Ecological Impact of Invasive Fish Species on Species and Ichthyocenotic Associations in Freshwater Aquatic Ecosystems of Romania †
Abstract
1. Introduction
- Increased competition with native species for trophic and spatial resources, with invasive species out-competing native species;
- High prolificacy and the ability to survive and self-perpetuate in new habitats faster than native species;
- Manifestation of the tendency to eliminate competing native species for the same space and food resources, both through non-aggressive competition and through aggression itself, achieved through an appropriate competitive informational apparatus: chemicalhumoral (hormones and exohormones), territorial and aggressive behavior, evolved reproductive and offspring rearing behavior, result of a selective pressure from the natural range, complex and evolved social or group behavior, etc.
2. Experimental
2.1. Materials
2.2. Methods
3. Results and Discussion
- I.
- In the associations made between Pseudorasbora parva and native peaceful species, the following was noted:
- Pseudorasbora parva shows a competitive feeding behavior superior to native cyprinids that allows for rapid feeding. During feeding, a non-aggressive competitive behavior appears, with rapid zigzag swimming sequences or aggressive fins nipping behavior of other cyprinids or even percids (Perca fluvialilis) [11,24];
- When food portions shrink, Pseudorasbora parva feeds on detritus, body mucus and fins of other cohabiting species. Under captive conditions, stress on the cohabiting native cyprinid species is induced [11];
- Pseudorasbora parva originates from the genetic center of the Cyprinidae family and is the result of efficient selection carried out throughout evolution in the area with the highest selective pressure within the Cyprinidae family. As such, the species has developed mechanisms of interspecific competition and survival that are adequate and extremely competitive—one could even say infallible— in the face of the indigenous European cyprinid species, which are practically disarmed in the face of this competitor;
