Quantum Biosensors on Chip: A Review from Electronic and Photonic Integrated Circuits to Future Integrated Quantum Photonic Circuits
Abstract
1. Introduction
2. Materials and Methods
3. Fundamentals of Quantum Technologies
3.1. Introduction to Quantum Concepts
3.2. Quantum Phenomena in Biomedical Contexts
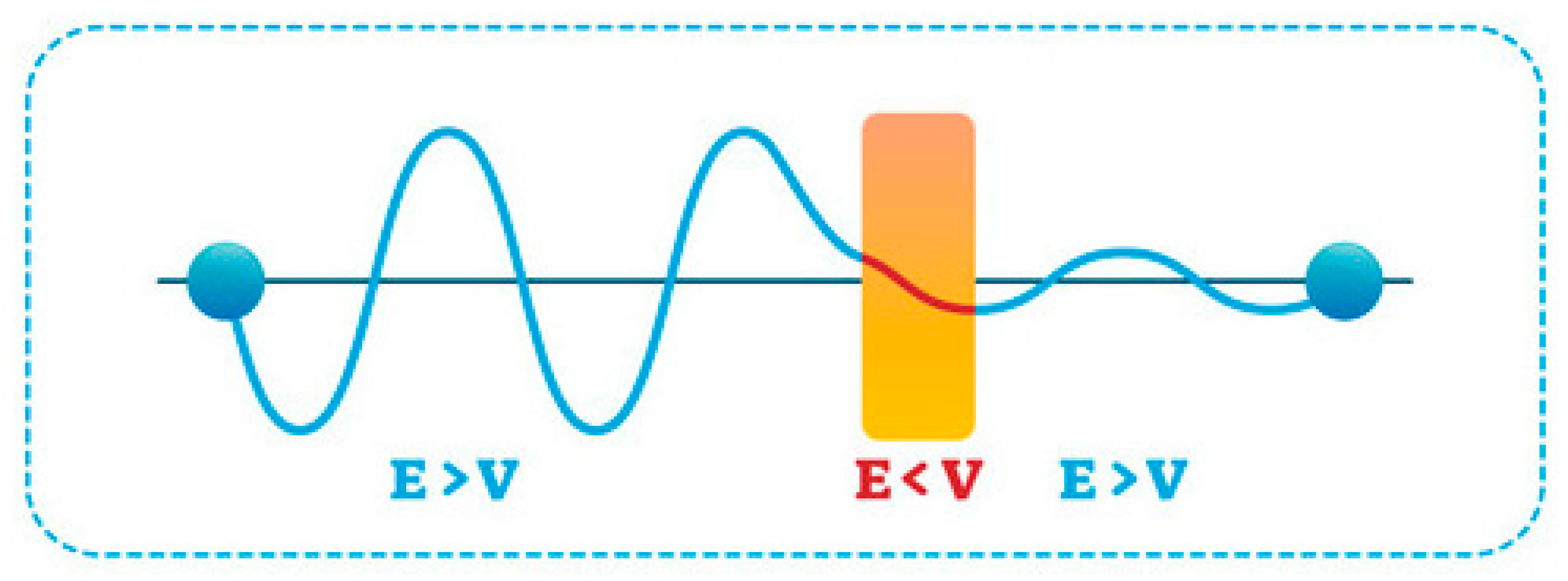
3.2.1. Quantum Tunnelling
3.2.2. Quantum Coherence
3.2.3. Quantum Entanglement and Superposition
3.3. Quantum Devices: Qubits, Sensors, and Photons
| Class | Operating Principle | Implementations | Biomedical Applications | Advantages | Limitations |
|---|---|---|---|---|---|
| Qubits | Superposition, Entanglement | Trapped ions, Superconducting circuits, Silicon spin qubits, NV centers, Topological qubits [34,35,36,37] | Molecular sensing, Biomedical imaging [38] | Scalable, High fidelity | Decoherence, Cryogenic needs, Fabrication complexity |
| Quantum Sensors | Quantum coherence, Interference | NV center Magnetometers [62] | Brain imaging, Nanoscopic magnetic sensing [39] | High sensitivity, Room-temperature | Integration challenges, SNR issues |
| Quantum Photonic | Single-photon | On-chip photon sources, CMOS-compatible biosensors [41,42] | Optical biosensing, Quantum communication | Compact, Fast, Low noise, CMOS-compatible | Photon loss, Detection inefficiency |
3.4. Interfacing Quantum and Classical Systems
4. Quantum Biosensing Systems
4.1. Quantum Sensing Principles
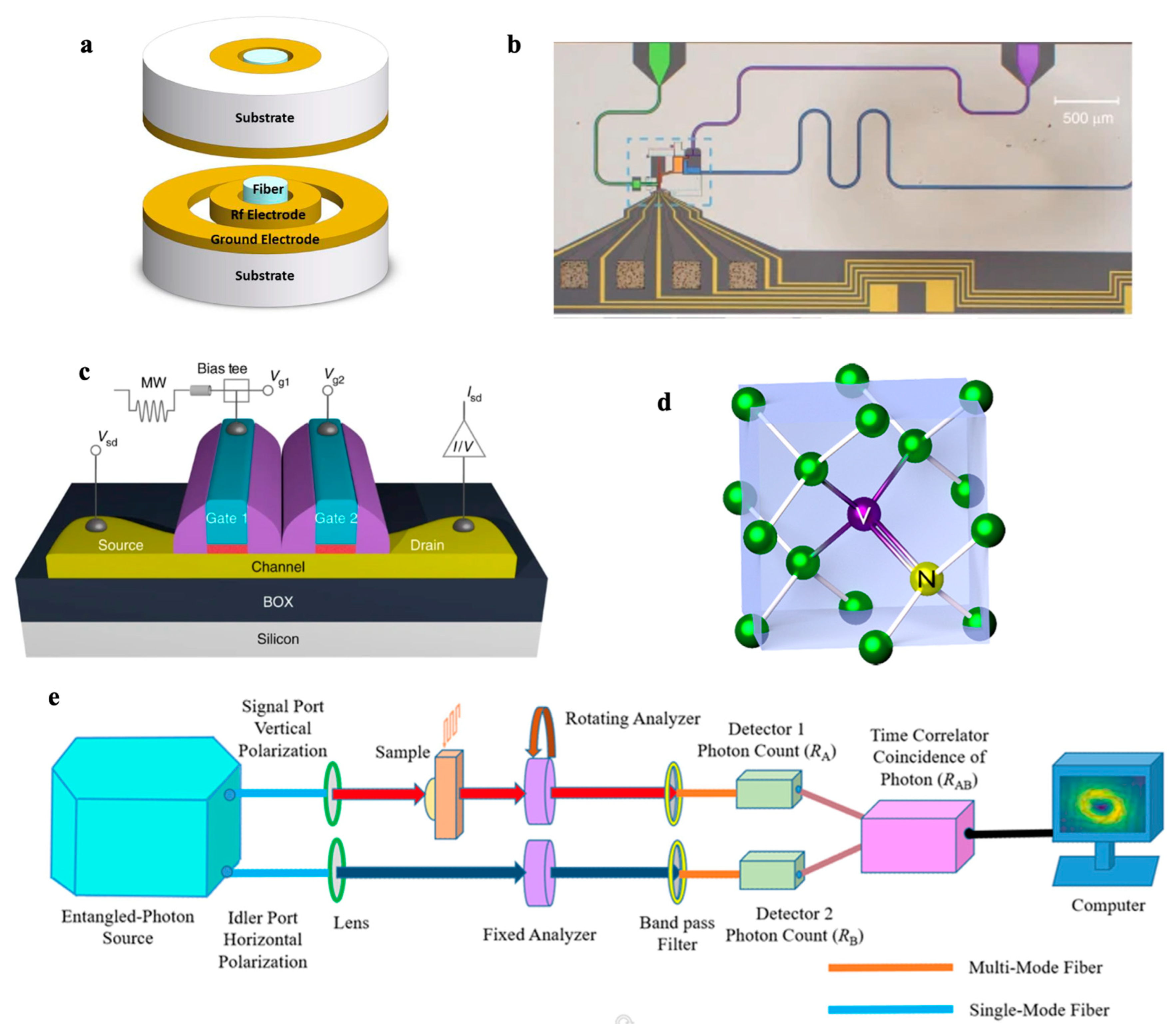
4.2. Quantum Plasmonic Biosensors
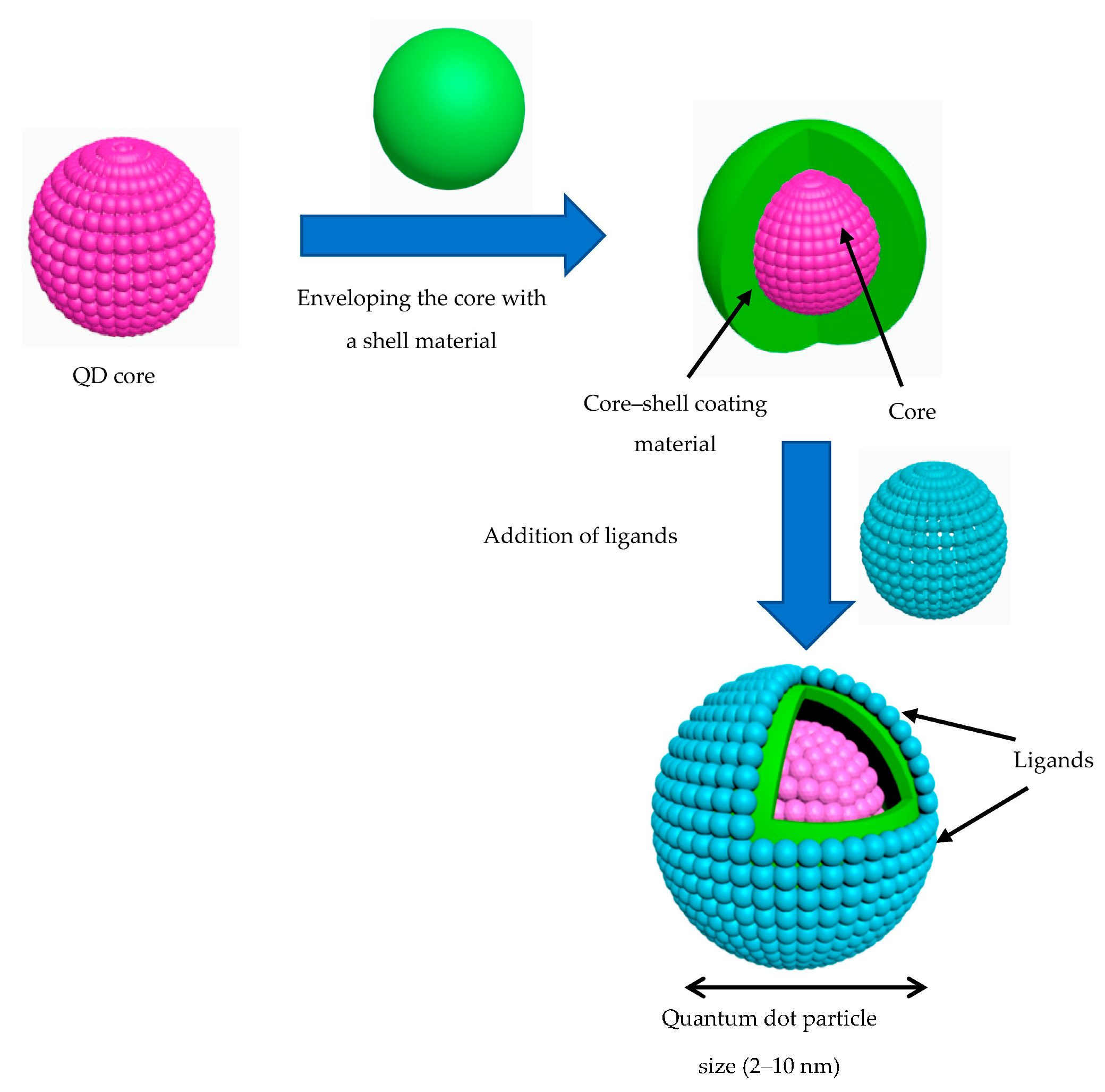
4.3. Quantum Dot (QD) Biosensors
4.4. Nitrogen-Vacancy (NV) Center Diamond Biosensors
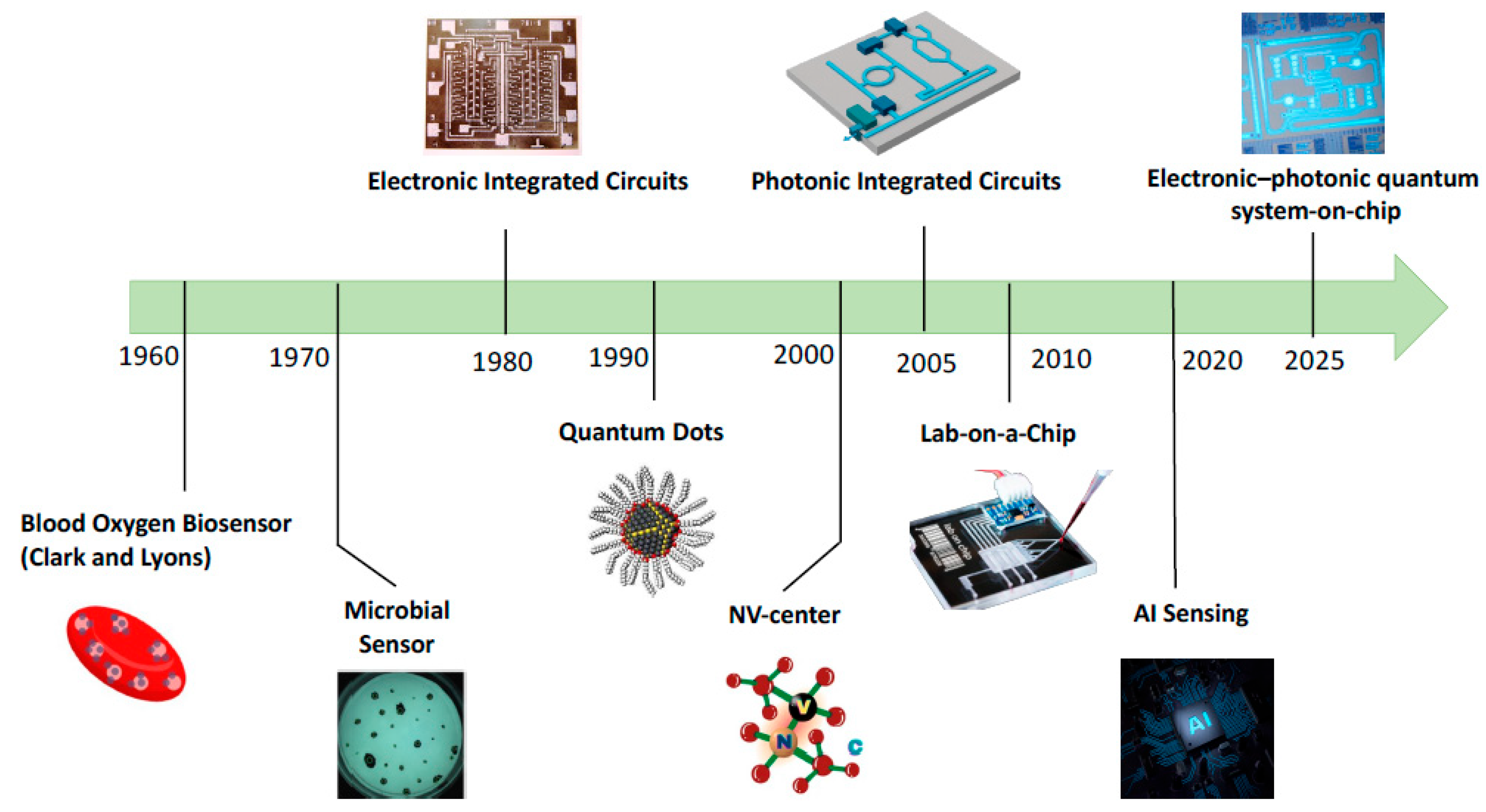
5. On-Chip Quantum Biosensing
5.1. From Electronic Chips to Photonic Circuits
5.2. Photonic Integrated Circuits for Biosensing

5.3. Toward Integrated Quantum Photonics (IQPs)
6. Research Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Analog-to-Digital Converter |
| Au | Gold |
| ClO− | Hypochlorite ion |
| CMOS | Complementary Metal–Oxide–Semiconductor |
| Co3O4 | Cobalt Oxide |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| E. coli | Escherichia coli |
| EIC | Electronic Integrated Circuit |
| ER | Extinction Ratio |
| FLS | Fibroblast-like Synoviocyte |
| FSR | Free Spectral Range |
| GaAs | Gallium Arsenide |
| GaN | Gallium Nitride |
| GQD | Graphene Quantum Dot |
| hBN | Hexagonal Boron Nitride |
| HIV | Human Immunodeficiency Virus |
| HL | Heisenberg Limit |
| IC | Integrated Circuit |
| InP | Indium Phosphide |
| IQP | Integrated Quantum Photonic |
| LiNbO3 | Lithium Niobate |
| LLOQ | Lower Limit of Quantification |
| LOD | Limit of Detection |
| MEG | Magnetoencephalography |
| MEMS | Microelectromechanical Systems |
| MZI | Mach–Zehnder Interferometer |
| NbN | Niobium Nitride |
| NCQD | Nitrogen-doped Carbon Quantum Dot |
| NV | Nitrogen-Vacancy |
| PCB | Printed Circuit Board |
| PDMS | Polydimethylsiloxane |
| PIC | Photonic Integrated Circuit |
| PN | P-type/N-type Semiconductor Junction |
| QD(s) | Quantum Dot(s) |
| Q-factor | Quality Factor |
| Qubit | Quantum-bit |
| RF | Radio Frequency |
| RIU | Refractive Index Unit |
| RNA | Ribonucleic Acid |
| Si3N4 | Silicon Nitride |
| SNR | Signal-to-Noise Ratio |
| SNSPD | Superconducting Nanowire |
| Single-Photon Detector | |
| SOI | Silicon-On-Insulator |
| SPAD | Single-Photon Avalanche Diode |
| SPD | Single-Photon Detector |
| SQL | Standard Quantum Limit |
| S-SiQD | Sulfhydryl-functionalized Silicon |
| Quantum Dot | |
| TMDs | Transition Metal Dichalcogenides |
| TSV | Through-Silicon-Via |
| ZnO | Zinc Oxide |
References
- Sasidhar, B. Advancements In Biosensor Technologies: From Nanobiosensors To Biocompatible And Optical Systems For Clinical And Environmental Applications. World J. Pharm. Sci. 2025, 13, 1–300. [Google Scholar]
- Hassan, M.M.; Xu, Y.; Zareef, M.; Li, H.; Chen, Q. Recent Progress in Chemometrics Driven Biosensors for Food Application. TrAC Trends Anal. Chem. 2022, 156, 116707. [Google Scholar] [CrossRef]
- Athanassov, A.S. The Little Transistor And The Ccas Revolution. In Proceedings of the Electronics’ 2007, Sozopol, Bulgaria, 19–21 September 2007. [Google Scholar]
- Kim, K.; Kim, J.-H.; Gweon, S.; Kim, M.; Yoo, H.-J. A 0.5-V Sub-10-μW 15.28-mΩ/√Hz Bio-Impedance Sensor IC With Sub-1° Phase Error. IEEE J. Solid-State Circuits 2020, 55, 2161–2173. [Google Scholar] [CrossRef]
