Abstract
Aquaculture water was exposed to very low dosages of copper sulfate and chloroquine sulphate in order to observe their purgative effects at minimal concentrations, where both substances are commonly employed for their antimicrobial and antiparasitic properties in fish farming. It was observed that the well-known treatment using cooper sulphate is effective even at a low dosage, while the proposed novel treatment using chloroquine was not. The present study highlights the need of effective treatment while avoiding compromising medicine misusage which greatly leads to host resistance.
1. Introduction
Chloroquine has been proven effective in the past against the protozoan parasites Plasmodium falciparium and Plasmodium vivax, and it is considered one of the first medicines against malaria. However, due to the development of drug resistance that was first observed in the early 1960s, the antimalarial drug is now considered ineffective in nearly all areas of the world where falciparum malaria is transmitted [1].
The creation of chloroquine as an antimalarial medication and the later emergence of drug-resistant plasmodium strains significantly influenced global public health in the 20th century [2,3,4]. Still, the effectiveness of the drug was observed in other protozoa, such as in the virulent enteric parasite Entamoeba histolytica, which causes extraintestinal amebiasis in humans [5]. Interestingly enough, humans appear to be the only host for E. histolytica, as thus far, no other environmental or animal reservoirs of the parasite have been detected [6,7].
Such is not the case for fish parasites. Most of the many fish parasites are harmless to humans [8,9]. Fish parasites are generally safely digested when consumed along with their hosts. However, some fish parasites in larval stages in freshwater or marine teleost can represent a zoonotic risk if the infected fish are eaten raw or partially cooked. These parasites typically have a piscivorous mammalian carnivore as their usual final host and can infect humans due to the low host specificity of their adult stage [10,11]. The main groups of fish parasites known to be potentially dangerous to humans belong to the helminth groups, such as cestoda, trematoda, nematoda, and, less commonly, acanthocephalan [12].
Unlike chloroquine, cooper sulphate pentahydrate (CuSO4·5H2O) does not confer host resistance; the mechanism of its toxicity is based on induced oxidative stress. Furthermore, copper ions bind to functional groups of protein molecules and cause protein denaturation, producing cell damage and leakage. Following membrane degradation, the copper ions also penetrate into the cell and induce further damage [13].
CuSO4·5H2O is one of the few approved water treatments and the only therapeutic option for food animals in the US [14,15]. It is suggested that combined with chloroquine (delivered at 10 mg/L), it is the only known effective treatment against external ciliates from the Ichthyophthirius and cryptocaryon genera, as well as on dinoflagellates such as Piscinoodinium and Amyloodinium [16,17]. However, Chloroquine has some supporting evidence, but it is not definitive [18], and such combined treatment is not well documented. Maintaining good water quality is essential for successful aquaculture [19]. Disinfectants and sanitizers, which are chemically based, help manage this aspect effectively, though their effectiveness against pathogens varies. Therapeutic chemicals are versatile in preventing and treating diseases in fish and shellfish when used properly, but their misuse can cause unintended and adverse effects [20,21].
The purpose of this paper is to evaluate the compounds in low doses on normal aquatic microscopic organisms, to establish the lowest concentration needed. And to present data demonstrating how such research can contribute to a deeper understanding of their potential synergy.
2. Experimental
2.1. Materials
Chloroquine sulphate (C1650000) was purchased as European Pharmacopoeia-certified reference standard compound from European Directorate for the Quality of Medicines & HealthCare. CuSO4·5H2O was purchased from Sigma at analytical grade purity (Copper (II) sulfate pentahydrate, purum p.a., crystallized, ≥99.0%, Sigma, Merck KGaA, Darmstadt, Germany). Both CuSO4·5H2O and chloroquine concentrations were prepared at 12.5, 25, 50, and 100 µM, followed by a wavelength spectral scan using an Implen NP80 spectrophotometer (Implen GmbH, Munich, Germany) to confirm their chemical identity (Figure 1A,B).
