lab-scale study. The other limitation was that gas sampling was performed at optimum and ideal conditions. Also, the fugitive N2O emissions were considered. According to the calibration test, the gas leakage ratio was figured as 0.05 (0.5%). This ratio was added to the measurement values.
2. Materials and Method
Experimental Procedure/Methodology/System Description
Experimental procedure was based on N2O measurement before and after biochar adsorption and wastewater analyses [11] (Figure 1). Also, N2O adsorption by biochar was tried to validate the uptake capacity of biochar. The feedstock was the malt-sprout which was sourced by a brewery industry. The temperatures in a fluidized slow pyrolysis bed reactor were 300 (B1), 400 (B2), and 550 °C (B3). The nitrogen (N2) was used as the fluidization gas in the pyrolysis system. Before pyrolysis, the feedstock was dried in an incubator at 80 °C for 60 min.
Figure 1.
Experimental planning.
N2O measurement was performed using gas chromatography (GC) equipped with an electron capture detector (GC-ECD). The concentration of N2O was determined by the GC-ECD. N2O sampling was realized seasonally (winter, spring, summer, autumn). N2O emission determination tool was depended on IPCC Tier-1 method (Equation (1)) [2]. In Equation (1), GHG represents the N2O emission (kg CO2e/d). N defined the N2O concentration. Global Warming Potential of N2O was shown as GWP. GWP of N2O is 273 [2], and K was the Henry’s Law coefficient.
GHG = N × GWP × K
In gas adsorption process by biochar, the malt-sprout-derived biochar was the adsorbent and the adsorbate was N2O. Langmuir isotherm model (Equation (2)) [12] was used to analyze the empirical N2O adsorption by biochar related to winter season. Collected N2O in winter was adsorbed by each biochar to validate the uptake capacity.
qe = (qmKLP)/(1 + KLP)
In Equation (2), the equilibrium and maximum adsorption capability were defined as qe and qm (mmol/g), respectively. KL (1/atm) and P (atm) were the Langmuir coefficient and partial pressure of N2O, respectively.
The correspondence between the N2O emission and wastewater quality parameters was investigated by Monte Carlo simulation using @RISK 6.0 version software. The simulation was based on one run and 1000 iterations. The simulation was given in Equation (3). The correlation coefficient (C) was figured out according to this tool.
C = Riskoutput(Lognormal) + RiskLognorm (GHG; TKN, NH4-N, Q, TSS, COD,pH,DO)
The inputs were N2O emission (GHG) and the wastewater quality parameters which were DO, TKN, NH4-N, Q, TSS, COD, pH. Also, an uncertainty analysis was performed by Monte Carlo methodology to validate the correspondence and the simulation (Equation (4)).
Degree of meaningful = Riskoutput(Lognormal) + RiskLognorm (C, GHG)
3. Results and Discussion
3.1. GHG Reduction Amounts
The values of GHG emissions related to before and after biochar adsorption are shown in Figure 2. In winter, N2O emission had the peak value due to higher dissolved O2 in wastewater. By contrast, N2O had the lowest value due to lower dissolved O2 in summer. According to the elemental analysis, the nitrogen content (12.5%) of malt-sprout-derived biochar can trigger the N2O uptake.
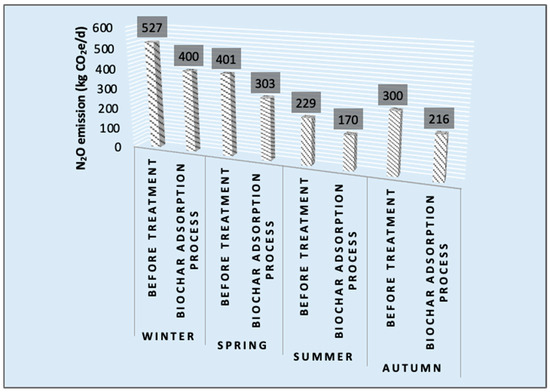
Figure 2.
Seasonal variation of N2O measurement values.
A reduction of 25.6% was reported for N2O emission based on N2O measurement. Table 1 shows the reduction amounts of N2O emissions for each biochar, in detail.

Table 1.
N2O removal amounts (%) by biochar (based on measurement results).
3.2. N2O Adsorption by Biochar
The maximum N2O adsorption capacity was corresponded to B1 (8.49 mmol/g) in winter. Brunauer, Emmet, and Teller (BET) analyses results corroborated the gas adsorption result. The B2 had the 8 mmol/g of N2O adsorption capacity, and the lowest adsorption capacity belonged to the B3 which fabricated at the highest temperature (7.91 mmol/g).