- Alhoshany, A.; Sivashankar, S.; Mashraei, Y.; Omran, H.; Salama, K.N. A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-Μm CMOS Technology for Cancer Biomarker Detection. Sensors 2017, 17, 1942. [Google Scholar] [CrossRef]
- Al Mamun, K.A.; Islam, S.K.; Hensley, D.K.; McFarlane, N. A Glucose Biosensor Using CMOS Potentiostat and Vertically Aligned Carbon Nanofibers. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; Doerr, C.; Kish, F. Optical Fiber Telecommunications VIA: Chapter 2. In Semiconductor Photonic Integrated Circuit Transmitters and Receivers; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-806056-8. [Google Scholar]
- Prieto, F.; Sepúlveda, B.; Calle, A.; Llobera, A.; Domínguez, C.; Abad, A.; Montoya, A.; Lechuga, L.M. An Integrated Optical Interferometric Nanodevice Based on Silicon Technology for Biosensor Applications. Nanotechnology 2003, 14, 907. [Google Scholar] [CrossRef]
- Ning, S.; Chang, H.-C.; Fan, K.-C.; Hsiao, P.-Y.; Feng, C.; Shoemaker, D.; Chen, R.T. A Point-of-Care Biosensor for Rapid Detection and Differentiation of COVID-19 Virus (SARS-CoV-2) and Influenza Virus Using Subwavelength Grating Micro-Ring Resonator. Appl. Phys. Rev. 2023, 10, 21410. [Google Scholar] [CrossRef]
- Bryan, M.R.; Butt, J.N.; Ding, Z.; Tokranova, N.; Cady, N.; Piorek, B.; Meinhart, C.; Tice, J.; Miller, B.L. A Multiplex “Disposable Photonics” Biosensor Platform and Its Application to Antibody Profiling in Upper Respiratory Disease. ACS Sens. 2024, 9, 1799–1808. [Google Scholar] [CrossRef]
- Sharma, S.; Roy, S. A Survey on Design and Synthesis Techniques for Photonic Integrated Circuits. J. Supercomput. 2021, 77, 4332. [Google Scholar] [CrossRef]
- NASA-Industry Team Creates and Demonstrates First Quantum Sensor for Satellite Gravimetry; NASA: Washington, DC, USA, 2018.
- Lerch, S.; Reinhard, B.M. Quantum Plasmonics: Optical Monitoring of DNA-Mediated Charge Transfer in Plasmon Rulers. Adv. Mater. 2016, 28, 2030–2036. [Google Scholar] [CrossRef]
- Wei, N.; Sun, Y.-C.; Guo, X.-F.; Wang, H. Synthesis of Sulfhydryl Functionalized Silicon Quantum Dots with High Quantum Yield for Imaging of Hypochlorite in Cells and Zebrafish. Microchim. Acta 2022, 189, 329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Feng, Y.; Guo, H.; Wen, H.; Tang, J.; Liu, J. High-Sensitivity DC Magnetic Field Detection with Ensemble NV Centers by Pulsed Quantum Filtering Technology. Opt. Express 2020, 28, 16191–16201. [Google Scholar] [CrossRef]
- Miladić, S. Electrical Control of a Spin Qubit in InSb Nanowire Quantum Dots: Strongly Suppressed Spin Relaxation in High Magnetic Field. Phys. Rev. B 2020, 101. [Google Scholar] [CrossRef]
- Stavrou, V.N. Electron Relaxation in a Double Quantum Dot through Two-Phonon Processes. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef]
- Kramnik, D.; Wang, I.; Ramesh, A.; Fargas Cabanillas, J.M.; Gluhović, Ð.; Buchbinder, S.; Zarkos, P.; Adamopoulos, C.; Kumar, P.; Stojanović, V.M.; et al. Scalable Feedback Stabilization of Quantum Light Sources on a CMOS Chip. Nat. Electron. 2025, 8, 620–630. [Google Scholar] [CrossRef]
- Hughes, C.; Isaacson, J.; Perry, A.; Sun, R.F.; Turner, J. What Is a Qubit? In Quantum Computing for the Quantum Curious; Springer International Publishing: Cham, Switzerland, 2021; pp. 7–16. ISBN 978-3-030-61601-4. [Google Scholar]
- Robert, A.; Barkoutsos, P.K.; Woerner, S.; Tavernelli, I. Resource-Efficient Quantum Algorithm for Protein Folding. npj Quantum Inf. 2021, 7, 38. [Google Scholar] [CrossRef]
- Sajjan, M.; Li, J.; Selvarajan, R.; Sureshbabu, S.H.; Kale, S.S.; Gupta, R.; Singh, V.; Kais, S. Quantum Machine Learning for Chemistry and Physics. Chem. Soc. Rev. 2022, 51, 6475–6573. [Google Scholar] [CrossRef]
- Sawaya, N.P.D.; Huh, J. Quantum Algorithm for Calculating Molecular Vibronic Spectra. J. Phys. Chem. Lett. 2019, 10, 3586–3591. [Google Scholar] [CrossRef]
- Huh, J.; Guerreschi, G.G.; Peropadre, B.; McClean, J.R.; Aspuru-Guzik, A. Boson Sampling for Molecular Vibronic Spectra. Nat. Photon. 2015, 9, 615–620. [Google Scholar] [CrossRef]
- Barr, A.J.; Fabbrichesi, M.; Floreanini, R.; Gabrielli, E.; Marzola, L. Quantum Entanglement and Bell Inequality Violation at Colliders. Prog. Part. Nucl. Phys. 2024, 139, 104134. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Chaudhuri, B. Application of Quantum Entanglement to Explain the Healing Mechanism by Highly Diluted Homoeopathic Medicines. Indian J. Res. Homoeopath. 2025, 19, 3–14. [Google Scholar] [CrossRef]
- He, M. Entanglement-Enhanced Bioimaging and Sensing. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 2024. [Google Scholar]