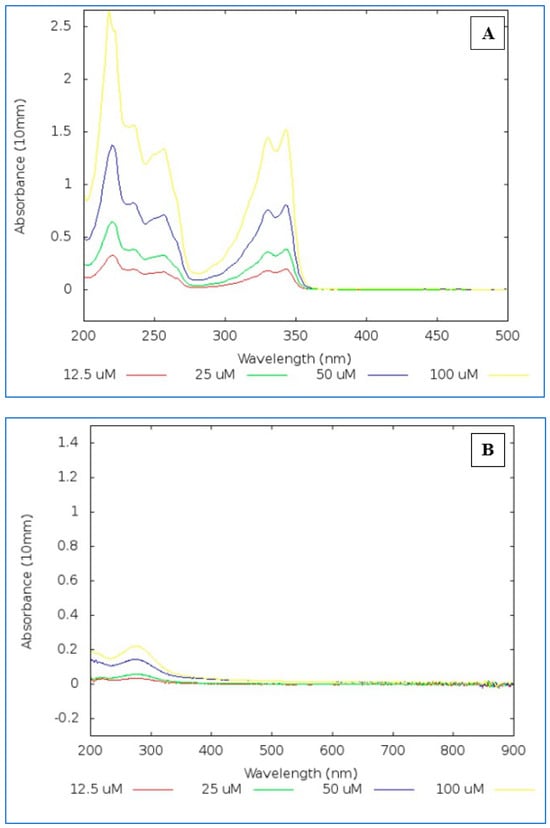
Figure 1.
(A) Chloroquine sulphate spectra at 200–500 nm. (B) CuSO4·5H2O spectra at 200–900 nm.
Chloroquine sulphate has distinctive spectra with absorption peak at 343 nm [22] while CuSO4·5H2O has less distinct spectra with a peak at 275 nm at the same concentrations, the peak being less evident due to the five water molecules for every one molecule of CuSO4.
Water samples were collected in sterile 15 mL tubes from recirculating aquaculture systems (RAS) that hosted Oreochromis niloticus for over 6 months. Water samples were collected in sterile 15 mL tubes from recirculating aquaculture systems (RAS) housing Oreochromis niloticus for over 6 months, with 10% of the water replenished weekly using chlorinated water.
2.2. Methods
The water samples were centrifuged at 4000 rpm for 4 min and the supernatant was (Corning, Sigma-Aldrich Inc., St. Louis, MO, USA) and resuspended in 1 mL of distilled water prior to investigation under a light microscope. The microscopical observations were performed using an Olympus IX71 (Tokyo, Japan) coupled with an Mshot imaging system (M28, Guangzhou, China).
Exposure took place in duplicate, starting with 12.5 µM of chloroquine and 12.5 µM of CuSO4·5H2O, and then followed by double dosage after every 5 min.
3. Results and Discussion
Chloroquine exposure was ineffective on all investigated organisms at both 12.5 µM and 25 µM, in contrast to CuSO4·5H2O, which demonstrated effectiveness at 25 µM. Control specimens were observed as healthy and motile (Figure 2), and no change in motility was observed in the mixture containing 12.5 µM of chloroquine (Figure 3) during 5–10 min of exposure. A ceasing of motility was observed after 5 min of exposure to 12.5 µM of CuSO4·5H2O (Figure 4). The same effect was observed for other specimens (Figure 5, Figure 6, Figure 7 and Figure 8), where CuSO4·5H2O was proven to be highly effective, unlike chloroquine sulfate.
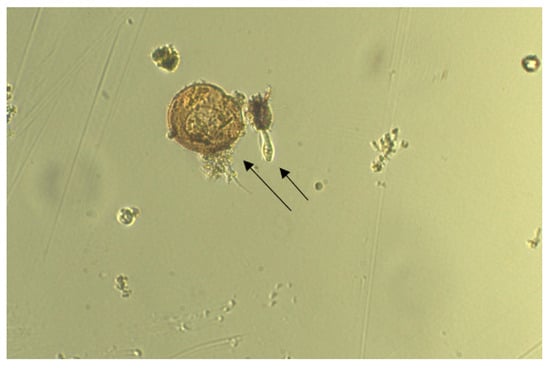
Figure 2.
Control specimen of two organisms. Arrows indicate the location of organisms within the sample.
