Synthesis and Photocatalytic Performance of g-C3N4/ZnO Nanocomposites for the Efficient Degradation of Dyes Under Sunlight †
Abstract
1. Introduction
2. Methods and Materials
2.1. Chemicals
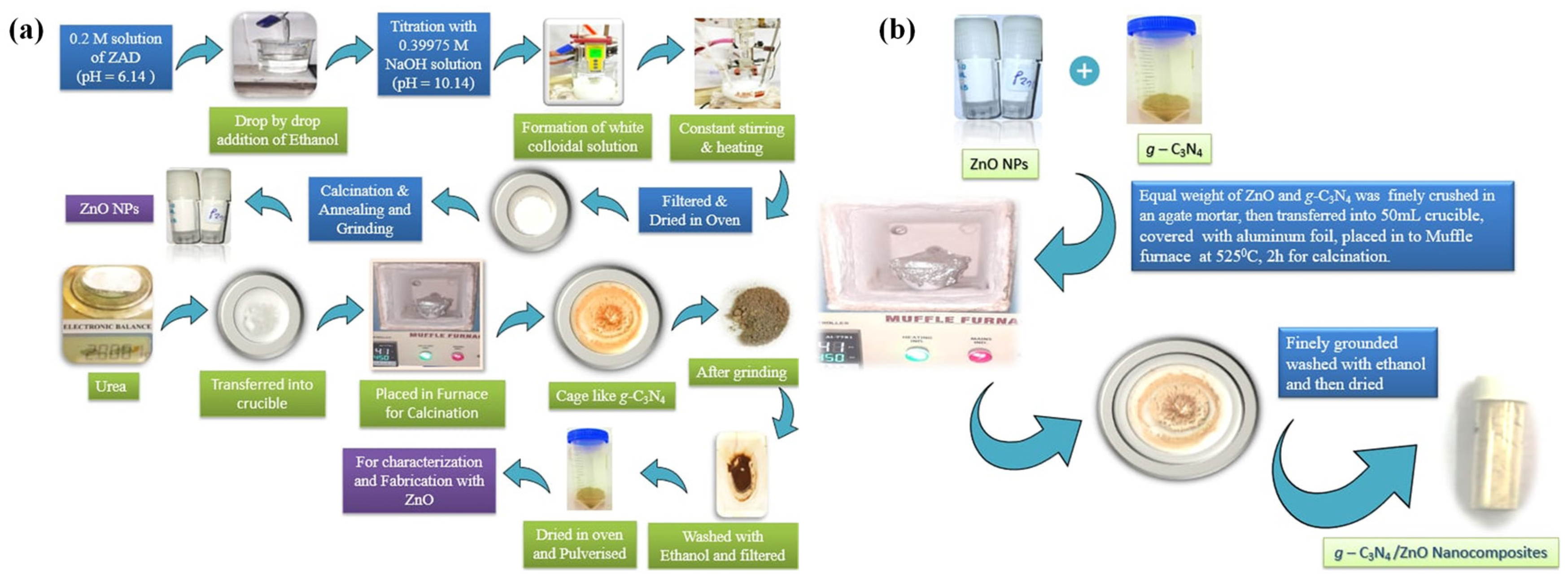
2.2. Synthesis of ZnO and g-C3N4
2.3. Synthesis of g-C3N4/ZnO Nanocomposite
2.4. Photocatalytic Test Evaluation
2.5. Characterizations
3. Result and Discussion
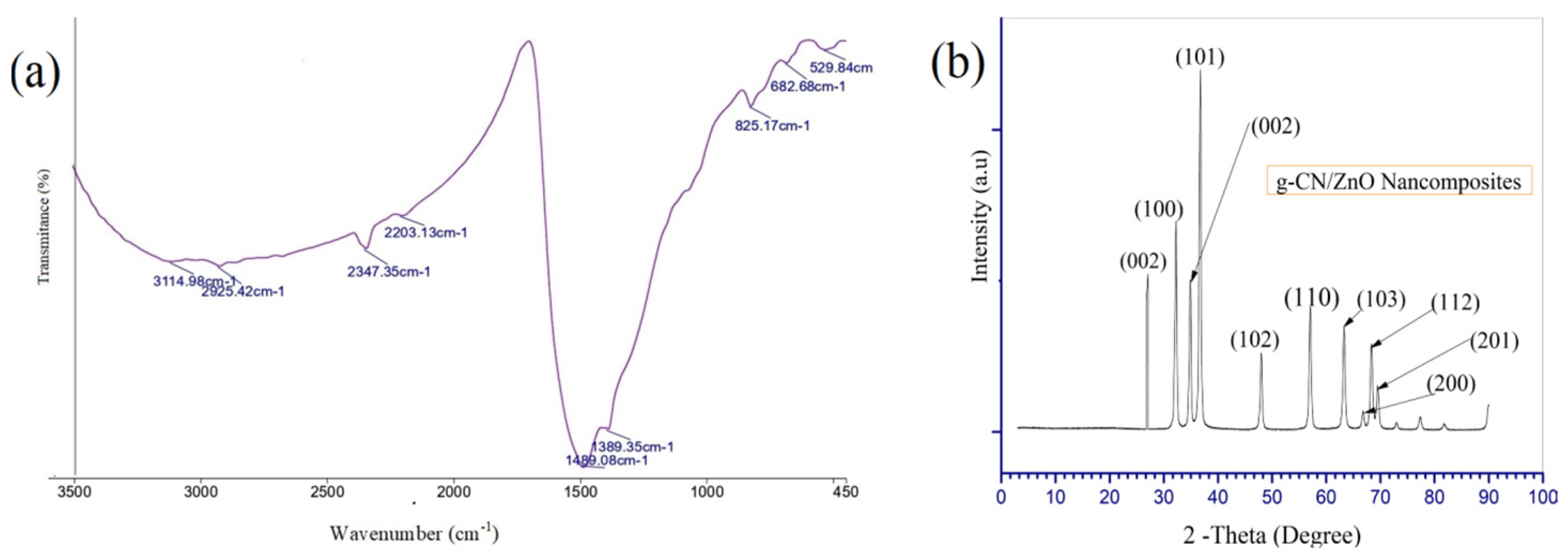
3.1. Fourier Transform Infrared (FTIR) Analysis
3.2. Phase Analysis and Characterization of Crystalline Structures
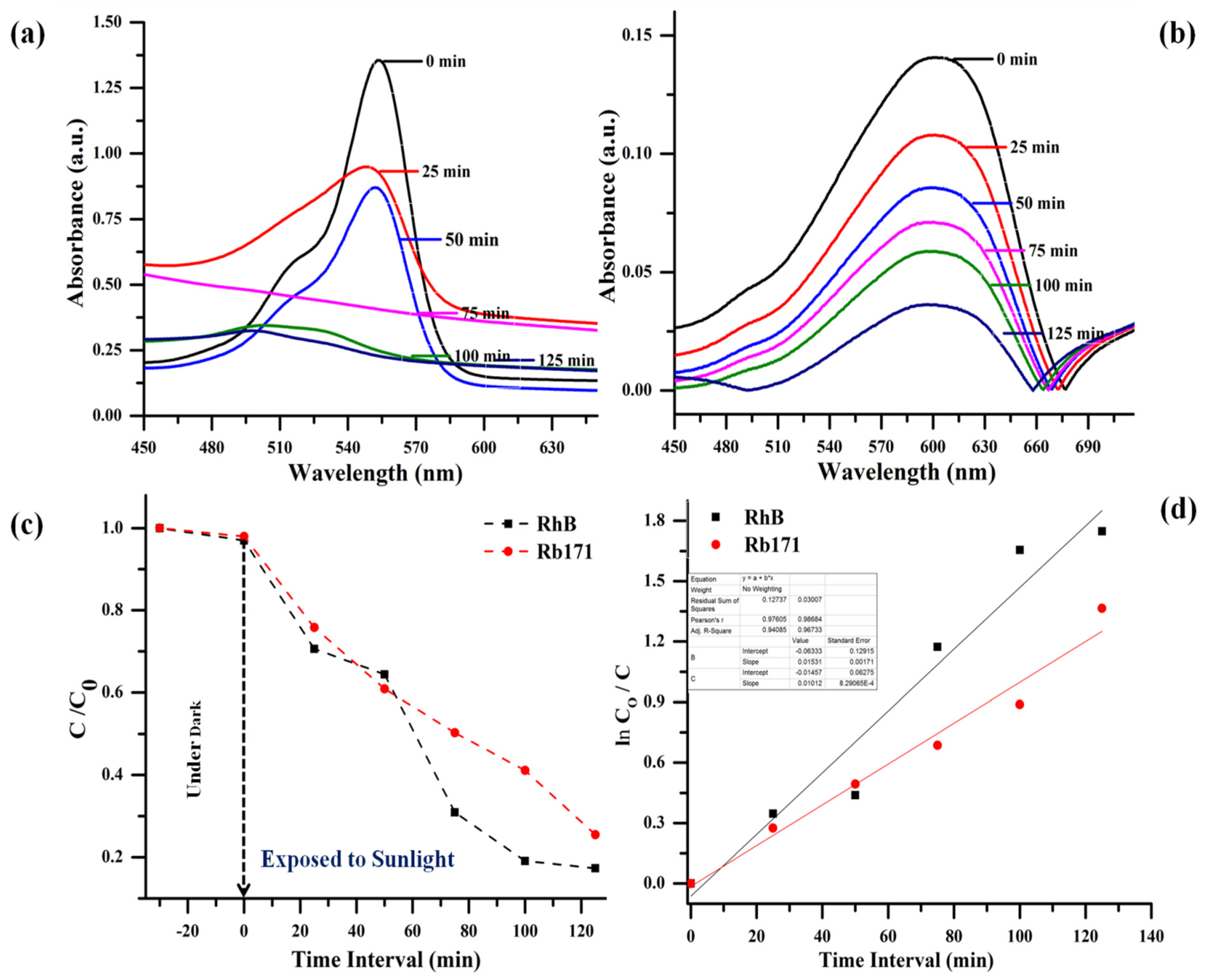
3.3. Evaluation of Photocatalytic Potential of g-C3N4/ZnO Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagar, A.; Pradeep, T. Clean Water through Nanotechnology: Needs, Gaps, and Fulfillment. ACS Nano 2020, 14, 6420–6435. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Polonio, C.Z.F.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Zollinger, H. Color chemistry. In Synthesis, Properties and Applications of Organic Dyes and Pigments, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Chequer, F.M.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Carvalho, J.; Boldrin Zanoni, M.V.; de Oliveir, D.P. Textile Dyes: Dyeing Process and Environmental Impact; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Y.Z. Decolorization and biodegradability of photocatalytic treated azo dyes and wool textile wastewater. Chemosphere 1999, 39, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Ansari, N.; Ali, A.; Akhtar, M.S.; Hasan, S.; Khatoon, T.; Khan, A.R.; Wabaidur, S.M.; Rahman, Q.I. Green synthesis of zinc oxide marigold shaped clusters using Eucalyptus globulus leaf extract as robust photocatalyst for dyes degradation under sunlight. Mater. Sci. Semicond. Process. 