Nasal Inflammation and Brain Bioenergetics: Does Chronic Rhinosinusitis Accelerate Neurodegeneration?
Abstract
1. Introduction
2. Methods
Study Selection and Data Extraction
3. Results
3.1. Association Between CRS and Neurodegeneration
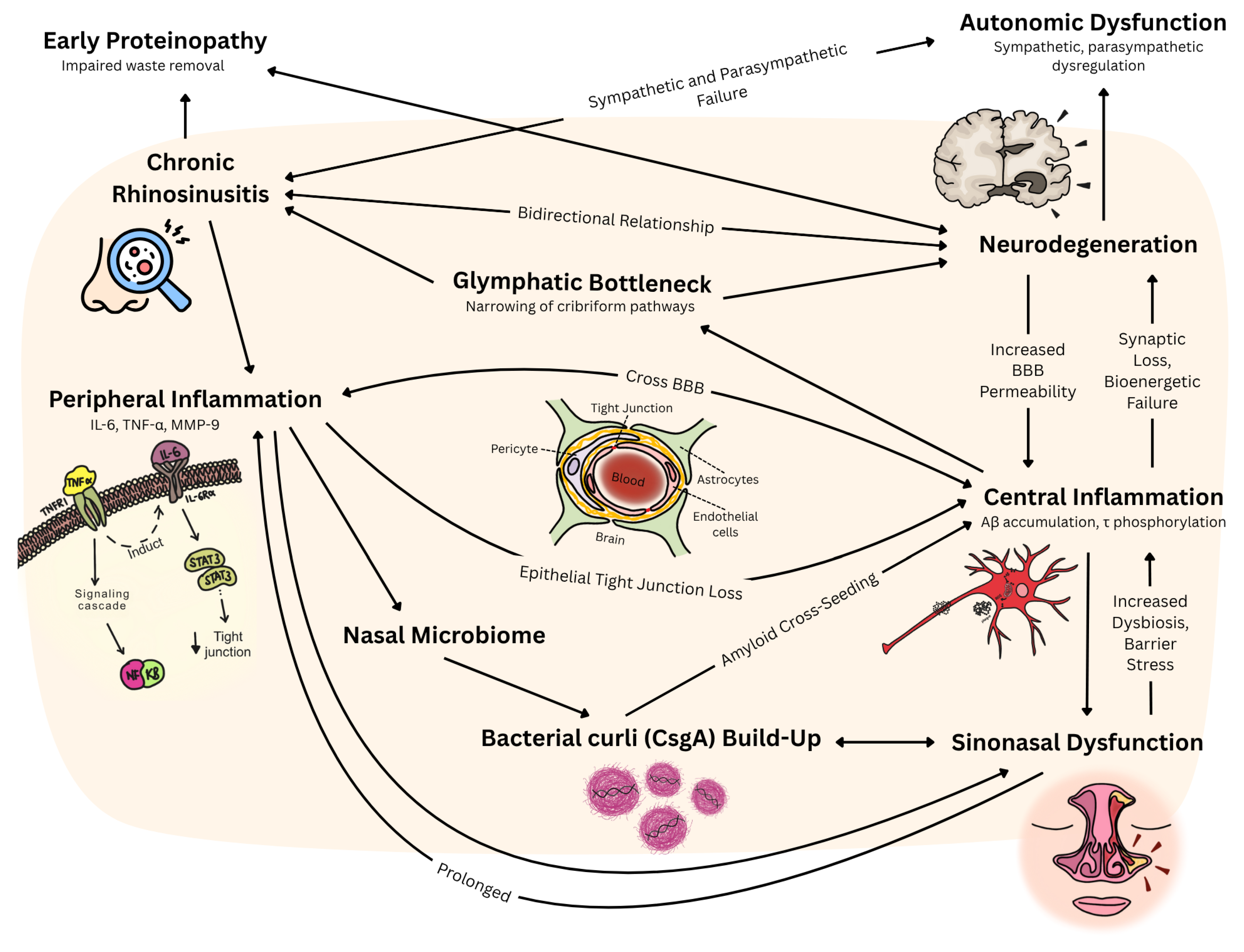
3.2. Inflammatory Mechanism Between CRS and Neurodegeneration
4. Discussion
4.1. Clinical Significance
4.2. Current Evidence Base and Its Limitations
4.3. Inflammatory Cascade: Cytokine-Mediated Blood–Brain Barrier Disruption
4.3.1. CRS-Driven Neuroinflammation
4.3.2. Neurodegeneration-Induced Sinonasal Dysfunction
4.4. Molecular Mechanisms: Kinase Signaling and Post-Translational Modifications
4.4.1. Bidirectional Molecular Pathways and Neuroinflammation
4.4.2. The Role of Non-Coding RNA in CRS and Neurodegeneration
4.4.3. Bidirectional Neuro-Sinonasal Network Dysfunction
4.5. Microbiome-Mediated Cross-Seeding: Bacterial Amyloid as Pathological Bridge
4.5.1. Bacterial Amyloid Cross-Seeding Mechanisms
4.5.2. Pathogenic Microbiome Alterations in Neurodegeneration
4.6. Olfactory–Limbic Axis: Proteinopathy Initiation and Clearance Dysfunction
4.6.1. Early Proteinopathy in Olfactory Structures
4.6.2. Compromised Cerebrospinal Fluid Clearance Mechanisms
4.7. Therapeutic Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Search Parameters | Specifications |
|---|---|
| Date of search | 8 July 2025 |
| Databases searched | PubMed, Cochrane library, Web of Science, Embase, CENTRAL (Cochrane Central register of controlled trials) |
| Search terms utilized | “Chronic Rhinosinusitis”, “Rhinosinusitis”, “Sinonasal dysfunction”, “Neurodegeneration”, “Mild Cognitive Impairment”, “Alzheimer’s Disease”, “Parkinson’s Disease”, “Dementia”, “Bidirectional pathway”, “Biomarkers”, “Inflammatory markers”, “Bioenergetics”, “Therapeutic targets”, “Management pathways”, “Mitochondrial dysfunction”, “Autonomic dysfunction”, “Microbiome”, “Dysbiosis”, “Research gaps” |
| Timeframe | January 2000 to July 2025 |
| Inclusion criteria | English studies investigating the CRS–neurodegeneration pathway |
| Exclusion criteria | Editorials, opinion pieces, conference abstracts |
References
- Min, H.K.; Lee, S.; Kim, S.; Son, Y.; Park, J.; Kim, H.J.; Lee, J.; Lee, H.; Smith, L.; Rahmati, M.; et al. Global Incidence and Prevalence of Chronic Rhinosinusitis: A Systematic Review. Clin. Exp. Allergy 2024, 55, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.J.; Leow, Y.J.; Vipin, A.; Sandhu, G.K.; Kandiah, N. Associations Between GFAP, Aβ42/40 Ratio, and Perivascular Spaces and Cognitive Domains in Vascular Cognitive Impairment. Int. J. Mol. Sci. 2025, 26, 3541. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.J.; Leow, Y.J.; Vipin, A.; Kumar, D.; Kandiah, N. Impact of White Matter Hyperintensities on domain-specific cognition in Southeast Asians. Alzheimer’s Dement. 2023, 19, e082267. [Google Scholar] [CrossRef]