- Michaud, A. Critical Analysis of the Origins of Heisenberg’s Uncertainty Principle. J. Mod. Phys. 2024, 15, 765–795. [Google Scholar] [CrossRef]
- Gurjar, V.; Rajan, A.; Chaturvedi, A.; Tiwari, R.; Ratre, P.; Mishra, P. Deep Learning-Enabled Quantum Imaging: Future-Ready Nanosensing Technologies for Preventive Health Interventions. Comput. Struct. Biotechnol. Rep. 2025, 2, 100053. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Baniasadi, F. Quantum for Biology: Spectroscopy and Sensing. Innov. Emerg. Technol. 2024, 11. [Google Scholar] [CrossRef]
- Mauranyapin, N.P.; Terrasson, A.; Bowen, W.P. Quantum Biotechnology. Adv. Quantum Technol. 2022, 5, 2100139. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, H.; Schmieg, R.; Oesterle, A.; Polzik, E.S. Entanglement-Enhanced Magnetic Induction Tomography. Phys. Rev. Lett. 2023, 130, 203602. [Google Scholar] [CrossRef]
- Castillo, J.C.R. Differential Equations: Fundamentals, Solution Methods, and Applications in Dynamical Systems and Chaos Theory. Ibero Cienc. Rev. Científica Y Académica 2025, 4, 22–42. [Google Scholar] [CrossRef]
- Dal Lin, C.; Romano, P.; Iliceto, S.; Tona, F.; Vitiello, G. On Collective Molecular Dynamics in Biological Systems: A Review of Our Experimental Observations and Theoretical Modeling. Int. J. Mol. Sci. 2022, 23, 5145. [Google Scholar] [CrossRef]
- Matarèse, B.F.E.; Purushotham, A. Quantum Oncology. Quantum Rep. 2025, 7, 9. [Google Scholar] [CrossRef]
- Sutcliffe, M.J.; Scrutton, N.S. Enzymology Takes a Quantum Leap Forward. Philos. Trans. A Math. Phys. Eng. Sci. 2000, 358, 367–386. [Google Scholar] [CrossRef]
- Niazi, S.K. The Quantum Paradox in Pharmaceutical Science: Understanding Without Comprehending—A Centennial Reflection. Int. J. Mol. Sci. 2025, 26, 4658. [Google Scholar] [CrossRef]
- Slocombe, L.; Sacchi, M.; Al-Khalili, J. An Open Quantum Systems Approach to Proton Tunnelling in DNA. Commun. Phys. 2022, 5, 109. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Proton Tunneling in DNA and Its Biological Implications. Rev. Mod. Phys. 1963, 35, 724–732. [Google Scholar] [CrossRef]
- Ali, A.; Naeem, M.Y.; Selamoglu, Z.; Naqvi, M.R. Exploring Quantum in Cancer Biology: A Comprehensive Review of Nontrivial Quantum Events. Arch. Razi Inst. 2025, 80, 395–400. [Google Scholar] [CrossRef]
- Nicolini, C.; Sivozhelezov, V. Quantum Effects and Genetic Code: Dynamics and Information Transfer in DNA. arXiv 2006. [Google Scholar] [CrossRef]
- Adams, B.; Sinayskiy, I.; van Grondelle, R.; Petruccione, F. Quantum Tunnelling in the Context of SARS-CoV-2 Infection. Sci. Rep. 2022, 12, 16929. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.W.; Toutounji, M. Vibration Assisted Electron Tunnelling in COVID-19 Infection Using Quantum State Diffusion. Sci. Rep. 2024, 14, 12152. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N.; Zhou, H.; Urbach, E.K.; Turner, M.J.; Walsworth, R.L.; Lukin, M.D.; Park, H. Quantum Sensors for Biomedical Applications. Nat. Rev. Phys. 2023, 5, 157–169. [Google Scholar] [CrossRef]
- Schofield, H.; Boto, E.; Shah, V.; Hill, R.M.; Osborne, J.; Rea, M.; Doyle, C.; Holmes, N.; Bowtell, R.; Woolger, D.; et al. Quantum Enabled Functional Neuroimaging: The Why and How of Magnetoencephalography Using Optically Pumped Magnetometers. Contemp. Phys. 2022, 63, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Goryanin, I.I.; Damms, B.; Vesnin, S.; Shevelev, O.; Gorya, I. Exploring the Interface of Microwave Technology, Quantum Computing and Neuroscience. Preprints 2024. [Google Scholar] [CrossRef]
- Allert, R.D.; Briegel, K.D.; Bucher, D.B. Advances in Nano- and Microscale NMR Spectroscopy Using Diamond Quantum Sensors. Chem. Commun. 2022, 58, 8165–8181. [Google Scholar] [CrossRef]
- Georgiev, D.D. Quantum Information in Neural Systems. Symmetry 2021, 13, 773. [Google Scholar] [CrossRef]
- Parmar, S.J.; Parmar, V.R.; Verma, J.; Roy, S.; Bhattacharya, P. Quantum Computing: Exploring Superposition and Entanglement for Cutting-Edge Applications. In Proceedings of the 2023 16th International Conference on Security of Information and Networks (SIN), Rajasthan, India, 20–21 November 2023; pp. 1–6. [Google Scholar]
- Salloum, H.; Lukin, R.; Mazzara, M. Quantum Computing in Drug Discovery: A Review of Quantum Annealing and Gate-Based Approaches. In Proceedings of the ICOMP 2024 Conference, Innopolis, Russia, 20 September 2024. [Google Scholar]