- In our experiments in captivity we have discovered a very complex behavioral arsenal (defense and dissimulation behavior from predators manifested by a zigzag dance with short swimming sequences and short turns; shelter behavior realized in about 3–5 s with high speed of reaction to aggressors and specific and non-specific natural predators, and complex breeding behavior realized by group territorialism and choice of breeding areas guarded in common by several males, even if sexual selection is also manifested among them) [11,25].
- II.
- Carassius auratus gibelio exhibits aggressive, competitive behavior that manifests itself especially during feeding, in captivity conditions in aquaria and in the ponds of SCDP NucetDambovita, chasing away from feeding sites Cyprinus carpio, Aristichthys nobilis and Ctenopharyngodon idella specimens by an aggressive zigzag dance with short aggressive zigzag swimming sequences [26].Aggressive behavior has two phases [11]:
- Ritualized phase, with short zigzag dances of 2–3 s;
- Actual aggressive phase with interspecific fights by using the dorsal spine (first dorsal ray) in competition for food, in order to attack intruders from other species or even smaller conspecifics.
- III.
- Lepomis gibbosus exhibits territorial and aggressive behavior very similar to species of the family Cichlidae [11,27]. The territoriality of the males is realized on territories with a radius of 15–25 cm, the intensity of the aggressive behavior being the highest in the center of the territory or in the place in the territory where there is a hollow shelter (rock and root). It was observed that nests were built at a distance of 30–40 cm from each other in captivity, in large aquaria of 2000 × 700 × 600 mm (about eight different territories) In natural environment, it was reported thousands of nests in the I.O.R Balta Alba Lake in Bucharest and Blasova Lake in the Small Island of Braila [11,27].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genovesi, P.; Shine, C. European strategy on invasive alien species-Convention on the Conservation of European Wildlife and Habitats. Nat. Environ. 2004, 137, 1–68. [Google Scholar]
- Genovesi, P.; Shine, C. European strategy on invasive alien species. In Proceedings of the 3rd Draft-Convention on the Conservation of European Wildlife and Habitats, Strasbourg, France, 5 September 2003; pp. 1–50. [Google Scholar]