- Wang, J.D.J.; Chan, C.K.M.; Chua, W.Y.; Chao, Y.; Chan, L.; Tan, E.K. A Systemic Review and Meta-Analysis of the Risk of Venous Thromboembolic Events in Parkinson’s Patients. Eur. J. Neurol. 2025, 32, e70047. [Google Scholar] [CrossRef]
- Chua, W.Y.; Wang, J.D.J.; Chan, C.K.M.; Chan, L.; Tan, E.K. Risk of aspiration pneumonia and hospital mortality in Parkinson disease: A systematic review and meta-analysis. Eur. J. Neurol. 2024, 31, e16449. [Google Scholar] [CrossRef]
- Yang, B.; Gu, M.; Hong, C.; Zou, X.-Y.; Zhang, J.-Q.; Yuan, Y.; Qiu, C.-Y.; Lu, M.-P.; Cheng, L. Integrated machine learning and bioinformatic analysis of mitochondrial-related signature in chronic rhinosinusitis with nasal polyps. World Allergy Organ. J. 2024, 17, 100964. [Google Scholar] [CrossRef]
- Kathiresan, D.S.; Balasubramani, R.; Marudhachalam, K.; Jaiswal, P.; Ramesh, N.; Sureshbabu, S.G.; Puthamohan, V.M.; Vijayan, M. Role of Mitochondrial Dysfunctions in Neurodegenerative Disorders: Advances in Mitochondrial Biology. Mol. Neurobiol. 2025, 62, 6827–6855. [Google Scholar] [CrossRef]
- Dan, X.; Wechter, N.; Gray, S.; Mohanty, J.G.; Croteau, D.L.; Bohr, V.A. Olfactory dysfunction in aging and neurodegenerative diseases. Ageing Res. Rev. 2021, 70, 101416. [Google Scholar] [CrossRef]
- Di Francesco, V.; Chua, A.J.; Bleier, B.S.; Amiji, M.M. Effective Nose-to-Brain Delivery of Blood-Brain Barrier Impermeant Anti-IL-1β Antibody via the Minimally Invasive Nasal Depot (MIND) Technique. ACS Appl. Mater. Interfaces 2024, 16, 69103–69113. [Google Scholar] [CrossRef]
- Song, H.; Zou, J.; Sun, Z.; Pu, Y.; Qi, W.; Sun, L.; Li, Q.; Yuan, C.; Wang, X.; Gao, X.; et al. Nasal microbiome in relation to olfactory dysfunction and cognitive decline in older adults. Transl. Psychiatry 2025, 15, 122. [Google Scholar] [CrossRef]
- Gao, E.Y.; Tan, B.K.J.; Chan, K.L.; Chia, C.X.Y.; Tan, J.-W.; Yeo, B.S.Y. Chronic rhinosinusitis and cognition: A systematic review and meta-analysis. Rhinol. J. 2025, 63, 514–522. [Google Scholar] [CrossRef]
- Son, D.-S.; Kim, J.-I.; Kim, D.-K. A Longitudinal Study Investigating Whether Chronic Rhinosinusitis Influences the Subsequent Risk of Developing Dementia. J. Pers. Med. 2024, 14, 1081. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Nie, M.; Wang, B.; Duan, S.; Huang, Q.; Wu, N.; Chen, Z.; Zhao, H.; Han, Y. Intrinsic brain abnormalities in chronic rhinosinusitis associated with mood and cognitive function. Front. Neurosci. 2023, 17, 1131114. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Edwards, T.S.; Hinson, V.K.; Soler, Z.M. Prevalence of Rhinorrhea in Parkinson Disease. Neurol. Clin. Pract. 2022, 12, e75–e81. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Xavier, L.d.L.; Bernstein, J.D.; Simonyan, K.; Bleier, B.S. Association of Sinonasal Inflammation with Functional Brain Connectivity. JAMA Otolaryngol. Neck Surg. 2021, 147, 534–543. [Google Scholar] [CrossRef]