- Chow, J.C.L. Quantum Computing in Medicine. Med. Sci. 2024, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Pokhrel, S.R. Modeling Quantum Machine Learning for Genomic Data Analysis. arXiv 2025. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X. Efficient Quantum Algorithm for Lattice Protein Folding. Quantum Sci. Technol. 2025, 10, 15056. [Google Scholar] [CrossRef]
- Kiani, B.T.; Villanyi, A.; Lloyd, S. Quantum Medical Imaging Algorithms. arXiv 2020. [Google Scholar] [CrossRef]
- Smith, M.C.; Leu, A.D.; Miyanishi, K.; Gely, M.F.; Lucas, D.M. Single-Qubit Gates with Errors at the 10−7 Level. Phys. Rev. Lett. 2025, 134, 230601. [Google Scholar] [CrossRef]
- Hawaldar, S.; Shahi, P.; Carter, A.L.; Rey, A.M.; Bollinger, J.J.; Shankar, A. Bilayer Crystals of Trapped Ions for Quantum Information Processing. Phys. Rev. X 2024, 14, 031030. [Google Scholar] [CrossRef]
- Chiu, N.-C.; Trapp, E.C.; Guo, J.; Abobeih, M.H.; Stewart, L.M.; Hollerith, S.; Stroganov, P.L.; Kalinowski, M.; Geim, A.A.; Evered, S.J.; et al. Continuous Operation of a Coherent 3000-Qubit System. Nature 2025. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Noiri, A.; Nakajima, T.; Kobayashi, T.; Tarucha, S. Quantum Error Correction with Silicon Spin Qubits. Nature 2022, 608, 682–686. [Google Scholar] [CrossRef]
- Hernández-Gómez, S.; Fabbri, N. Quantum Control for Nanoscale Spectroscopy With Diamond Nitrogen-Vacancy Centers: A Short Review. Front. Phys. 2021, 8, 610868. [Google Scholar] [CrossRef]
- Jin, K.-H.; Jiang, W.; Sethi, G.; Liu, F. Topological Quantum Devices: A Review. Nanoscale 2023, 15, 12787–12817. [Google Scholar] [CrossRef] [PubMed]
- AbuGhanem, M. Superconducting Quantum Computers: Who Is Leading the Future? AbuGhanem EPJ Quantum Technol. 2025, 12, 102. [Google Scholar] [CrossRef]
- Simon, D.S. Solid-State Qubits. In Introduction to Quantum Science and Technology; Simon, D.S., Ed.; Springer Nature: Cham, Switzerland, 2025; pp. 715–729. ISBN 978-3-031-81315-3. [Google Scholar]
- Leveraging Nanomaterials for Measurements at the Quantum Limit-ProQuest. Available online: https://www.proquest.com/openview/7fde4142cf741375e4f13c5b8dbede0a/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 22 July 2025).
- Abraheem, S.M.; Ali, M.E.; Abuali, R.M. Emerging Trends in Quantum Sensors: Applications in Defense and Communication. Middle East J. Pure Appl. Sci. (MEJPAS) 2025, 1, 19–37. [Google Scholar]
- Kaur, T.; Peace, D.; Romero, J. On-Chip High-Dimensional Entangled Photon Sources. J. Opt. 2025, 27, 023001. [Google Scholar] [CrossRef]
- Butt, M.A.; Imran Akca, B.; Mateos, X. Integrated Photonic Biosensors: Enabling Next-Generation Lab-on-a-Chip Platforms. Nanomaterials 2025, 15, 731. [Google Scholar] [CrossRef]
- Hosseini, M. Silicon Germanium BiCMOS Integrated Circuits for Scalable Cryogenic Sensing Applications. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2022. [Google Scholar]
- Sadhu, B.; Tien, K.; Chakraborty, S.; Frank, D.; Rosno, P.; Moertl, D.; Yeck, M.; Bulzacchelli, J.; Frolov, D.; Underwood, D.; et al. Cryogenic CMOS Circuits for Future Scaled Quantum Computing Systems: Challenges and Solutions. In Proceedings of the 2025 IEEE Custom Integrated Circuits Conference (CICC), Boston, MA, USA, 13–17 April 2025; pp. 1–3. [Google Scholar]
- Brennan, J.C.; Barbosa, J.; Li, C.; Ahmad, M.; Imroze, F.; Rose, C.; Karar, W.; Stanley, M.; Heidari, H.; Ridler, N.M.; et al. Classical Interfaces for Controlling Cryogenic Quantum Computing Technologies. arXiv 2025. [Google Scholar] [CrossRef]
- Elshaari, A.W.; Pernice, W.; Srinivasan, K.; Benson, O.; Zwiller, V. Hybrid Integrated Quantum Photonic Circuits. Nat. Photonics 2020, 14, 285–298. [Google Scholar] [CrossRef]
- Kim, J.-H.; Aghaeimeibodi, S.; Carolan, J.; Englund, D.; Waks, E. Hybrid Integration Methods for On-Chip Quantum Photonics. Opt. Opt. 2020, 7, 291–308. [Google Scholar] [CrossRef]
- Lange, W. Quantum Computing with Trapped Ions. In Encyclopedia of Complexity and Systems Science; Springer: New York, NY, USA, 2009; pp. 7218–7249. ISBN 978-0-387-30440-3. [Google Scholar]
- Siddiqi, I. Engineering High-Coherence Superconducting Qubits. Nat. Rev. Mater. 2021, 6, 875–891. [Google Scholar] [CrossRef]
- Burkard, G.; Ladd, T.D.; Pan, A.; Nichol, J.M.; Petta, J.R. Semiconductor Spin Qubits. Rev. Mod. Phys. 2023, 95, 025003. [Google Scholar] [CrossRef]
- Nanophotonic Integration and Engineering of Defect Qubits in Diamond-ProQuest. Available online: https://www.proquest.com/openview/546efb33185488642fc5d08a7d9e12fd/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 22 July 2025).