- Directorate of Culture and Cultural and Natural Heritage. Bern Convention action on invasive alien species in Europe. In Proceedings of the Convention on the Conservation of European Wildlife and Habitats, Strasbourg, France, 7 January 2008; pp. 1–6. [Google Scholar]
- Busnita, T.; Popescu-Gorj, A.; Dumitriu, M.; Manea, G.; Luscan, S.; Matei, D. Primele incercari de aclimatizare a coregonilor in apele R.P.R. Bul. Institutului Cercet. Piscic. 1957, 16, 5–19. [Google Scholar]
- Banarescu, P. Pisces-Osteichthyes, Fauna, R.P.R.; Editura Academiei, R.P.R.: Bucharest, Romania, 1964; Volume XIII. [Google Scholar]
- Banarescu, P. Zoogeography of Freshwaters; Aula Verlag: Wiesbaden, Germany, 1990. [Google Scholar]
- Manea, G.I. Aclimatization of New Fishes and Other Aquatic Organisms; Ceres Publishing House: Bucharest, Romania, 1985. [Google Scholar]
- Angelescu, N. Pestii bufalo in apele Romaniei. Van. Pesc. Sport 1982, 2, 12. [Google Scholar]
- Decei, P. Pastravii apelor noastre. Van. Pesc. Sport. 1986, 7, 4–5. [Google Scholar]
- Giurca, R. Noi oaspeti la Nucet: Pestii pisica. Van. Pesc. Sport. 1978, 10, 4. [Google Scholar]
- Craciun, N. Cercetari de Etologie Comparata a Unor Pesti Marini si Dulcicoli din Fauna Romaniei. Ph.D. Thesis, Institutul de Biologie al Academiei Române, Bucharest, Romania, 1999. [Google Scholar]
- Deák, G.; Sadica, I.; Matei, M.; Holban, E.; Raischi, M.; Jawdhari, A. The Preference of Anadromous Sturgeon Species Regarding Temperature and Depth Parameters during Spawning Migration in the Lower Danube. E3S Web Conf. 2023, 437, 02005. [Google Scholar] [CrossRef]
- Holban, E.; Deak, G.; Matache, R.; Dănălache, T.; Matei, M.; Boboc, M.; Raischi, M.; Gheorghe, I.; Keresztesi, S.A.; Kilár, F. Identification of Sturgeon Behavior in Different Hydromorphodynamic Conditions Resulting from the Implementation of Hydrotechnical Arrangements. Int. J. Conserv. Sci. 2022, 13, 743–750. [Google Scholar]
- Matei, M.; Laslo, L.; Ciobotaru, N.; Musat, C.; Boboc, M.; Raisch, M.; Deak, G. Assessment of Pressures Caused by Climate Changes on Wetlands in Romania Based on MAES Framework. Int. J. Environ. Sci. 2016, 1, 265–271. [Google Scholar]