There were some studies related to estimation and mitigation of N2O emission resulting from wastewater treatment [13,14,15,16,17]. Unlike these studies, this study focused on mitigation of N2O emission from wastewater treatment using a unique biochar. This research is novel in that biochar was practiced to reduce the N2O emission originated from wastewater treatment.
The studies on mitigation of N2O emission used biological treatment techniques. The control and operations of biological systems are more difficult by comparing with biochar applications. Also, there is no N2O accumulation in the biochar process. The other main advantages of biochar applications are low cost and regeneration and reuse capability.
4. Conclusions
This research established that malt-sprout-derived biochar can ensure the removal of N2O which was the major air pollutant. Even so, practices dependent on biochar use in large-scale wastewater treatment plants should be further researched in order to reuse this valuable waste in future research in terms of circular economy aims and perspective. On average, a 25.6% reduction was reported in N2O emission by malt-sprout-derived biochar. The regeneration capacity of biochar should be investigated after the biochar adsorption process to contribute to the circular economy principles in future research.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- IPCC. Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- IPCC. IPCC Sixth Assessment Report. Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC: Cambridge, UK, 2022. [Google Scholar]
- EU European (EU) Commission. A Clean Planet for All—A European Strategic Long-Term Vision for a Prosperous, Modern, Competitive and Climate Neutral Economy; European Green Deal; (COM (2018) 773); European Commission: Brussels, Belgium, 2018. [Google Scholar]
- European Union (EU) Commission. Report on GREEN DEAL Framework and Fit for 55 Legislation Package; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Yapıcıoğlu, P.S.; Yalçın, H.; Yeşilnacar, M.İ. Experimental and statistical modeling of the effect of process modification and wastewater characterization on greenhouse gas emissions for a dairy industry wastewater treatment plant: A minimization approach. J. Water Clim. Change 2023, 14, 3313–3328. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Qambrani, N.A.; Rahman, M.M.; Won, S. Biochar properties and eco-friendly applications for climate change mit-igation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Yapıcıoğlu, P.S.; Yeşilnacar, M.İ. Experimental Design, Statistical Analysis, and Modeling of the Reduction in Methane Emissions from Dam Lake Treatment Using Agro-Industrial Biochar: A New Methane Capture Index. Water 2024, 16, 2792. [Google Scholar] [CrossRef]
- Sadhu, M.; Bhattacharya, P.; Vithanage, M.; Sudhakar, P.P. Adsorptive removal of fluoride using biochar—A potential application in drinking water treatment. Sep. Purif. Technol. 2021, 278, 119106. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Zhu, S.; Li, S.; Wei, C. Effects of biochar on anaerobic treatment systems: Some perspectives. Bioresour. Technol. 2022, 367, 128226. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA, 1999. [Google Scholar]
- Metcalf, E. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill: Boston, MA, USA, 2014. [Google Scholar]
- Khalil, M.; AlSayed, A.; Liu, Y.; Vanrolleghem, P.A. Machine learning for modeling N2O emissions from wastewater treatment plants: Aligning model performance, complexity, and interpretability. Water Res. 2023, 245, 120667. [Google Scholar] [CrossRef] [PubMed]
- Gruber, W.; von Känel, L.; Vogt, L.; Luck, M.; Biolley, L.; Feller, K.; Joss, A. Estimation of countrywide N2O emissions from wastewater treatment in Switzerland using long-term monitoring data. Water Res. X 2021, 13, 100122. [Google Scholar] [CrossRef] [PubMed]
- Gruber, W.; Villez, K.; Kipf, M.; Wunderlin, P.; Siegrist, H.; Vogt, L.; Joss, A. N2O emission in full-scale wastewater treatment: Proposing a refined monitoring strategy. Sci. Total Environ. 2020, 699, 134157. [Google Scholar] [CrossRef] [PubMed]
- Shukuru, B.N.; Politaeva, N.A. Mitigating CH4 and N2O emissions from domestic and industrial wastewater. Renew. Sustain. Energy Rev. 2025, 210, 115203. [Google Scholar] [CrossRef]
- Duan, H.; van den Akker, B.; Thwaites, B.J.; Peng, L.; Herman, C.; Pan, Y.; Ye, L. Mitigating nitrous oxide emissions at a full-scale wastewater treatment plant. Water Res. 2020, 185, 116196. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).