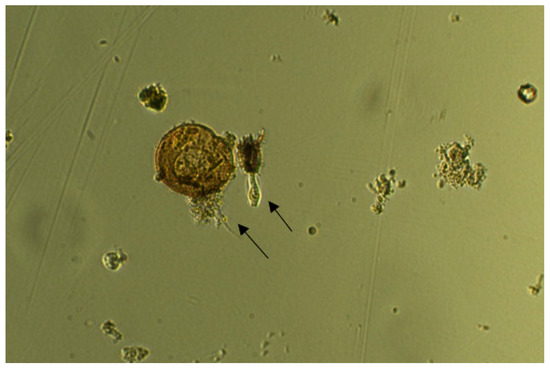
Figure 3.
Organisms exposed to 12.5 µM of chloroquine sulfate for 5–10 min. Arrows indicate areas of altered motility.
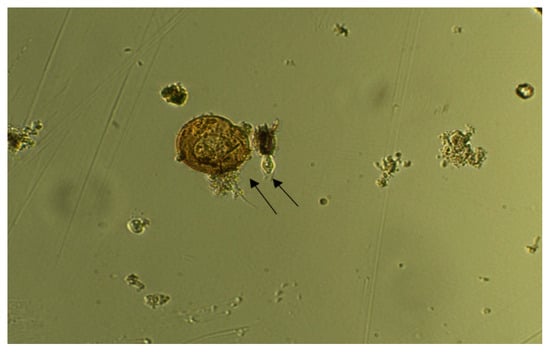
Figure 4.
Organisms after 5 min of exposure to 12.5 µM of CuSO4·5H2O. Arrows indicate two Organisms.
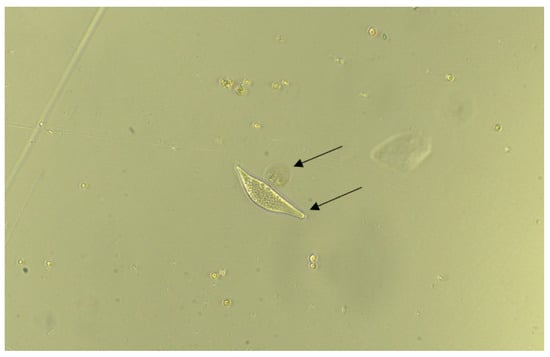
Figure 5.
Control image of two species. Arrows indicate the location of the two organisms.
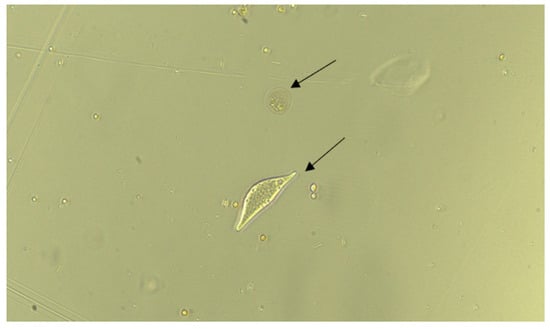
Figure 6.
The arrows show the location of two organisms exposed to 12.5 µM of chloroquine sulfate for 5 min.
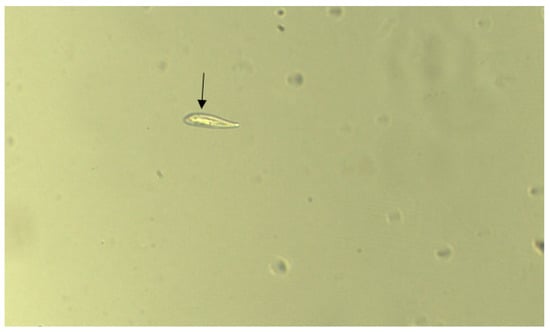
Figure 7.
The arrow shows the location of one of the Organisms exposed to 12.5 µM of CuSO4·5H2O for less than 5 min.