- Timbergen, N. The Biological Bases of Behavior. In Psychhobiology; W.H. Freeman and Co.: San Francisco, CA, USA, 1952; pp. 5–9. [Google Scholar]
- Md Nasir, N.A.N.; Zakarya, I.A.; Kamaruddin, S.A.; Mohammad Aminul Islam, A.K. Advances and Future Prospects on Biotechnological Approaches Towards Azolla for Environmental Sustainability. Pertanika J. Trop. Agric. Sci. 2022, 45, 595–609. [Google Scholar] [CrossRef]
- Deák, G.; Daescu, V.; Holban, E.; Marinescu, P.; Tanase, G.S.; Csergo, R.; Daescu, A.I.; Gaman, S. Health–environment Relation: A Key Issue of Romanian Environmental Protection. J. Environ. Prot. Ecol. 2015, 16, 304–315. [Google Scholar]
- Jawdhari, A.; Deák, G.; Mihăilescu, D.F.; Crăciun, N.; Staicu, A.C.; Stanca, I.; Cozorici, D.; Fendrihan, S.; Pop, C.-E.; Mernea, M. Ingested Microplastics Can Act as Microbial Vectors of Ichthyofauna. Microbiol. Res. 2024, 15, 614–625. [Google Scholar] [CrossRef]
- Danalache, T.; Holban, E.; Deák, G.; Parlog, C.; Matache, R.; Cudalbeanu, M.; Nicolae, C.G. Findings of the Fish Inventory within River Danube Lower from 2008 to 2020. Curr. Trends Nat. Sci. 2020, 9, 117–127. [Google Scholar] [CrossRef]
- Holban, E.; Danalache, T.; Deák, G.; Parlog, C.; Matache, R.; Cudalbeanu, M.; Nicolae, C.G. Ecological Characterization of The Fish Communities Within Lower Danube River. Curr. Trends Nat. Sci. 2020, 9, 107–116. [Google Scholar] [CrossRef]
- Azman, A.; Ng, F.C.; Zawawi, M.H.; Abas, A.; Mohd Remy Rozainy, M.A.Z.; Abustan, I.; Adlan, M.N.; Tam, W.L. Effect of Barrier Height on the Design of Stepped Spillway Using Smoothed Particle Hydrodynamics and Particle Image Velocimetry. KSCE J. Civ. Eng. 2020, 24, 451–470. [Google Scholar] [CrossRef]
- Reid, C.H.; Vandergoot, C.S.; Stevens, E.D.; Bowker, J.; Cooke, S.J. On the Electroimmo-bilization of Fishes for Research and Practice: Opportunities, Challenges, and Research Need. Fisheries 2019, 44, 576–585. [Google Scholar] [CrossRef]
- Coman, V.; Voicu, M.; Laslo, L.; Rotaru, A.; Matei, M.; Bara, N.; Enache, N.; Boboc, M.; Deak, G.; Tanciu, S.; et al. General Framework for Ecosystem Assessment for Measures to Adapt and Mitigate the Effects of Climate Change. IOP Conf. Ser. Earth Environ. Sci. 2020, 616, 012013. [Google Scholar] [CrossRef]
