The Influence of Mechanochemical Synthesis Method on Photodegradability Characteristics of Hydroxyapatite/Zinc Oxide Composite †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used for the Development of Hydroxyapatite/Zinc Oxide Composite
2.2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Supin, K.K.; Namboothiri, M.P.P.; Vasundhara, M. Enhanced photocatalytic activity in ZnO nanoparticles developed using novel Lepidagathis ananthapuramensis leaf extract. RSC Adv. 2023, 13, 1497–1515. [Google Scholar]
- Xian, B.C.C.; Kang, C.W.; Wahab, M.A.; Zainol, M.R.R.M.A.; Baharudin, F. Evaluation of low impact development and best management practices on peak flow reduction using SWMM. IOP Conf. Ser. Earth Environ. Sci 2021, 646, 012045. [Google Scholar] [CrossRef]
- Ramli, N.; Hamid, H.A.; Yahaya, A.S.; Ul-Saufie, A.Z.; Noor, N.M.; Seman NA, A.; Kamarudzaman, A.N.; Deák, G. Performance of Bayesian Model Averaging (BMA) for Short-Term Prediction of PM10 Concentration in the Peninsular Malaysia. Atmosphere 2023, 14, 311. [Google Scholar] [CrossRef]
- Ilie, M.; Deák, G.; Marinescu, F.; Ghita, G.; Tociu, C.; Matei, M.; Covaliu, C.I.; Raischi, M.; Yusof, S.Y. Detection of Emerging Pollutants Oxytetracycline and Paracetamol and the Potential Aquatic Ecological Risk Associated with their Presence in Surface Waters of the Arges-Vedea, Buzau-Ialomita, Dobrogea-Litoral River Basins in Romania. IOP Conf. Ser. Earth Environ. Sci. 2020, 616, 012016. [Google Scholar] [CrossRef]
- Maria, C.; Deák, G.; Tudor, G.; Holban, E.; Zamfir, C.; Ivanov, A.A.; Grigore, G.; Rahim, N. Investigation of Microplastics Presence in the Dambovita River. Int. J. Conserv. Sci. 2023, 14, 663–670. [Google Scholar] [CrossRef]