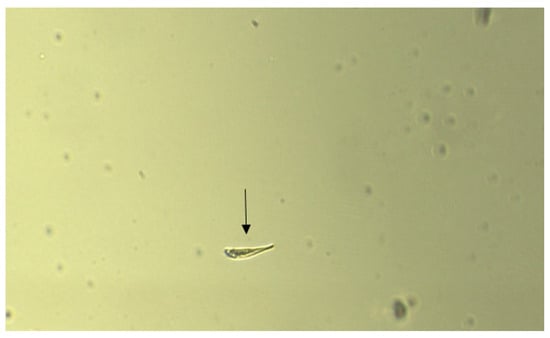
Figure 8.
The arrow shows the location of one of the Organisms following 5 min of exposure to 12.5 µM of CuSO4·5H2O.
It is important to note that out of 25 µM of CuSO4·5H2O, only 63.92% is represented by CuSO4, with the rest being water; Thus, the effective purgative concentration appears to consist solely of CuSO4. This observation aligns with the information provided by Aquavet®, (Aquavet Ltd., London, UK), which states that ¼ ppm is required to affect flagellates (protozoa) such as Dinobryon sp., Synura sp., Uroglena sp., and Volvox sp. [23].
4. Conclusions
Drinkable water or wastewater chemical treatments vary greatly and have been improved significantly in recent years, yet aquaculture water treatments require improvement to support increasingly higher demands as well as to face the climate change challenges that are to come. Therefore, further studies on simple and cost-effective treatments that do not induce genetic resistance to pathogens or toxicity to fish are needed.
We conclude that the use of chloroquine in aquaculture is not recommended, as reports in the literature state that host resistance can quickly be developed. Furthermore, the purgative effects of chloroquine are visible only at very high concentrations (usually above 10 mg/L, as reported by AquaVet [24]), a fact which poses an increased risk in pathogen resistance development. Therefore, as the drug still has clinical applications, it should be set aside in favor of CuSO4, which has proven to be an effective and low-cost treatment which does not induce host resistance and has passed the test of time.
Author Contributions
Conceptualization, G.D. and A.J.; methodology, A.J. and E.H.; software, I.S.; validation, G.D.; formal analysis, G.D. and I.S.; investigation, A.J.; resources, G.D.; data curation, G.D.; writing—original draft preparation, A.J., G.C., S.S., G.G. and B.Z.; writing—review and editing, A.J. and E.H.; visualization, G.D.; supervision, G.D.; project administration G.D and E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out through the Core Program BIO-CliMission of the National Plan for Research, Development and Innovation 2022–2027, carried out with the support of MCID, project no. PN 23 31 02 01., contract no. 44N/2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
This work has been carried out through the Core Program BIO-CliMission of the National Plan for Research, Development, and Innovation 2022–2027, carried out with the support of MCID, project no. PN 23 31 02 01.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- CDC. Drug Resistance in the Malaria-Endemic World. Available online: https://www.cdc.gov/malaria/php/public-health-strategy/drug-resistance.html (accessed on 20 July 2024).
- Wellems, T.E.; Plowe, C.V. Chloroquine-resistant malaria. J. Infect. Dis. 2001, 184, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Ursos, L.M.; Roepe, P.D. Chloroquine resistance in the malarial parasite, Plasmodium falciparum. Med. Res. Rev. 2002, 22, 465–491. [Google Scholar] [CrossRef]
- Deak, G.Y.; Daescu, V.; Holban, E.; Marinescu, P.; Tanase, G.S.; Csergo, R.; Daescu, A.I.; Gaman, S. Health-environment relation: A key issue of Romanian environmental protection. J. Environ. Prot. Ecol. 2015, 16, 304–315. [Google Scholar]
- Sulfonamides. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Morf, L.; Singh, U. Entamoeba histolytica: A snapshot of current research and methods for genetic analysis. Curr. Opin. Microbiol. 2012, 15, 469–475. [Google Scholar] [CrossRef]