- Bless, R. Einsichten in Die Okologie der Elritze Phoxinus Phoxinus; Landwirtschaftsvlg Münster: Bad Godesberg, Bonn, Germany, 1992. [Google Scholar]
- Partridge, B. The Structure and Social Role of Fish Schools. Sci. Am. 1982, 246, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Timms, A.M. The effects of Visio non the general Locomotor Behavior of the Goldfish (Carassius auratus). Anim. Behav. 1976, 2, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Chiszar, D.; Windel, J.T. Predation by Bluegill Sunfish (Lepomis gibbosus) upon Mealworm larvae (Tenebrio molitor). Anim. Behav. 1973, 3, 536–544. [Google Scholar] [CrossRef]
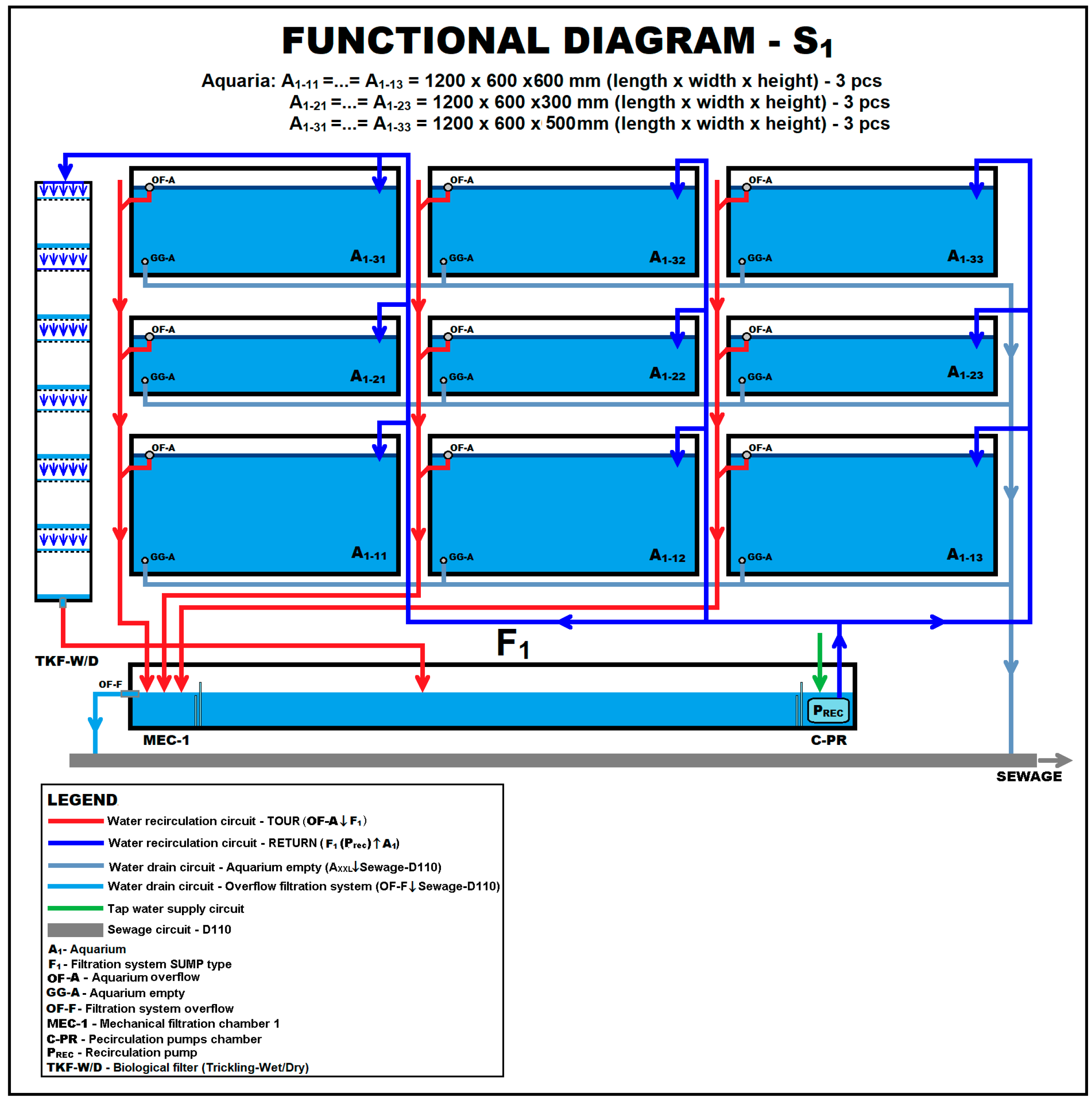
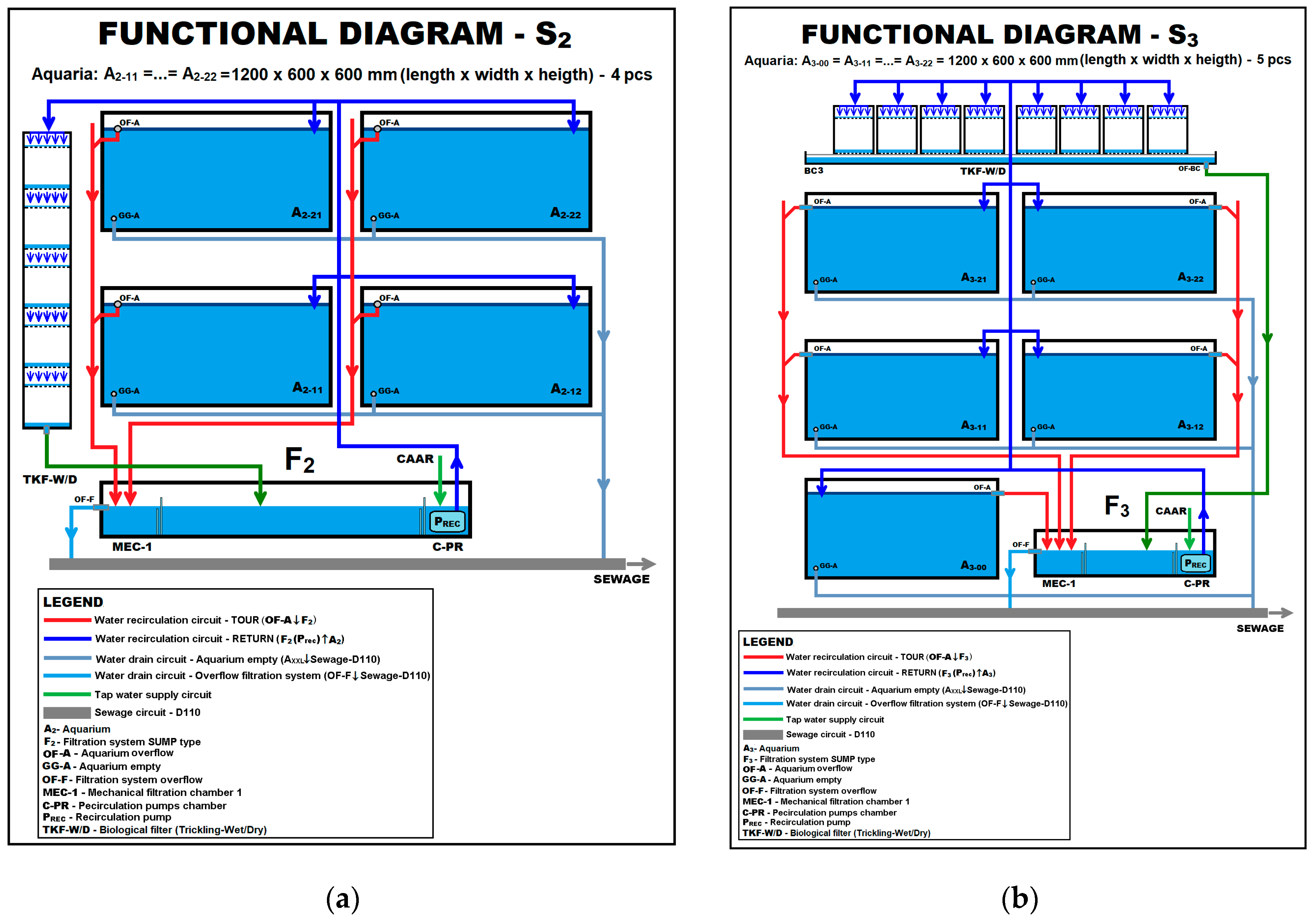
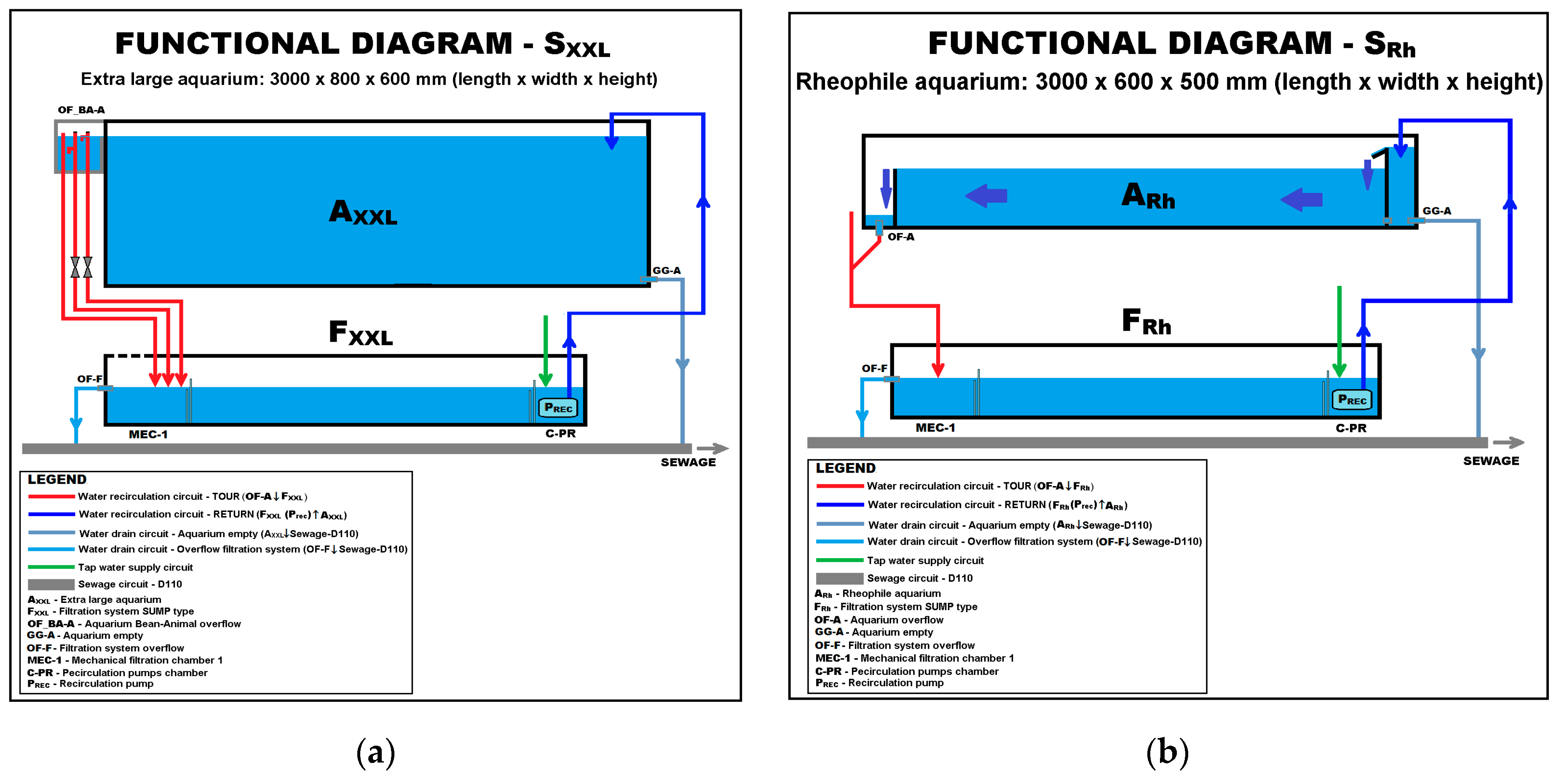
| Crt. No. | Species | Date and Place of Introduction | Purpose of Introduction | Effects Produced and Reported |
|---|---|---|---|---|
| 1. | Oncorhynchusmikiss | 1885, anonymous [9] | Aquaculture | In the trout farms |
| 2. | Salvelinusfontinalis | 1906, Moldova River [5,6,9] | Aquaculture | Stabilized |
| 3. | Carassius auratus gibellio | 1912, Moldova River [5,6] | Ornamental (aquaristics) and economic | Stabilized (has become invasive) |
| 4. | Gambusia holbrooki | 1927, Mangalia Lake [5,6] | Combating malaria | Stabilized |
| 5. | Lepomis gibbosus | 1929, Lower Danube Basin [5,6] | Natural pathways | Stabilized (has become invasive) |
| 6. | Ictalurusnebulosus | 1934, Tisa river [5,6] | Natural pathways | Stabilized (tending towards becoming invasive in Hungary) |
| 7. | Coregonus lavaretus | 1956, SCDP NucetDambovita; Tarcau River [4] | Aquaculture | Only in extensive aquaculture in mountain reservoirs |
| 8. | Coregonusalbula | 1956, SCDP NucetDambovita; Tarcau River [4] | Aquaculture | |
| 9. | Pseudorasbora parva | 1960, SCDP NucetDambovita [5,6] | Incidentally, together with the embryonated eggs of Asian cyprinid species used in aquaculture | Stabilized (has become invasive) |
| 10. | Ctenopharyngodon idella | 1960–1962, SCDP NucetDambovita [7] | Aquaculture | Stabilized |