2024, 173, e108087. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabbet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Nehra, S.P.; Sharma, A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019, 9, 15381–15391. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Dong, Y.P.; Chu, X.F. Synthesis of ZnO/g-C3N4 Nanocomposite and Its Electrochemical Application in Hydrogen Peroxide Detection. Russ. J. Electrochem. 2021, 57, 808–815. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Xiao, M.; Liu, F.; Xu, X.; Du, E. Construction of direct solid-state Z-scheme g-C3N4/BiOI with improved photocatalytic activity for microcystin-LR degradation. J. Mater. Res. 2018, 33, 201–212. [Google Scholar] [CrossRef]
- Chen, X.Y.; Pan, Q.J.; Guo, Y.R. One-pot synthesis of the g-C3N4/S-doped ZnO composite with assistance of sodium lignosulfonate and its enhanced photodegradation on organic pollutants. Mater. Res. Bull. 2018, 107, 164–170. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Gobile, N.; Oyewo, O.A.; Makgato, S.S. Photocatalytic reduction of hexavalent chromium using Zn2SnO4–ZnO modified g-C3N4 composite. Results Eng. 2023, 20, 101521. [Google Scholar] [CrossRef]
- Bian, S.W.; Ma, Z.; Song, W.G. Preparation and characterization of carbon nitride nanotubes and their applications as catalyst supporter. J. Phys. Chem. C 2009, 113, 8668–8672. [Google Scholar] [CrossRef]