- Jung, H.-J.; Lee, J.-Y.; Choi, Y.-S.; Choi, H.-G.; Wee, J.-H. Chronic rhinosinusitis and progression of cognitive impairment in dementia. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 147–151. [Google Scholar] [CrossRef]
- Wee, J.H.; Yoo, D.M.; Byun, S.H.; Hong, S.J.; Park, M.W.; Choi, H.G. Association between neurodegenerative dementia and chronic rhinosinusitis: A nested case-control study using a national health screening cohort. Medicine 2020, 99, e22141. [Google Scholar] [CrossRef]
- Rowan, N.R.; Schlosser, R.J.; Storck, K.A.; Ganjaei, K.G.; Soler, Z.M. The impact of medical therapy on cognitive dysfunction in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 738–745. [Google Scholar] [CrossRef]
- Yoo, F.; Schlosser, R.J.; Storck, K.A.; Ganjaei, K.G.; Rowan, N.R.; Soler, Z.M. Effects of endoscopic sinus surgery on objective and subjective measures of cognitive dysfunction in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 1135–1143. [Google Scholar] [CrossRef]
- Domellöf, M.E.; Lundin, K.-F.; Edström, M.; Forsgren, L. Olfactory dysfunction and dementia in newly diagnosed patients with Parkinson’s disease. Park. Relat. Disord. 2017, 38, 41–47. [Google Scholar] [CrossRef]
- Hauser, L.J.; Chandra, R.K.; Li, P.; Turner, J.H. Role of tissue eosinophils in chronic rhinosinusitis–associated olfactory loss. Int. Forum Allergy Rhinol. 2017, 7, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Alt, J.A.; Mace, J.C.; Smith, T.L.; Soler, Z.M. Endoscopic Sinus Surgery Improves Cognitive Dysfunction in Patients with Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Kikuchi, A.; Hirayama, K.; Nishio, Y.; Hosokai, Y.; Kanno, S.; Hasegawa, T.; Sugeno, N.; Konno, M.; Suzuki, K.; et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: A 3 year longitudinal study. Brain J. Neurol. 2012, 135, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.L.; Koeppe, R.A.; Bohnen, N.I. Rhinorrhea: A common nondopaminergic feature of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 320–323. [Google Scholar] [CrossRef]
- Perić, A.; Vojvodić, D.; Radulović, V.; Vukomanović-Đurđević, B.; Miljanović, O. Correlation between cytokine levels in nasal fluid and eosinophil counts in nasal polyp tissue in asthmatic and non-asthmatic patients. Allergol. Immunopathol. 2011, 39, 133–139. [Google Scholar] [CrossRef]
- Huang, W.-H.; Hung, Y.-W.; Hung, W.; Lan, M.-Y.; Yeh, C.-F. Murine model of eosinophilic chronic rhinosinusitis with nasal polyposis inducing neuroinflammation and olfactory dysfunction. J. Allergy Clin. Immunol. 2024, 154, 325–339.e3. [Google Scholar] [CrossRef]