- Nasir, N.A.N.M.; Zakarya, I.A.; Kamaruddin, S.A.; Islam, A.K.M.A. Advances and future prospects on biotechnological approaches towards Azolla for environmental sustainability. Pertanika J. Trop. Agri. Sci. 2022, 45, 595–609. [Google Scholar] [CrossRef]
- Xian, B.C.C.; Kang, C.W.; Ab Wahab, M.; Zainol, M.R.R.M.A.; Baharudin, F. Evaluation of low impact development and best management practices on peak flow reduction using SWMM. IOP Conf. Ser. Earth Environ. Sci 2021, 646, 012045. [Google Scholar] [CrossRef]
- Deák, G.; Holban, E.; Sadîca, I.; Jawdhari, A. Sturgeon Parasites: A Review of Their Diversity and Distribution. Diversity 2024, 16, 163. [Google Scholar] [CrossRef]
- Deák, G.; Matache, R.; Prangate, R.; Dumitrescu, G.; Holban, E.; Lupea, L.; Norlia, N.R.; Ibrahim, M. Risk of contamination of sturgeon species along the Lower Danube with AcIV-E virus from sturgeons raised in aquaculture systems. IOP Conf. Ser. Earth Environ. Sci 2023, 1216, 012012. [Google Scholar] [CrossRef]
- Matache, R.; Deak, G.; Jawdhari, A.; Sadica, I.; Pop, C.E.; Fendrihan, S.; Craciun, N. First insights of the Danube sturgeon (Acipenser gueldenstaedtii) skin adherent microbiota. bioRxiv 2024. [Google Scholar] [CrossRef]
- Aloo, P.A. Health problems associated with consumption of fish and the role of aquatic environments in the transmission of human diseases. Afr. J. Health Sci. 2000, 7, 107–113. [Google Scholar]
- Abdulhusein, G.; Ramteke, P. Investigations on parasitic diseases in fish of river Yamuna during the summer season. European Acad. Res. 2014, 2, 10057–10097. [Google Scholar]
- Adams, A.M.; Murrell, K.D.; Cross, J.H. Parasites of fish and risks to public health. Rev. Sci. Tech. 1997, 16, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Jawdhari, A.; Sadîca, I.; Matei, M.; Boboc, M.; Holban, E.; Laslo, L.; Mihăilescu, D.F. Assessment of Conservation Status of Petroleuciscus Borysthenicus Celensis From Gurban River, Romania By Identification of Parasites and Bacteria. Int. J. Conserv. Sci. 2024, 15, 1115–1128. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Veterinary, M. Parasitic Disease of Fishes. Available online: https://www.msdvetmanual.com/exotic-and-laboratory-animals/aquarium-fish/parasitic-diseases-of-fish#Flagellates_v23355077 (accessed on 5 July 2024).
- Matthews, J.L. Common diseases of laboratory zebrafish. Methods Cell Biol. 2004, 77, 617–643. [Google Scholar]
- Laufer, M.K.; Thesing, P.C.; Eddington, N.D.; Masonga, R.; Dzinjalamala, F.K.; Takala, S.L.; Taylor, T.E.; Plowe, C.V. Return of chloroquine antimalarial efficacy in Malawi. N. Engl. J. Med. 2006, 355, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E. General relationship between water quality and aquaculture performance in ponds. In Fish Diseases Prevention and Control Strategies, 1st ed.; Jeney, G., Ed.; Elsevier: Auburn, AL, USA, 2017; pp. 147–166. [Google Scholar] [CrossRef]
- Dinesh, R.; Anand, C.; John, K.; George, M.; Bharathi, S.; Kumar, J. An overview of chemicals and drugs in aquaculture disease management. Indian J. Anim. Health 2022, 62, 1–20. [Google Scholar] [CrossRef]
- Desta, K.; Amare, M. Validated UV-Visible spectrometry using water as a solvent for determination of chloroquine in tablet samples. Chem. Int. 2017, 3, 288–295. [Google Scholar]
- Ciucă, V.C.; Safta, V.V.; Rusănescu, C.O.; Paraschiv, G.; Deák, G.; Ilie, M.; Cănănău, S. Rapid environmental impact assessment of penicillin G in a veterinary product using an original software method and monitoring by SPE-Online-UHPLC-MS/MS. Molecules 2023, 28, 6227. [Google Scholar] [CrossRef]
- AquaVet. Proof Five Cooper Sulfate. Available online: http://legacy.picol.cahnrs.wsu.edu/~picol/pdf/OR/60867.pdf (accessed on 20 July 2016).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).