| 11. | Mylopharyngodon piceus | 1960–1962, SCDP NucetDambovita [7] | Aquaculture | On fish farms and in Danube Delta |
| 12. | Hypophthalmichthys molitrix | 1960–1962, SCDP NucetDambovita [7] | Aquaculture | Stabilized |
| 13. | Aristichthys nobilis | 1960–1962, SCDP Nucet–Dambovita [7] | Aquaculture | Stabilized |
| 14. | Poecilia reticulata | 1975–1977, Oradea—1 Mai Lake | Ornamental | Disappeared |
| 15. | Ichthyobus cyprinellus | 1978, 1980, SCDP NucetDambovita [8] | Aquaculture | Disappeared |
| 16. | Ictiobus bubalus | 1978–1980, SCDP NucetDambovita [8] | Aquaculture | Disappeared |
| 17. | Ictiobus niger | 1978–1980, SCDP NucetDambovita [8] | Aquaculture | Disappeared |
| 18. | Iclalurus punctatus | 1980, SCDP NucetDambovita [8,10] | Aquaculture | Only in artificial ponds |
| 19. | Polyodon spathula | 1992, SCDP NucetDambovita; Acvares lasi | Aquaculture | In intensive aquaculture |
| 20. | Cyprinus carpio var. KOI | 1992, SCDP NucetDambovita, from Statiunea Piscicola Szarvasz, Budapest (Hungary) | Ornamental | - |
| 21. | Hybrids of Oncorhynchus mikiss × Salmo trutta fario | 2002–2003, Trout farm Oesti, Valsan River | Aquaculture | - |
| 22. | Mollietiisia sphenops | 1994, Oradea—1 Mai Lake | Ornamental | Disappeared |
| 23. | Xiphophonzs helleri | 1994, Oradea—1 Mai Lake | Ornamental | Disappeared |
| 24. | Clarias gariepinus | 1998–1999, Oradea | Intensive aquaculture | - |
| 25. | Hybrids of Acipenser sp. × Huso huso | After 1998 in private farms, brought from Italy and Germany | Aquaculture | - |
| 26. | Percotus glennii | 2002, Dornesti Suceava and Siret River | Natural pathways | Stabilized and invasive in all Middle and Upper Danube |
| 27. | Hybrids of Oreochromis niloticus × Oreochromis mossambica | 2010 | Aquaculture | - |
| Experiment No. | Species Association | Experimental System Used | Aquariums Setup | |
|---|---|---|---|---|
| Species | No. of Individuals | |||
| 1 | Pseudorasbora parva | Max, 25 | S1, S2, S3 | Aquatic submerged plants: Potamogeton crispus, Polygonium amphibium, and Vallisneria spiralis Aquatic floating plants: Nymphaea alba, Nuphar luteum, Hydrocharis morsus-ranae, Nymphoides peltata Salvinia natans Original invertebrate fauna: gastropods Limnaea sp., Planorbis sp. and Melanopsis palustris; and shells Anodonta cygnaea Substrate: sand, gravel, boulders and detritus Shelters: willow and Cyperaceae roots |