- Pal, G.; Ramirez, V.; Engen, P.A.; Naqib, A.; Forsyth, C.B.; Green, S.J.; Mahdavinia, M.; Batra, P.S.; Tajudeen, B.A.; Keshavarzian, A. Deep nasal sinus cavity microbiota dysbiosis in Parkinson’s disease. npj Park. Dis. 2021, 7, 111. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Yeon, S.H.; Choi, M.R.; Jang, Y.S.; Kim, J.A.; Oh, H.W.; Jun, X.; Park, S.K.; Heo, J.Y.; Rha, K.-S.; et al. Altered Mitochondrial Functions and Morphologies in Epithelial Cells Are Associated with Pathogenesis of Chronic Rhinosinusitis with Nasal Polyps. Allergy Asthma Immunol. Res. 2020, 12, 653–668. [Google Scholar] [CrossRef]
- Alt, J.A.; Sautter, N.B.; Mace, J.C.; Detwiller, K.Y.; Smith, T.L. Antisomnogenic cytokines, quality of life, and chronic rhinosinusitis: A pilot study. Laryngoscope 2014, 124, E107–E114. [Google Scholar] [CrossRef]
- Lane, A.P.; Turner, J.; May, L.; Reed, R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J. Neurosci. 2010, 30, 2324–2329. [Google Scholar] [CrossRef]
- Wang, J.D.J.; Chua, N.Y.M.; Chan, L.-L.; Tan, E.-K. Obstructive Sleep Apnea and Parkinson’s Disease: Bidirectional Clinical and Pathophysiologic Links. Int. J. Mol. Sci. 2025, 26, 3762. [Google Scholar] [CrossRef]
- D’aNdrea, F.; Tischler, V.; Dening, T.; Churchill, A. Olfactory stimulation for people with dementia: A rapid review. Dementia 2022, 21, 1800–1824. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Khan, T.; Reyes, D. A Prospective Study: Clinical Significance of Anticholinergic Nasal Sprays in Patients with Parkinson Disease Afflicted by Rhinorrhea (P04.149). Neurology 2013, 80, P04.149. [Google Scholar] [CrossRef]
- Mannion, J.M.; Segal, B.M.; McLoughlin, R.M.; Lalor, S.J. Respiratory tract Moraxella catarrhalis and Klebsiella pneumoniae can promote pathogenicity of myelin-reactive Th17 cells. Mucosal Immunol. 2023, 16, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bongianni, M.; Catalan, M.; Perra, D.; Fontana, E.; Janes, F.; Bertolotti, C.; Sacchetto, L.; Capaldi, S.; Tagliapietra, M.; Polverino, P.; et al. Olfactory swab sampling optimization for α-synuclein aggregate detection in patients with Parkinson’s disease. Transl. Neurodegener. 2022, 11, 37. [Google Scholar] [CrossRef]
- Kuzkina, A.; Rößle, J.; Seger, A.; Panzer, C.; Kohl, A.; Maltese, V.; Musacchio, T.; Blaschke, S.J.; Tamgüney, G.; Kaulitz, S.; et al. Combining skin and olfactory α-synuclein seed amplification assays (SAA)—Towards biomarker-driven phenotyping in synucleinopathies. npj Park. Dis. 2023, 9, 79. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.-M.; Cho, S.; Kang, J.-H.; Minn, Y.-K.; Park, H.; Choi, S.H. Amyloid beta in nasal secretions may be a potential biomarker of Alzheimer’s disease. Sci. Rep. 2019, 9, 4966. [Google Scholar] [CrossRef]
- Yoo, S.-J.; Son, G.; Bae, J.; Kim, S.Y.; Yoo, Y.K.; Park, D.; Baek, S.Y.; Chang, K.-A.; Suh, Y.-H.; Lee, Y.-B.; et al. Longitudinal profiling of oligomeric Aβ in human nasal discharge reflecting cognitive decline in probable Alzheimer’s disease. Sci. Rep. 2020, 10, 11234. [Google Scholar] [CrossRef]