- Doicin, I.E.; Preda, M.D.; Neacsu, I.A.; Ene, V.L.; Birca, A.C.; Vasile, B.S.; Andronescu, E. Tailoring Zinc Oxide Nanoparticles via Microwave-Assisted Hydrothermal Synthesis for Enhanced Antibacterial Properties. Appl. Sci. 2024, 14, 7854. [Google Scholar] [CrossRef]
- Daescu, A.; Holban, E.; Boboc, M.; Raischi, M.; Matei, M.; Ilie, M.; Deak, G.; Daescu, V. Performant technology to remove organic and inorganic pollutants from wastewaters. J Environ. Prot. Ecol. 2017, 18, 304–312. [Google Scholar]
- Jihane Labrag, E.B.; Oulguidoum, A.; Robert, D.; Laghzizil, A.; Nunzi, J.M. Porous and Bifunctional ZnO-Hydroxyapatite Nanostructure for Photocatalytic Degradation of Paracetamol and Methylene Blue in Water. Iran. J. Catal. 2021, 11, 389–395. [Google Scholar]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment— Unexpected nitration side reactions—A serious environmental issue: A review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Loddo, V.; Bellardita, M.; Camera-Roda, G.; Parrino, F.; Palmisano, L. Chapter 1—Heterogeneous Photocatalysis: A Promising Advanced Oxidation Process. In Current Trends and Future Developments on (Bio) Membranes; Basile, A., Mozia, S., Molinari, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–43. [Google Scholar]
- Burlacu, I.F.; Deák, G.; Marcu, E.; Serre, I.P.; Balloy, D.; Halin, D.S.C. Photocatalytic degradation of a refractory water pollutant using nanosized catalysts. J. Environ. Prot. Ecol. 2020, 21, 571–578. [Google Scholar]