- Paul, D.R.; Gautam, S.; Panchal, P.; Nehra, S.P.; Choudhary, P.; Sharm, A. ZnO-Modified g-C3N4:A Potential Photocatalyst for Environmental Application. ACS Omega 2020, 5, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Hexagonal ZnO Nanorods Assembled Flowers for Photocatalytic Dye Degradation: Growth, Structural and Optical Properties. Superlattices Microstruct. 2013, 64, 495–506. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Mehta, S.K. Hydrothermal synthesis of Cr-doped SrTiO3 nanoparticles for rhodamine-B dye degradation under visible light illumination. Colloid Polym. Sci. 2017, 295, 933–937. [Google Scholar] [CrossRef]
- Nihal Kumari, C.; Sharma, R.; Sharma, M.; Dogra, S.D.; Sharma, I.; Choudhary, B.C.; Goswamy, J.K. gCN/ZnO nanocomposite as an efficient sunlight active photocatalyst for Rhodamine B dye degradation. Hybrid Adv. 2025, 8, 100346. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Haman, S.; Ali, A.; Mehta, S.K.; Raja, M.A.; Ahmad, N.; Khan, A.R.; Muddassir, M. Synthesis and characterizations of nitrogen (N) doped strontium titanate (SrTiO3) nanoparticles for enhanced visible light driven photocatalytic degradation. J. Nanosci. Nanotechnol. 2020, 20, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
| Properties | Reactive Blue (Rb171) | Rhodamine B |
|---|---|---|
| Chemical Formula | C40H23O19 N15Na6S6Cl2 | C28H31ClN2O3 |
| Molecular Weight (g/mol) | 1419.83 approx. | 479.02 approx |
| Dye Type | Anionic (Acidic) | Cationic (Basic) |