- Márquez Seco, A.; Takahashi, H.; Keller, M. Novel Ion Trap Design for Strong Ion-Cavity Coupling. Atoms 2016, 4, 15. [Google Scholar] [CrossRef]
- Scarlino, P.; van Woerkom, D.J.; Mendes, U.C.; Koski, J.V.; Landig, A.J.; Andersen, C.K.; Gasparinetti, S.; Reichl, C.; Wegscheider, W.; Ensslin, K.; et al. Coherent Microwave-Photon-Mediated Coupling between a Semiconductor and a Superconducting Qubit. Nat. Commun. 2019, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Maurand, R.; Jehl, X.; Kotekar-Patil, D.; Corna, A.; Bohuslavskyi, H.; Laviéville, R.; Hutin, L.; Barraud, S.; Vinet, M.; Sanquer, M.; et al. A CMOS Silicon Spin Qubit. Nat. Commun. 2016, 7, 13575. [Google Scholar] [CrossRef]
- Nitrogen-Vacancy Center. Wikipedia. 2025. Available online: https://en.wikipedia.org/wiki/Nitrogen-vacancy_center (accessed on 1 October 2025).
- Samad, M.; Shimizu, M.; Hijikata, Y. Demonstration of Quantum Polarized Microscopy Using an Entangled-Photon Source. Photonics 2025, 12, 127. [Google Scholar] [CrossRef]
- Degen, C.L.; Reinhard, F.; Cappellaro, P. Quantum Sensing. Rev. Mod. Phys. 2017, 89, 035002. [Google Scholar] [CrossRef]
- Xavier, J.; Yu, D.; Jones, C.; Zossimova, E.; Vollmer, F. Quantum Nanophotonic and Nanoplasmonic Sensing: Towards Quantum Optical Bioscience Laboratories on Chip. Nanophotonics 2021, 10, 1387–1435. [Google Scholar] [CrossRef]
- Baraeinejad, B.; Forouzesh, M.; Babaei, S.; Naghshbandi, Y.; Torabi, Y.; Fazliani, S. Design and Implementation of an IoT-Based Respiratory Motion Sensor. arXiv 2024. [Google Scholar] [CrossRef]
- Torabi, Y.; Shirani, S.; Reilly, J.P. Manikin-Recorded Cardiopulmonary Sounds Dataset Using Digital Stethoscope. arXiv 2024. [Google Scholar] [CrossRef]
- Huang, J.; Zhuang, M.; Lee, C. Entanglement-Enhanced Quantum Metrology: From Standard Quantum Limit to Heisenberg Limit. Appl. Phys. Rev. 2024, 11, 031302. [Google Scholar] [CrossRef]
- Lee, C.; Lawrie, B.; Pooser, R.; Lee, K.-G.; Rockstuhl, C.; Tame, M. Quantum Plasmonic Sensors. Chem. Rev. 2021, 121, 4743–4804. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, Mechanisms, and Application of Carbon Quantum Dots in Sensors: A Review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Schirhagl, R.; Chang, K.; Loretz, M.; Degen, C.L. Nitrogen-Vacancy Centers in Diamond: Nanoscale Sensors for Physics and Biology. Annu. Rev. Phys. Chem. 2014, 65, 83–105. [Google Scholar] [CrossRef]
- Hildebrandt, N. Biofunctional Quantum Dots: Controlled Conjugation for Multiplexed Biosensors. ACS Nano 2011, 5, 5286–5290. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials Enhanced Surface Plasmon Resonance for Biological and Chemical Sensing Applications. Chem. Soc. Rev. 2014, 43, 3426. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, M.; Omran, B.; Whitehead, K.; Baek, K.-H. Superior Properties and Biomedical Applications of Microorganism-Derived Fluorescent Quantum Dots. Molecules 2020, 25, 4486. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, Y.; Pan, J.; Zhao, Y.; Chen, Y.; Ren, X.; Ma, H.; Wei, Q.; Du, B. A Photoelectrochemical Biosensor for Fibroblast-like Synoviocyte Cell Using Visible Light-Activated NCQDs Sensitized-ZnO/CH3NH3PbI3 Heterojunction. Biosens. Bioelectron. 2016, 77, 330–338. [Google Scholar] [CrossRef]
- Roushani, M.; Ghanbari, K.; Jafar Hoseini, S. Designing an Electrochemical Aptasensor Based on Immobilization of the Aptamer onto Nanocomposite for Detection of the Streptomycin Antibiotic. Microchem. J. 2018, 141, 96–103. [Google Scholar] [CrossRef]
- Muthusankar, G.; Devi, R.K.; Gopu, G. Nitrogen-Doped Carbon Quantum Dots Embedded Co3O4 with Multiwall Carbon Nanotubes: An Efficient Probe for the Simultaneous Determination of Anticancer and Antibiotic Drugs. Biosens. Bioelectron. 2020, 150, 111947. [Google Scholar] [CrossRef] [PubMed]
- Saadati, A.; Hassanpour, S.; Bahavarnia, F.; Hasanzadeh, M. A Novel Biosensor for the Monitoring of Ovarian Cancer Tumor Protein CA 125 in Untreated Human Plasma Samples Using a Novel Nano-Ink: A New Platform for Efficient Diagnosis of Cancer Using Paper Based Microfluidic Technology. Anal. Methods 2020, 12, 1639–1649. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Garranzo-Asensio, M.; Barderas, R.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Simultaneous Amperometric Immunosensing of the Metastasis-Related Biomarkers IL-13Rα2 and CDH-17 by Using Grafted Screen-Printed Electrodes and a Composite Prepared from Quantum Dots and Carbon Nanotubes for Signal Amplification. Microchim. Acta 2019, 186, 411. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Bezinge, L.; Gliddon, H.D.; Huang, D.; Dold, G.; Gray, E.R.; Heaney, J.; Dobson, P.J.; Nastouli, E.; Morton, J.J.L.; et al. Spin-Enhanced Nanodiamond Biosensing for Ultrasensitive Diagnostics. Nature 2020, 587, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Soleyman, R.; Kohandel, M.; Cappellaro, P. SARS-CoV-2 Quantum Sensor Based on Nitrogen-Vacancy Centers in Diamond. Nano Lett. 2022, 22, 43–49. [Google Scholar] [CrossRef]
- Sharmin, R.; Hamoh, T.; Sigaeva, A.; Mzyk, A.; Damle, V.G.; Morita, A.; Vedelaar, T.; Schirhagl, R. Fluorescent Nanodiamonds for Detecting Free-Radical Generation in Real Time during Shear Stress in Human Umbilical Vein Endothelial Cells. ACS Sens. 2021, 6, 4349–4359. [Google Scholar] [CrossRef]
- Sánchez Toural, J.L.; Marzoa, V.; Bernardo-Gavito, R.; Pau, J.L.; Granados, D. Hands-On Quantum Sensing with NV− Centers in Diamonds. C 2023, 9, 16. [Google Scholar] [CrossRef]
- Torabi, Y.; Shirani, S.; Reilly, J.P.; Gauvreau, G.M. MEMS and ECM Sensor Technologies for Cardiorespiratory Sound Monitoring—A Comprehensive Review. Sensors 2024, 24, 7036. [Google Scholar] [CrossRef]
- Jang, B.; Cao, P.; Chevalier, A.; Ellington, A.; Hassibi, A. A CMOS Fluorescent-Based Biosensor Microarray. In Proceedings of the 2009 IEEE International Solid-State Circuits Conference-Digest of Technical Papers, San Francisco, CA, USA, 8–12 February 2009; pp. 436–437. [Google Scholar]
- Li, H.; Parsnejad, S.; Ashoori, E.; Thompson, C.; Purcell, E.K.; Mason, A.J. Ultracompact Microwatt CMOS Current Readout With Picoampere Noise and Kilohertz Bandwidth for Biosensor Arrays. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 35–46. [Google Scholar] [CrossRef]
- Mehdipoor, M.; Ghavifekr, H.B. A Novel Microfluidics Integrated Biosensor Based on a MEMS Resonator. Microsyst. Technol. 2020, 26, 3821–3828. [Google Scholar] [CrossRef]
- Sang, S.; Witte, H. A Novel PDMS Micro Membrane Biosensor Based on the Analysis of Surface Stress. Biosens. Bioelectron. 2010, 25, 2420–2424. [Google Scholar] [CrossRef]
- Kurmendra; Kumar, R. MEMS Based Cantilever Biosensors for Cancer Detection Using Potential Bio-Markers Present in VOCs: A Survey. Microsyst. Technol. 2019, 25, 3253–3267. [Google Scholar] [CrossRef]
- Timurdogan, E.; Alaca, B.E.; Kavakli, I.H.; Urey, H. MEMS Biosensor for Detection of Hepatitis A and C Viruses in Serum. Biosens. Bioelectron. 2011, 28, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Bharati, M.; Rana, L.; Gupta, R.; Sharma, A.; Jha, P.K.; Tomar, M. Realization of a DNA Biosensor Using Inverted Lamb Wave MEMS Resonator Based on ZnO/SiO2/Si/ZnO Membrane. Anal. Chim. Acta 2023, 1249, 340929. [Google Scholar] [CrossRef] [PubMed]
- Silicon Photonics Circuit Design: Methods, Tools and Challenges-Bogaerts-2018-Laser & Photonics Reviews-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/lpor.201700237?casa_token=-QraakcNN34AAAAA%3AATwiaMvGhO3eUQBB7Qvq2Mie-PHDmWnDgbr5f6qctFqmftYZ7s-ZJVSVX4EaG-if79n7PLXe78JlQuzE (accessed on 23 July 2025).