- Kato, A.; Peters, A.T.; Stevens, W.W.; Schleimer, R.P.; Tan, B.K.; Kern, R.C. Endotypes of chronic rhinosinusitis: Relationships to disease phenotypes, pathogenesis, clinical findings, and treatment approaches. Allergy 2022, 77, 812–826. [Google Scholar] [CrossRef]
- Readhead, B.; Haure-Mirande, J.-V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron 2018, 99, 64–82.e7. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Collins, L.E.; McLoughlin, A.; Cummins, P.M. Tumour necrosis factor-α-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory interleukin-6. J. Neurochem. 2016, 136, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Kotan, D.; Tatar, A.; Aygul, R.; Ulvi, H. Assessment of nasal parameters in determination of olfactory dysfunction in Parkinson’s disease. J. Int. Med. Res. 2013, 41, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood–brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef] [PubMed]
- de Leon, M.J.; Li, Y.; Okamura, N.; Tsui, W.H.; Saint-Louis, L.A.; Glodzik, L.; Osorio, R.S.; Fortea, J.; Butler, T.; Pirraglia, E.; et al. Cerebrospinal Fluid Clearance in Alzheimer Disease Measured with Dynamic PET. J. Nucl. Med. 2017, 58, 1471–1476. [Google Scholar] [CrossRef]
- Bachert, C.; Hicks, A.; Gane, S.; Peters, A.T.; Gevaert, P.; Nash, S.; Horowitz, J.E.; Sacks, H.; Jacob-Nara, J.A. The interleukin-4/interleukin-13 pathway in type 2 inflammation in chronic rhinosinusitis with nasal polyps. Front. Immunol. 2024, 1356298. [Google Scholar] [CrossRef]
- Victores, A.J.; Chen, M.; Smith, A.; Lane, A.P. Olfactory loss in chronic rhinosinusitis is associated with neuronal activation of c-Jun N-terminal kinase. Int. Forum Allergy Rhinol. 2017, 8, 415–420. [Google Scholar] [CrossRef]
- Leon, M.; Troscianko, E.T.; Woo, C.C. Inflammation and olfactory loss are associated with at least 139 medical conditions. Front. Mol. Neurosci. 2024, 17, 1455418. [Google Scholar] [CrossRef]
- Nemec, C.M.; Singh, A.K.; Ali, A.; Tseng, S.C.; Syal, K.; Ringelberg, K.J.; Ho, Y.-H.; Hintermair, C.; Ahmad, M.F.; Kar, R.K.; et al. Noncanonical CTD kinases regulate RNA polymerase II in a gene-class-specific manner. Nat. Chem. Biol. 2019, 15, 123–131. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol. Dis. 2015, 84, 39–49. [Google Scholar] [CrossRef]
- Guo, N.; Wang, X.; Xu, M.; Bai, J.; Yu, H.; Zhang, L. PI3K/AKT signaling pathway: Molecular mechanisms and therapeutic potential in depression. Pharmacol. Res. 2024, 206, 107300. [Google Scholar] [CrossRef]
- Yan, L.; Wang, M.; Yang, F.; Wang, Y.; Wang, S.; So, K.-F.; Zhang, L. Physical exercise mediates a cortical FMRP–mTOR pathway to improve resilience against chronic stress in adolescent mice. Transl. Psychiatry 2023, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, C.-Y.; Tao, Y.-J.; Cheng, L. Epigenetic modifications in chronic rhinosinusitis with and without nasal polyps. Front. Genet. 2023, 13, 1089647. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sahu, M.; Srivastava, D.