- Lindino, C.A.; Batalioto, C.F.; Hoss, D.; Schuranck, S.C.H. Degradação do Agrotóxico Connect® com Fotocatalisador Hidroxiapatita. Ciência Nat. 2016, 38, 1570–1579. [Google Scholar] [CrossRef][Green Version]
- Burdusel, A.C.; Neacsu, I.A.; Birca, A.C.; Chircov, C.; Grumezescu, A.M.; Holban, A.M.; Curutiu, C.; Ditu, L.M.; Stan, M.; Andronescu, E. Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration. J. Funct. Biomater. 2023, 14, 378. [Google Scholar] [CrossRef]
- Reddy, M.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite photocatalytic degradation of calmagite (an azo dye) in aqueous suspension. Appl. Catal. B Environ. 2007, 69, 164–170. [Google Scholar] [CrossRef]
- Corami, A.; Mignardi, S.; Ferrini, V. Cadmium removal from single- and multi-metal (Cd + Pb + Zn + Cu) solutions by sorption on hydroxyapatite. J. Colloid Interface Sci. 2008, 317, 402–408. [Google Scholar] [CrossRef]
- Gatou, M.A.; Kontoliou, K.; Volla, E.; Karachalios, K.; Raptopoulos, G.; Paraskevopoulou, P.; Lagopati, N.; Pavlatou, E.A. Optimization of ZnO Nanoparticles’ Synthesis via Precipitation Method Applying Taguchi Robust Design. Catalysts 2023, 13, 1367. [Google Scholar] [CrossRef]
- Burlacu, I.F.; Favier, L.; Matei, E.; Predescu, C.; Deák, G. Successful elimination of a refractory emergent organic compound from aqueous system using different catalytic materials. UPB Sci. Bull. 2019, 81, 217–225. [Google Scholar]
- El Bekkali, C.; Bouyarmane, H.; El Karbane, M.; Masse, S.; Saoiabi, A.; Coradin, T.; Laghzizil, A. Zinc oxide-hydroxyapatite nanocomposite photocatalysts for the degradation of ciprofloxacin and ofloxacin antibiotics. Coll. Surf. A Colloid Surf. A Physicochem. Eng. Asp. 2018, 539, 364–370. [Google Scholar] [CrossRef]