| Solubility in water at 25 °C | Soluble | Soluble |
| Dye | Degradation Efficiency (%) | Sunlight Exposure (min) | Half-Life Time (min) | Rate Constant (min−1) | R2 Value |
|---|---|---|---|---|---|
| RhB | 82.50 | 125 | 45.27 | 0.01531 | 0.89207 |
| Rb171 | 74.46 | 68.49 | 0.01012 | 0.96733 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suneel; Khan, M.A.; Tabassum, N.; Khan, A.R.; Feroz, Z.; Rahman, Q.I. Synthesis and Photocatalytic Performance of g-C3N4/ZnO Nanocomposites for the Efficient Degradation of Dyes Under Sunlight. Environ. Earth Sci. Proc. 2025, 32, 18. https://doi.org/10.3390/eesp2025032018
Suneel, Khan MA, Tabassum N, Khan AR, Feroz Z, Rahman QI. Synthesis and Photocatalytic Performance of g-C3N4/ZnO Nanocomposites for the Efficient Degradation of Dyes Under Sunlight. Environmental and Earth Sciences Proceedings. 2025; 32(1):18. https://doi.org/10.3390/eesp2025032018
Chicago/Turabian StyleSuneel, Mohd Arsh Khan, Neda Tabassum, Abdul Rahman Khan, Zainab Feroz, and Qazi Inamur Rahman. 2025. "Synthesis and Photocatalytic Performance of g-C3N4/ZnO Nanocomposites for the Efficient Degradation of Dyes Under Sunlight" Environmental and Earth Sciences Proceedings 32, no. 1: 18. https://doi.org/10.3390/eesp2025032018
APA StyleSuneel, Khan, M. A., Tabassum, N., Khan, A. R., Feroz, Z., & Rahman, Q. I. (2025). Synthesis and Photocatalytic Performance of g-C3N4/ZnO Nanocomposites for the Efficient Degradation of Dyes Under Sunlight. Environmental and Earth Sciences Proceedings, 32(1), 18. https://doi.org/10.3390/eesp2025032018