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Post-translational modifications: Regulators of neurodegenerative proteinopathies. Ageing Res. Rev. 2021, 68, 101336. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.K.; Hanner, A.S.; Starost, M.F.; Springer, D.; Mastracci, T.L.; Mirmira, R.G.; Park, M.H. Neuron-specific ablation of eIF5A or deoxyhypusine synthase leads to impairments in growth, viability, neurodevelopment, and cognitive functions in mice. J. Biol. Chem. 2021, 297, 101333. [Google Scholar] [CrossRef]
- Park, M.H.; Kar, R.K.; Banka, S.; Ziegler, A.; Chung, W.K. Post-translational formation of hypusine in eIF5A: Implications in human neurodevelopment. Amino Acids 2022, 54, 485–499. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, T.; Wei, G.; Que, Y.; Wang, W.; Kong, Y.; Xie, T.; Chen, X. Role of Histone Post-Translational Modifications in Inflammatory Diseases. Front. Immunol. 2022, 13, 852272. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Xiao, T.S. Post-translational regulation of inflammasomes. Cell. Mol. Immunol. 2016, 14, 65–79. [Google Scholar] [CrossRef]
- Wang, H.; Do, D.C.; Liu, J.; Wang, B.; Qu, J.; Ke, X.; Luo, X.; Tang, H.M.; Tang, H.L.; Hu, C.; et al. Functional role of kynurenine and aryl hydrocarbon receptor axis in chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2018, 141, 586–600.e6. [Google Scholar] [CrossRef]
- Qu, J.; Mei, Q.; Niu, R. Oxidative CaMKII as a potential target for inflammatory disease (Review). Mol. Med. Rep. 2019, 20, 863–870. [Google Scholar] [CrossRef]
- Hansen, M.H.; Sadredini, M.; Hasic, A.; Anderson, M.E.; Sjaastad, I.; Stokke, M.K. CaMKII and reactive oxygen species contribute to early reperfusion arrhythmias, but oxidation of CaMKIIδ at methionines 281/282 is not a determining factor. J. Mol. Cell. Cardiol. 2023, 175, 49–61. [Google Scholar] [CrossRef]
- Begum, R.; Thota, S.; Abdulkadir, A.; Kaur, G.; Bagam, P.; Batra, S. NADPH oxidase family proteins: Signaling dynamics to disease management. Cell. Mol. Immunol. 2022, 19, 660–686. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.R.; Lee, H.J.; Jung, Y.H.; Kim, J.S.; Chae, C.W.; Kim, S.Y.; Han, H.J. Ethanol-activated CaMKII signaling induces neuronal apoptosis through Drp1-mediated excessive mitochondrial fission and JNK1-dependent NLRP3 inflammasome activation. Cell Commun. Signal. 2020, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Ashpole, N.M.; Song, W.; Brustovetsky, T.; Engleman, E.A.; Brustovetsky, N.; Cummins, T.R.; Hudmon, A. Calcium/Calmodulin-dependent Protein Kinase II (CaMKII) Inhibition Induces Neurotoxicity via Dysregulation of Glutamate/Calcium Signaling and Hyperexcitability. J. Biol. Chem. 2012, 287, 8495–8506. [Google Scholar] [CrossRef] [PubMed]
- Sałaciak, K.; Koszałka, A.; Żmudzka, E.; Pytka, K. The Calcium/Calmodulin-Dependent Kinases II and IV as Therapeutic Targets in Neurodegenerative and Neuropsychiatric Disorders. Int. J. Mol. Sci. 2021, 22, 4307. [Google Scholar] [CrossRef]