- Kugarajah, V.; Hadem, H.; Ojha, A.K.; Ranjan, S.; Dasgupta, N.; Mishra, B.N.; Dharmalingam, S. Chapter 1—Fabrication of nanomaterials. In Food, Medical, and Environmental Applications of Nanomaterials; Pal, K., Sarkar, A., Sarkar, P., Bandara, N., Jegatheesan, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–39. [Google Scholar]
- Tanji, K.; Navio, J.A.; Chaqroune, A.; Naja, J.; Puga, F.; Hidalgo, M.C.; Kherbeche, A. Fast photodegradation of rhodamine B and caffeine using ZnO-hydroxyapatite composites under UV-light illumination. Catal. Today 2022, 388–389, 176–186. [Google Scholar] [CrossRef]
- Dumitrescu, C.R.; Neacsu, I.A.; Surdu, V.A.; Nicoara, A.I.; Codrea, C.I.; Pop, C.E.; Trusca, R.; Andronescu, E. Maturation of Hydroxyapatite from Biogenic Calcium Source—A Comparative Study. UPB. Sci. Bull. Ser. B 2022, 84, 19–30. [Google Scholar]
- Deák, G.; Dumitru, F.D.; Moncea, M.A.; Panait, A.M.; Baraitaru, A.G.; Olteanu, M.V.; Boboc, M.G.; Stanciu, S. Synthesis of ZnO nanoparticles for water treatment applications. Int. J. Conserv. Sci. 2019, 10, 343–350. [Google Scholar]
- Walters, C.; Keeney, A.; Wigal, C.; Johnson, C.; Cornelius, R. The Spectrophotometric Analysis and Modeling of Sunscreens. J. Chem. Educ. 1997, 74, 99. [Google Scholar] [CrossRef]
- Sheng, G.; Qiao, L.; Mou, Y. Enhanced activity of silver modified thin-film TiO2 photocatalysts. J. Environ. Eng. 2011, 137, 611–616. [Google Scholar] [CrossRef]

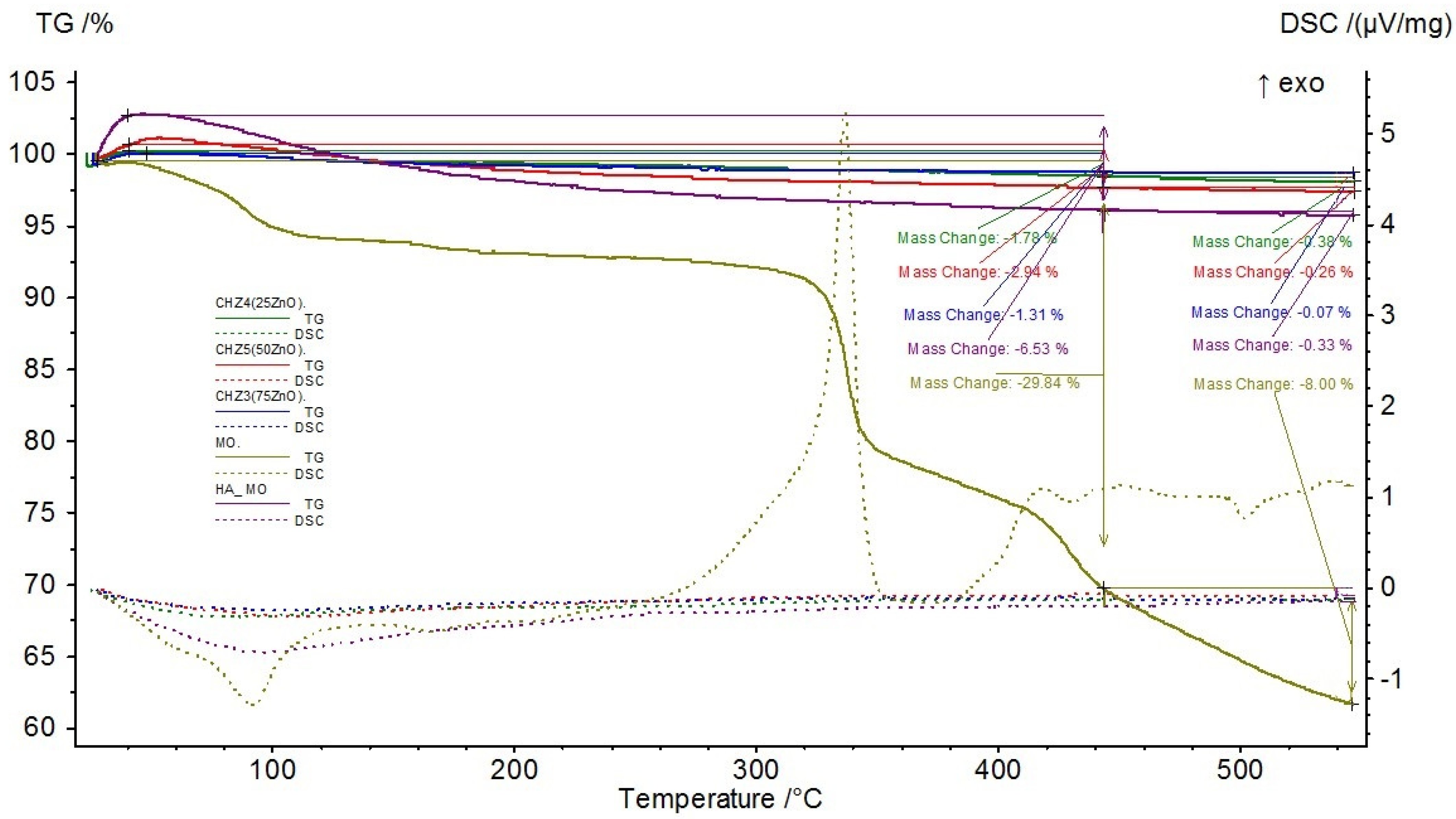
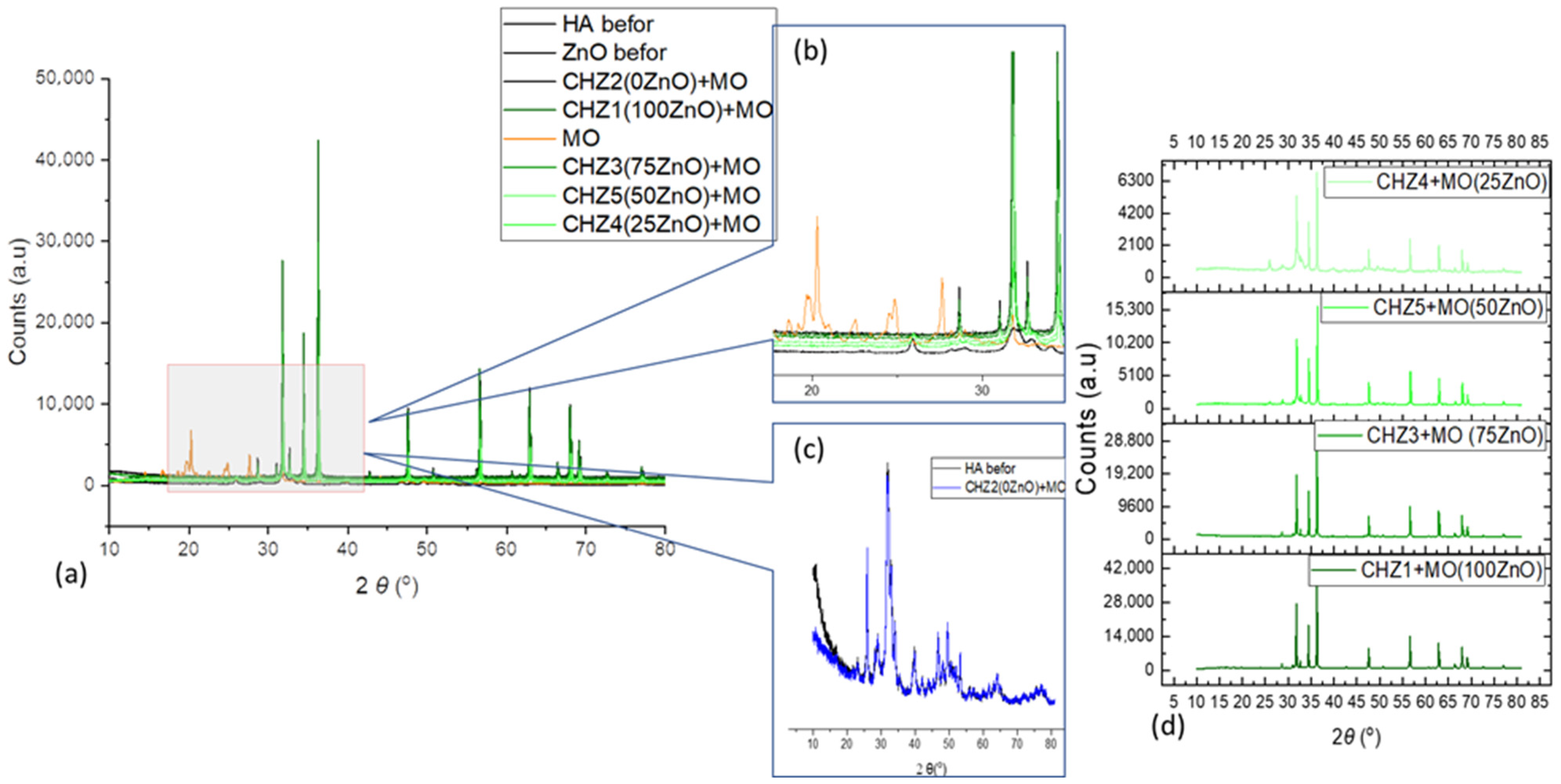
| Sample Code | ZnO [%] | HA [%] |