| Carassius auratus gibelio | Max, 25 | |||
| Cyprinus carpio | Max, 25 | |||
| 2 | Pseudorasbora parva | Max, 20 | S1, S2, S3 | |
| Carassius auratus gibelio | Max, 20 | |||
| Lepomis gibbosus | Max, 20 | |||
| Cyprinus carpio | Max,20 | |||
| 3 | Pseudorasbora parva | 50 | SXXL | |
| Carassius auratus gibelio | 50 | |||
| Lepomis gibbosus | 20 | |||
| Ctenopharyngodon idella | 10 | |||
| Mylopharyngodon piceus | 5 | |||
| Hypophthalmichthys molitrix | 10 | |||
| Aristichthys nobilis | 5 | |||
| 4 | Pseudorasbora parva | 100 | SRh | |
| Esox lucius | 10 | |||
| 5 | Pseudorasbora parva | 100 | SRh | |
| Silurus glanis | 10 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crăciun, N.; Hanganu, L.-D.; Sadîca, I.; Cucu, G.; Stegărescu, S.; Gheorghe, I.-P.; Raischi, M. Ecological Impact of Invasive Fish Species on Species and Ichthyocenotic Associations in Freshwater Aquatic Ecosystems of Romania. Environ. Earth Sci. Proc. 2025, 33, 4. https://doi.org/10.3390/eesp2025033004
Crăciun N, Hanganu L-D, Sadîca I, Cucu G, Stegărescu S, Gheorghe I-P, Raischi M. Ecological Impact of Invasive Fish Species on Species and Ichthyocenotic Associations in Freshwater Aquatic Ecosystems of Romania. Environmental and Earth Sciences Proceedings. 2025; 33(1):4. https://doi.org/10.3390/eesp2025033004
Chicago/Turabian StyleCrăciun, Nicolai, Lucian-Dorin Hanganu, Isabela Sadîca, George Cucu, Sorin Stegărescu, Ionut-Petrache Gheorghe, and Marius Raischi. 2025. "Ecological Impact of Invasive Fish Species on Species and Ichthyocenotic Associations in Freshwater Aquatic Ecosystems of Romania" Environmental and Earth Sciences Proceedings 33, no. 1: 4. https://doi.org/10.3390/eesp2025033004
APA StyleCrăciun, N., Hanganu, L.-D., Sadîca, I., Cucu, G., Stegărescu, S., Gheorghe, I.-P., & Raischi, M. (2025). Ecological Impact of Invasive Fish Species on Species and Ichthyocenotic Associations in Freshwater Aquatic Ecosystems of Romania. Environmental and Earth Sciences Proceedings, 33(1), 4. https://doi.org/10.3390/eesp2025033004