- Shin, W.H.; Chung, K.C. Death-associated Protein Kinase 1 Phosphorylates α-Synuclein at Ser129 and Exacerbates Rotenone-induced Toxic Aggregation of α-Synuclein in Dopaminergic SH-SY5Y Cells. Exp. Neurobiol. 2020, 29, 207–218. [Google Scholar] [CrossRef]
- Alsaadi, M.S. Role of DAPK1 in neuronal cell death, survival and diseases in the nervous system. Int. J. Dev. Neurosci. 2019, 74, 11–17. [Google Scholar] [CrossRef]
- Zhang, T.; Xia, Y.; Hu, L.; Chen, D.; Gan, C.-L.; Wang, L.; Mei, Y.; Lan, G.; Shui, X.; Tian, Y.; et al. Death-associated protein kinase 1 mediates Aβ42 aggregation-induced neuronal apoptosis and tau dysregulation in Alzheimer’s disease. Int. J. Biol. Sci. 2022, 18, 693–706. [Google Scholar] [CrossRef]
- Oueslati, A.; Schneider, B.L.; Aebischer, P.; Lashuel, H.A. Polo-like kinase 2 regulates selective autophagic alpha-synuclein clearance and suppresses its toxicity in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, E3945–E3954. [Google Scholar] [CrossRef]
- Wang, S.; Xu, B.; Liou, L.-C.; Ren, Q.; Huang, S.; Luo, Y.; Zhang, Z.; Witt, S.N. α-Synuclein disrupts stress signaling by inhibiting polo-like kinase Cdc5/Plk2. Proc. Natl. Acad. Sci. USA 2012, 109, 16119–16124. [Google Scholar] [CrossRef]
- Sung, C.C.; Lam, W.Y.; Chung, K.K.K. The role of polo-like kinases 2 in the proteasomal and lysosomal degradation of alpha-synuclein in neurons. FASEB J. 2024, 38, e70121. [Google Scholar] [CrossRef]
- Gata, A.; Neagoe, I.B.; Leucuta, D.-C.; Budisan, L.; Raduly, L.; Trombitas, V.E.; Albu, S. MicroRNAs: Potential Biomarkers of Disease Severity in Chronic Rhinosinusitis with Nasal Polyps. Medicina 2023, 59, 550. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, X.; Liang, X.; Wang, W.; Yang, Y.; Xu, F.; Lu, X.; Geng, D.; Li, M. MicroRNA-145-5p Regulates the Epithelial-Mesenchymal Transition in Nasal Polyps by Targeting Smad3. Clin. Exp. Otorhinolaryngol. 2024, 17, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kang, X.; Xiong, Y.; Luo, Q.; Dai, D.; Ye, J. Gene Expression Profiles of Circular RNAs and MicroRNAs in Chronic Rhinosinusitis With Nasal Polyps. Front. Mol. Biosci. 2021, 8, 643504. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, X.; Xing, X.; Bi, Z.; Wang, D.; Zhang, C.; Wei, L.; Jin, Y.; Xu, S. Advances in autonomic dysfunction research in Parkinson’s disease. Front. Aging Neurosci. 2025, 17, 1468895. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, H.; Biaggioni, I. Autonomic failure in neurodegenerative disorders. Semin. Neurol. 2003, 23, 351–363. [Google Scholar] [CrossRef]
- Engelhardt, E.; Laks, J. Alzheimer disease neuropathology:understanding autonomic dysfunction. Dement. Neuropsychol. 2008, 2, 183–191. [Google Scholar] [CrossRef]