|---|---|---|
| CHZ1 | 100 | 0 |
| CHZ2 | 0 | 100 |
| CHZ3 | 75 | 25 |
| CHZ4 | 25 | 75 |
| CHZ5 | 50 | 50 |
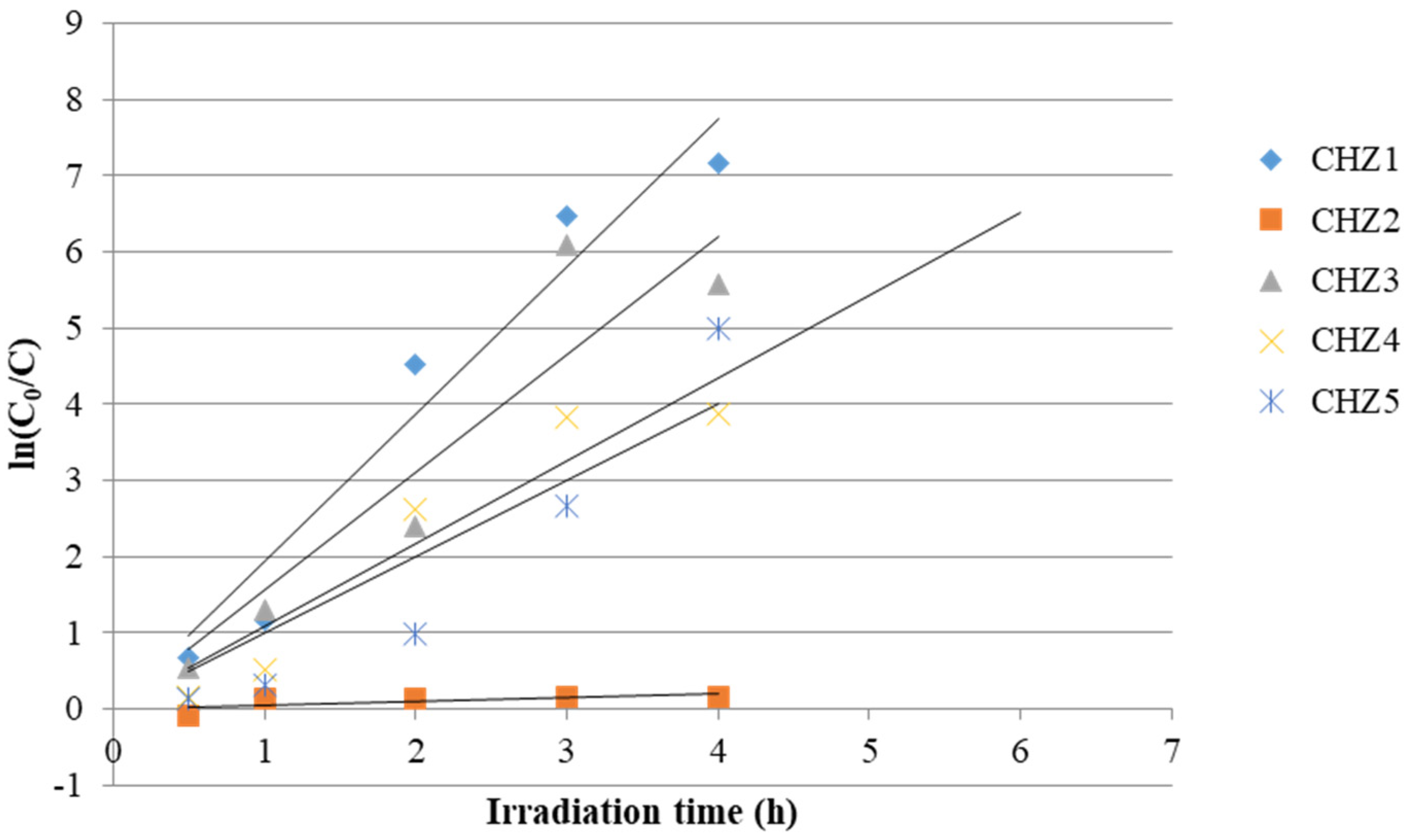
| Sample Code | k (h−1) | R2 |
|---|---|---|
| CHZ1 | 1.9356 | 0.9836 |
| CHZ2 | 0.0483 | 0.7778 |
| CHZ3 | 1.5493 | 0.9593 |
| CHZ4 | 1.0862 | 0.9665 |
| CHZ5 | 1.0013 | 0.9161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrescu, C.R.; Gheorghe, F.-D.; Matei, M.; Ștefan, L.-M.; Holban, E. The Influence of Mechanochemical Synthesis Method on Photodegradability Characteristics of Hydroxyapatite/Zinc Oxide Composite. Environ. Earth Sci. Proc. 2025, 33, 3. https://doi.org/10.3390/eesp2025033003
Dumitrescu CR, Gheorghe F-D, Matei M, Ștefan L-M, Holban E. The Influence of Mechanochemical Synthesis Method on Photodegradability Characteristics of Hydroxyapatite/Zinc Oxide Composite. Environmental and Earth Sciences Proceedings. 2025; 33(1):3. https://doi.org/10.3390/eesp2025033003
Chicago/Turabian StyleDumitrescu, Cristina Rodica, Florina-Diana Gheorghe, Monica Matei, Larisa-Mădălina Ștefan, and Elena Holban. 2025. "The Influence of Mechanochemical Synthesis Method on Photodegradability Characteristics of Hydroxyapatite/Zinc Oxide Composite" Environmental and Earth Sciences Proceedings 33, no. 1: 3. https://doi.org/10.3390/eesp2025033003
APA StyleDumitrescu, C. R., Gheorghe, F.-D., Matei, M., Ștefan, L.-M., & Holban, E. (2025). The Influence of Mechanochemical Synthesis Method on Photodegradability Characteristics of Hydroxyapatite/Zinc Oxide Composite. Environmental and Earth Sciences Proceedings, 33(1), 3. https://doi.org/10.3390/eesp2025033003