- Bogaerts, W.; Baets, R.; Dumon, P.; Wiaux, V.; Beckx, S.; Taillaert, D.; Luyssaert, B.; Van Campenhout, J.; Bienstman, P.; Van Thourhout, D. Nanophotonic Waveguides in Silicon-on-Insulator Fabricated with CMOS Technology. J. Light. Technol. 2005, 23, 401–412. [Google Scholar] [CrossRef]
- Juan Colás, J. Introduction to Label-Free Biosensing. In Dual-Mode Electro-photonic Silicon Biosensors; Juan Colás, J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 7–35. ISBN 978-3-319-60501-2. [Google Scholar]
- Claes, T. Advanced Silicon Photonic Ring Resonator Label-Free Biosensors. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2012. [Google Scholar]
- Heideman, R.G.; Lambeck, P.V. Remote Opto-Chemical Sensing with Extreme Sensitivity: Design, Fabrication and Performance of a Pigtailed Integrated Optical Phase-Modulated Mach–Zehnder Interferometer System. Sens. Actuators B Chem. 1999, 61, 100–127. [Google Scholar] [CrossRef]
- Vogelbacher, F.; Kothe, T.; Muellner, P.; Melnik, E.; Sagmeister, M.; Kraft, J.; Hainberger, R. Waveguide Mach-Zehnder Biosensor with Laser Diode Pumped Integrated Single-Mode Silicon Nitride Organic Hybrid Solid-State Laser. Biosens. Bioelectron. 2022, 197, 113816. [Google Scholar] [CrossRef]
- Densmore, A.; Vachon, M.; Xu, D.-X.; Janz, S.; Ma, R.; Li, Y.-H.; Lopinski, G.; Delâge, A.; Lapointe, J.; Luebbert, C.C.; et al. Silicon Photonic Wire Biosensor Array for Multiplexed Real-Time and Label-Free Molecular Detection. Opt. Lett. 2009, 34, 3598. [Google Scholar] [CrossRef]
- Crespi, A.; Gu, Y.; Ngamsom, B.; Hoekstra, H.J.W.M.; Dongre, C.; Pollnau, M.; Ramponi, R.; van den Vlekkert, H.H.; Watts, P.; Cerullo, G.; et al. Three-Dimensional Mach-Zehnder Interferometer in a Microfluidic Chip for Spatially-Resolved Label-Free Detection. Lab. Chip 2010, 10, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, A.; Mishra, R.D.; Babu, P.; Pandey, S.K.; Pal, M.K.; Kumar, M. Nanophotonic Ring Resonator Based on Slotted Hybrid Plasmonic Waveguide for Biochemical Sensing. IEEE Sens. J. 2023, 23, 5695–5702. [Google Scholar] [CrossRef]
- Singh, R.; Chack, D.; Priye, V. SNROW-Based Highly Sensitive Label-Free Surface Biosensor for Hepatitis B Detection. Appl. Opt. 2022, 61, 6510–6517. [Google Scholar] [CrossRef]
- Haron, M.H.; Berhanuddin, D.D.; Shaari, S.; Yeop Majlis, B.; Md Zain, A.R. The Design of Tunable Photonic Crystal Biosensor With the Integration of PN Phase Shifter Using PIC Design Approach. Preprints 2021. [Google Scholar] [CrossRef]
- Voronkov, G.; Zakoyan, A.; Ivanov, V.; Iraev, D.; Stepanov, I.; Yuldashev, R.; Grakhova, E.; Lyubopytov, V.; Morozov, O.; Kutluyarov, R. Design and Modeling of a Fully Integrated Microring-Based Photonic Sensing System for Liquid Refractometry. Sensors 2022, 22, 9553. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Lee, J.-S.; Scarcella, C.; Gradkowski, K.; Duperron, M.; Lu, H.; Zhao, Y.; Eason, C.; Morrissey, P.; Rensing, M.; et al. Photonic Packaging: Transforming Silicon Photonic Integrated Circuits into Photonic Devices. Appl. Sci. 2016, 6, 426. [Google Scholar] [CrossRef]
- Yang, J.; Tang, M.; Chen, S.; Liu, H. From Past to Future: On-Chip Laser Sources for Photonic Integrated Circuits. Light. Sci. Appl. 2023, 12, 16. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, X.; Lu, Y.; Zhu, S.; Ma, X.-S. Advances in Chip-Scale Quantum Photonic Technologies. Adv. Quantum Technol. 2021, 4, 2100068. [Google Scholar] [CrossRef]
- Mai, A.; Mai, C.; Steglich, P. From Lab-on-Chip to Lab-in-App: Challenges towards Silicon Photonic Biosensors Product Developments. Results Opt. 2022, 9, 100317. [Google Scholar] [CrossRef]
- Li, R.; Liu, F.; Lu, Q. Quantum Light Source Based on Semiconductor Quantum Dots: A Review. Photonics 2023, 10, 639. [Google Scholar] [CrossRef]
- Silverstone, J.W.; Bonneau, D.; Ohira, K.; Suzuki, N.; Yoshida, H.; Iizuka, N.; Ezaki, M.; Natarajan, C.M.; Tanner, M.G.; Hadfield, R.H.; et al. On-Chip Quantum Interference between Silicon Photon-Pair Sources. Nat. Photon 2014, 8, 104–108. [Google Scholar] [CrossRef]
- Ezawa, M. Electric Circuits for Universal Quantum Gates and Quantum Fourier Transformation. Phys. Rev. Res. 2020, 2, 023278. [Google Scholar] [CrossRef]
- Grottke, T.; Hartmann, W.; Schuck, C.; Pernice, W.H.P. Optoelectromechanical Phase Shifter with Low Insertion Loss and a 13π Tuning Range. Opt. Express 2021, 29, 5525–5537. [Google Scholar] [CrossRef]
- Li, Z.; Jin, X.; Yuan, C.; Wang, K. Photon Detector Technology for Laser Ranging: A Review of Recent Developments. Coatings 2025, 15, 798. [Google Scholar] [CrossRef]
- Dao, T.H.; Amanti, F.; Andrini, G.; Armani, F.; Barbato, F.; Bellani, V.; Bonaiuto, V.; Cammarata, S.; Campostrini, M.; Cornia, S.; et al. Single-Photon Detectors for Quantum Integrated Photonics. Photonics 2025, 12, 8. [Google Scholar] [CrossRef]
- Na, N.; Lu, Y.-C.; Liu, Y.-H.; Chen, P.-W.; Lai, Y.-C.; Lin, Y.-R.; Lin, C.-C.; Shia, T.; Cheng, C.-H.; Chen, S.-L. Room Temperature Operation of Germanium–Silicon Single-Photon Avalanche Diode. Nature 2024, 627, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Holzman, I.; Ivry, Y. Superconducting Nanowires for Single-Photon Detection: Progress, Challenges, and Opportunities. Adv. Quantum Technol. 2019, 2, 1800058. [Google Scholar] [CrossRef]
- Hao, H.; Zhao, Q.-Y.; Huang, Y.-H.; Deng, J.; Yang, F.; Ru, S.-Y.; Liu, Z.; Wan, C.; Liu, H.; Li, Z.-J.; et al. A Compact Multi-Pixel Superconducting Nanowire Single-Photon Detector Array Supporting Gigabit Space-to-Ground Communications. Light. Sci. Appl. 2024, 13, 25. [Google Scholar] [CrossRef]
- Stosch, J.H.; Kühler, T.; Griese, E. Optical Directional Coupler for Graded Index Waveguides in Thin Glass Sheets for PCB Integration. In Proceedings of the 2016 IEEE 20th Workshop on Signal and Power Integrity (SPI), Turin, Italy, 8–11 May 2016; pp. 1–4. [Google Scholar]
- Righini, G.C.; Liñares, J. Active and Quantum Integrated Photonic Elements by Ion Exchange in Glass. Appl. Sci. 2021, 11, 5222. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Derkach, I.; Hajomer, A.A.E.; Chin, H.-M.; Oruganti, A.N.; Andersen, U.L.; Usenko, V.; Gehring, T. Digital Reconstruction of Squeezed Light for Quantum Information Processing. npj Quantum Inf. 2025, 11, 71. [Google Scholar] [CrossRef]
- Oveisi, M.; Hosseinisangchi, S.; Heydari, P. A Study of Out-of-Band Emission in Digital Transmitters Due to PLL Phase Noise, Circuit Non-Linearity, and Bandwidth Limitation. IEEE Open J. Circuits Syst. 2023, 4, 283–294. [Google Scholar] [CrossRef]
- Hosseinisangchi, S. A Wide-Band Frequency Domain Near Infrared Spectroscopy System on Chip. Master’s Thesis, University of California, Irvine, CA, USA, 2024. [Google Scholar]
- Fünning, T.; Peczek, A.; Kroh, A.; Mai, C.; Paul, M.; Thomsen, F.; Tannenberg, R.; Schumann, C.; Weller, M.G.; Mai, A.; et al. Optimization of Local Backside Released Micro-Ring Resonators for Sensing Applications Using Silicon Photonic Integrated Circuits in a SOI Technology. In Proceedings of the Optical Sensors 2025, SPIE, Prague, Czech Republic, 23 May 2025; Volume 13527, pp. 183–190. [Google Scholar]



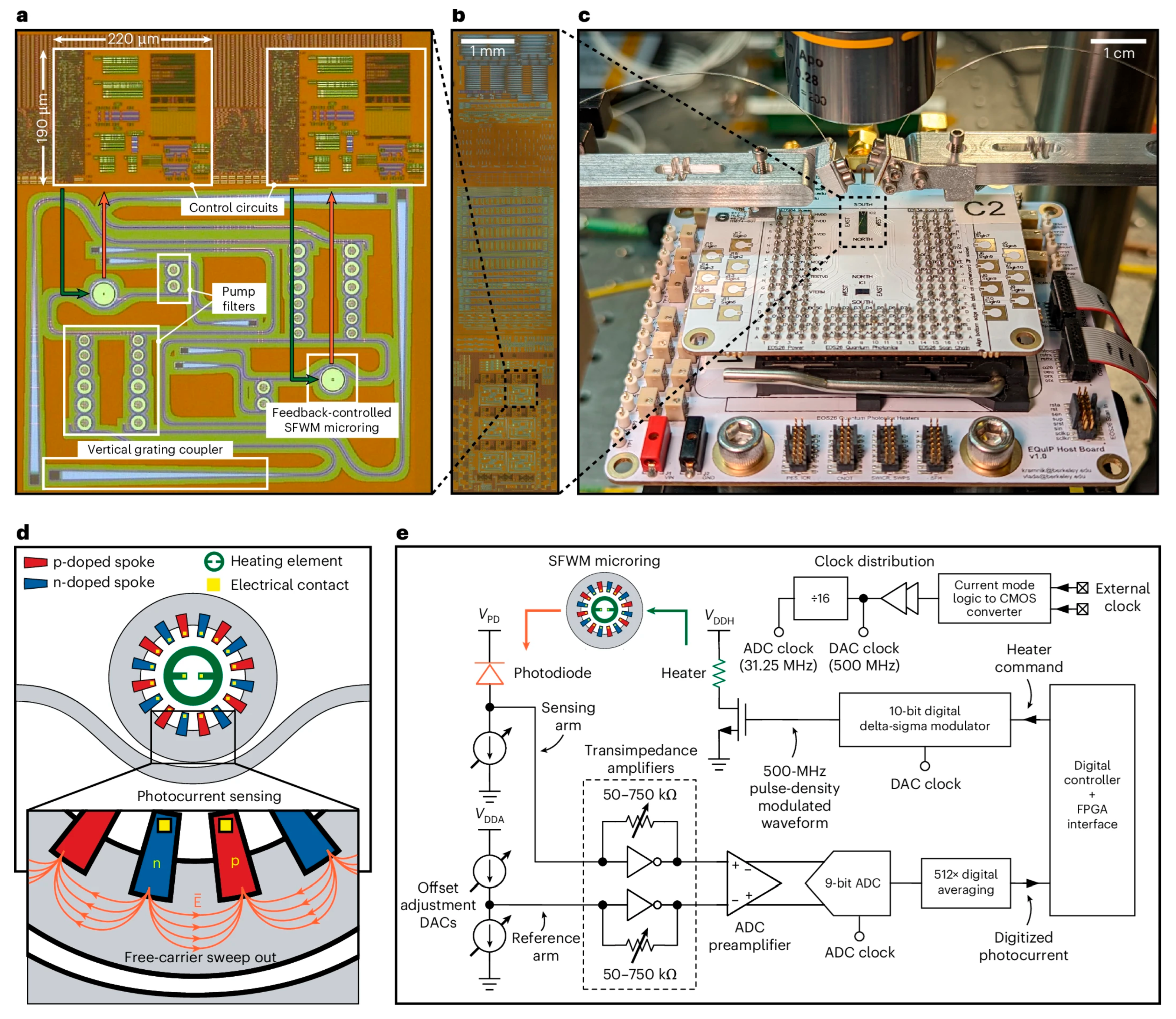
| Working Principle | Example | Temperature | Control Method | Latency | Pros | Cons |
|---|---|---|---|---|---|---|
| Superposition | Trapped Ions [71] | 10–100 mK | Laser pulses, RF fields | 10–500 µs | Precise control, High-fidelity entanglement | Bulky optics, Slow |
| Coherent Charge States | Superconducting Circuits [72] | 10–20 mK | Microwave pulses | 10–300 ns | Fast switching, Supports multi-qubit arrays | Low-temp, microwave crosstalk in dense arrays |
| Spin-Based | Silicon Spin Qubits [73] | 50 mK–1 K | GHz pulses | 1–10 µs | Compact, CMOS process fabrication | Low-temp, Noise-sensitive |
| Defect-Based Qubits | NV Centers [74] | 300–700 K | Optical and microwave drive | 1–500 µs | Room-temp | Weak signals, Hard on-chip integration |
| Quantum Photonic | Entangled Photons [70] | 273–300 K | Electro-optic/thermal tuning | 1–10 ns | Room-temp, Fast data rates | Complex setup, Optical loss |
| Criterion | Plasmonic | Quantum Dot (QD) | Nitrogen-Vacancy (NV) Center |
|---|---|---|---|
| Description | Uses metal nanoparticles to detect molecular interactions via shifts in light absorption or scattering. | Semiconductor nanocrystals that emit fluorescence when excited, with wavelength tunable by their size. | Defects in diamond that detect small magnetic or thermal changes through optical signals. |
| Biosensing Applications | Molecular binding, drug development. | Disease detection, biomarker sensing, bioimaging. | Magnetometry, viral detection, gene sensing, molecular tagging. |
| Sensitivity | Detects refractive index changes with high sensitivity. | Bright, tunable emission supports. | Spin-state readout enables single-molecule detection. |
| Limit of Detection (LOD ↓) | ~1 fM–pM | ~1 pM–nM | ~1 aM–fM |
| Multiplexing | Enabled by engineering distinct plasmonic structures with different resonance frequencies. | Easily achieved through emission tuning across QDs. | Achieved using NVs with varied spin states or spatial encoding. |
| Limitations | Background interference and non-specific binding; requires precise surface engineering. | Fluorescence blinking, toxicity concerns, and spectral instability. | Low photon collection efficiency, need for complex optical setups. |
| Parameter | Value |
|---|---|
| Nanoparticle material | Gold (Au) |
| Nanoparticle diameter | 38.5 ± 4.6 nm |
| Interparticle Gap Distance (S) | 0.5–2.8 nm (quantum regime) |
| DNA Linker Length | 40 nucleotide/80 base pair |
| DNA conductivity | σ0 = 7.8 S/m (for S < 3 nm) |
| Type | Method | Application | Specification | Working Principle | Ref |
|---|---|---|---|---|---|
| Quantum Dot (QD) Biosensors | GQD electrochemical aptasensor | Streptomycin antibiotics detections | LOD: 0.0033 pg/mL; Linear range: 0.01–812.21 pg/mL | QDs increase electrode conductivity and active area, enabling sensitive detection via electron transfer changes upon target binding. | [92] |
| NCQD, electrochemical sensor | Anticancer (flutamide) drugs detection in urine | LOD: 0.0169 μM; Linear range: 0.05–590 μM | NCQDs enhance electron transfer and surface area; quantum confinement in CQDs improves electrocatalytic sensing on the modified electrode | [93] | |
| GQD nano-ink immunosensor (Paper-based microfluidic) | Ovarian cancer (Carcinoma Antigen 125) biomarker detection in plasma | LLOQ: 0.001 U/mL; Linear range: 0.001–400 U/mL | Antibody-modified GQD nano-ink enables electrochemical detection via changes in current from antigen–antibody binding | [94] | |
| GQD electrochemical immunosensor (screen-printed electrodes) | Cancer metastasis biomarkers | LOD: 1.4 ng/mL (IL-13Rα2), 0.03 ng/mL (CDH-17) | GQDs enhance signal via peroxidase-like activity; hybrid nanocarriers amplify current from sandwich immunoassay | [95] | |
| NCQD perovskite Photoelectrochemical | FLS cells detection (Rheumatoid arthritis) | Linear range: 1 × 104–10 cells/mL; LOD: 2 cells/mL | QDs and perovskite enhance light absorption and charge separation, boosting photocurrent for ultrasensitive detection | [91] | |
| S-SiQD fluorescence probe | ClO− ion detection, imaging in cells and zebrafish | LOD: 13 nM; Linear range: 0.05–1.8 μM; Quantum yield: 38.5% | Surface sulfhydryl groups enable selective and rapid fluorescence quenching by ClO− | [14] | |
| NV-center Diamond Biosensors | NV-center diamond quantum magnetometer | High-sensitivity magnetic field detection | Sensitivity: 1 nT/√Hz | Optically initializes and reads NV spin states; pulsed protocol filters noise for ultrasensitive magnetometry (biomedical and chip-scale) | [15] |
| Spin-enhanced NV-center nanodiamond immunosensor | HIV-1 RNA detection (model biotin–avidin) | LOD: 8.2 × 10−19 M; ~100,000× more sensitive than gold-nanoparticles | Microwave-modulated NV center fluorescence separates signal from background, enabling record-low detection limits | [96] | |
| NV center nanodiamond quantum sensor | SARS-CoV-2 RNA detection | LOD: a few hundred RNA copies; <1% false negatives | NV centers in nanodiamonds detect magnetic noise from CRISPR-generated magnetic tags on RNA; optically read out | [97] | |
| NV center fluorescent nanodiamond relaxometry | Detection of free radicals (oxidative stress) in living cells | Single-cell resolution; real-time detection | NV center spin relaxation changes with local magnetic noise from free radicals. | [98] |
| Technology | Electronic Integrated Circuit (EIC) | Photonic Integrated Circuit (PIC) |
| Advantage | Compact, scalable, cost-effective, readily available | High speed, low power consumption, immunity to electromagnetic interference, support high levels of multiplexing. |
| Limitation | Susceptible to electrical noise, electrode drift, and electromagnetic interference, speed limitation. | Complex fabrication, need precise optical alignment, technology is still evolving. |
| Specifications | SNR: 15–30 dB Multiplexing: up to 100 channels Footprint: ~0.01–10 mm2 | Signal-to-Noise Ratio (SNR): 30–50 dB Multiplexing: 50–1000 channels Footprint: ~1–100 mm2 |
| Application | Glucose biosensors, protein assays, cardiac sensors | Nucleic acid detection, protein biomarker panels, virus assays |
| Type | Method | Working Principle | Specification | Application | Ref |
|---|---|---|---|---|---|
| CMOS | Bio-impedance IC | Impedance-based voltage/current sensing | Supply: 0.5 V; Power: <10 μW; Noise: 15.28 mΩ/√Hz; Phase error: <1° | Cardiovascular disease | [4] |
| Capacitive CMOS | Capacitance change due to biomolecular binding | Power: 2.1 μW; Capacitance range: 16.137 pF; Resolution: 4.5 fF | Cancer enzyme biomarker (oncology) | [5] | |
| CMOS fluorescence microarray | Fluorescent emission, photodiode readout | Excitation: 532 nm; Dark current: ~12 fA; ADC resolution: 14-bit; Array size: 16 × 16 | Genomics, DNA hybridization (bioassay platforms) | [101] | |
| CMOS potentiostat with carbon nanofiber | Amperometric current changes from glucose oxidation | Power: 71.7 μW; Sensitivity: 50–200 nA/mM; Electrode area: 0.09 mm2; Detection range: 0.5–7 μA | Diabetes, glucose | [6] | |
| CMOS picoamp current readout | Low-noise current readout for electrochemical biosensor arrays | Noise: 7.2 pA_rms; Power: 21 μW/channel; Area: 0.06 mm2/channel; Bandwidth: 11.5 kHz | Multiplexed biosensor readout, DNA sequencing | [102] | |
| MEMS | MEMS resonator + microfluidics | Resonant frequency shift due to particle mass | Frequency: 16.5 kHz; Displacement: 1.44 μm; Q-factor: 49; Sensitivity: 1 × 1011 Hz/kg | Digital microfluidics, droplet (lab-on-chip) | [103] |
| PDMS membrane | Membrane deflection from surface stress, interferometric readout | Membrane: 2.5 × 2.5 mm; Thickness: 35 μm; Young’s modulus: 12 kPa–2.5 MPa; Sensitivity: 0.56 × 10−5 N/m | E. coli (microbiology, pathogen screening) | [104] | |
| MEMS cantilever | Adsorption-induced stress, piezoresistive readout | LOD: 1 ppb (VOC); Cantilever: 150 × 500 μm; Resonant freq: ~12 kHz; Response time: <10 s | Cancer (lung, breast, prostate) | [105] | |
| MEMS cantilever | Resonant frequency shift of nickel cantilever | LOD: 0.1 ng/mL; Dynamic range: >1000×; Array: up to 16 cantilevers; Detection time: ~20 s | Hepatitis A/C virus, serum analysis | [106] | |
| MEMS ZnO Lamb wave resonator | Piezoelectric Lamb wave, frequency shift by DNA mass | Sensitivity: 310 Hz/ng/μL (DNA); LOD: 82 pg/μL; Frequency: 137 MHz; Membrane: 4.5 × 5.9 mm | DNA biosensing (meningitis pathogen) | [107] |
| Type | Method | Working Principle | Specification | Application | Ref |
|---|---|---|---|---|---|
| Mach–Zehnder Interferometer (MZI) | Si3N4 MZI + integrated laser source | Optical phase shift between split paths caused by binding events | Sensitivity: 6.8 × 10−6 RIU; LOD: ~1 ng/mL; Footprint: 3.5 × 0.6 mm2 | Streptavidin–biotin sensing | [113] |
| Spiral photonic wire MZI in SOI | Compact spiral MZI maximizes evanescent field–analyte interaction | LOD: 0.25 pg/mm2; Waveguide: 1.8 mm spiral; Channel width: 190 µm | Antibody–antigen detection | [114] | |
| 3D femtosecond-laser-written MZI | Vertical sensing arm intersects fluidic channel orthogonally | LOD: 1 × 10−4 RIU; Spatial resolution: 10 µm; Channel width: 150 µm | Resolved label-free sensing | [115] | |
| Si3N4 rib nanodevice MZI | Evanescent field senses refractive index shifts | LOD: 7 × 10−6 RIU; Sensor length: 15 mm; Core thickness: 250 nm | Water pollutants detection | [8] | |
| Ring Resonator | Subwavelength grating micro-ring | Antigen–antibody binding alters resonance in the ring | LOD: 1.31 fM; Detection time: 15 min; Q-factor: ~30,000 | SARS-CoV-2 and influenza | [9] |
| SiN ring resonator array with photonic packaging | Parallel resonance shift in functionalized rings | Q-factor: >4 × 104; ER: >20 dB; FSR: 2.54 nm | Respiratory antibody profiling | [10] | |
| Slotted plasmonic ring resonator | Enhanced field in slot increases sensitivity to refractive index | Sensitivity: 1609 nm/RIU; Spectral shift: 29.6 nm (ΔRI = 0.0184); Slot width: 10 nm | Water pollutants detection | [116] | |
| Rectangular semi-ring optical waveguide biosensor | Refractive index changes from biomolecular interaction shifts | LOD: 0.12 ng/mL; Linear range: 0.1–100 ng/mL; Sensor width: 800 nm | Hepatitis B virus | [117] | |
| Photonic crystal ring + PN phase shifter | Phase-tunable ring enables reconfigurable resonance sensing | Tuning range: 1.3–1.7 µm; Ring radius: 6 µm; Phase shift: π | General biomedical sensing | [118] | |
| Racetrack micro-ring resonator with dual ring readout | Refractive index changes shift resonance; monitored by intensity changes via secondary ring | Sensitivity: 110 nm/RIU; Delay: 10 ns at 100 MHz; Fabrication tolerance: ±8 nm | Liquid refractometry | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torabi, Y.; Shirani, S.; Reilly, J.P. Quantum Biosensors on Chip: A Review from Electronic and Photonic Integrated Circuits to Future Integrated Quantum Photonic Circuits. Microelectronics 2025, 1, 5. https://doi.org/10.3390/microelectronics1020005
Torabi Y, Shirani S, Reilly JP. Quantum Biosensors on Chip: A Review from Electronic and Photonic Integrated Circuits to Future Integrated Quantum Photonic Circuits. Microelectronics. 2025; 1(2):5. https://doi.org/10.3390/microelectronics1020005
Chicago/Turabian StyleTorabi, Yasaman, Shahram Shirani, and James P. Reilly. 2025. "Quantum Biosensors on Chip: A Review from Electronic and Photonic Integrated Circuits to Future Integrated Quantum Photonic Circuits" Microelectronics 1, no. 2: 5. https://doi.org/10.3390/microelectronics1020005
APA StyleTorabi, Y., Shirani, S., & Reilly, J. P. (2025). Quantum Biosensors on Chip: A Review from Electronic and Photonic Integrated Circuits to Future Integrated Quantum Photonic Circuits. Microelectronics, 1(2), 5. https://doi.org/10.3390/microelectronics1020005