- Malakar, P.; Shukla, S.; Mondal, M.; Kar, R.K.; Siddiqui, J.A. The nexus of long noncoding RNAs, splicing factors, alternative splicing and their modulations. RNA Biol. 2024, 21, 16–35. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Lu, J. Apoptosis and turnover disruption of olfactory sensory neurons in eosinophilic chronic rhinosinusitis. Front. Cell. Neurosci. 2024, 18, 1371587. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Cha, X.; Li, F.; Xu, Z.; Wu, J.; Liu, H.; Ren, W. Aging and chronic inflammation: Impacts on olfactory dysfunction-a comprehensive review. Cell. Mol. Life Sci. 2025, 82, 199. [Google Scholar] [CrossRef]
- Vezzani, A.; Fujinami, R.S.; White, H.S.; Preux, P.-M.; Blümcke, I.; Sander, J.W.; Löscher, W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016, 131, 211–234. [Google Scholar] [CrossRef]
- Pan, H.-H.; Hung, T.-W.; Tsai, J.-D.; Chen, H.-J.; Liao, P.-F.; Sheu, J.-N. Children with allergic rhinitis and a risk of epilepsy: A nationwide cohort study. Seizure 2020, 76, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.K. Recent Insights of Molecular Approaches to Study Brain Tumor Associated Seizure and Epilepsy. In Iterative International Publishers (IIP), 1st ed.; Selfypage Developers Pvt Ltd.: Chikkamagaluru, India, 2024; Volume 1, pp. 21–47. [Google Scholar] [CrossRef]
- Wang, C.; Lau, C.Y.; Ma, F.; Zheng, C. Genome-wide screen identifies curli amyloid fibril as a bacterial component promoting host neurodegeneration. Proc. Natl. Acad. Sci. USA 2021, 118, e2106504118. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σE-dependent sRNA RybB. Mol. Microbiol. 2016, 101, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Consonni, A.; Miglietti, M.; De Luca, C.M.G.; Cazzaniga, F.A.; Ciullini, A.; Dellarole, I.L.; Bufano, G.; Di Fonzo, A.; Giaccone, G.; Baggi, F.; et al. Approaching the Gut and Nasal Microbiota in Parkinson’s Disease in the Era of the Seed Amplification Assays. Brain Sci. 2022, 12, 1579. [Google Scholar] [CrossRef]
- Tremblay, C.; Serrano, G.E.; Intorcia, A.J.; Mariner, M.R.; Sue, L.I.; Arce, R.A.; Atri, A.; Adler, C.H.; Belden, C.M.; Shill, H.A.; et al. Olfactory Bulb Amyloid-β Correlates with Brain Thal Amyloid Phase and Severity of Cognitive Impairment. J. Neuropathol. Exp. Neurol. 2022, 81, 643–649. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Qian, J.; Muzikansky, A.; Monsell, S.E.; Montine, T.J.; Frosch, M.P.; Betensky, R.A.; Hyman, B.T. Thal Amyloid Stages Do Not Significantly Impact the Correlation Between Neuropathological Change and Cognition in the Alzheimer Disease Continuum. J. Neuropathol. Exp. Neurol. 2016, 75, 516–526. [Google Scholar] [CrossRef]
- Diez, I.; Ortiz-Terán, L.; Ng, T.S.C.; Albers, M.W.; Marshall, G.; Orwig, W.; Kim, C.-M.; Bueichekú, E.; Montal, V.; Olofsson, J.; et al. Tau propagation in the brain olfactory circuits is associated with smell perception changes in aging. Nat. Commun. 2024, 15, 4809. [Google Scholar] [CrossRef]
- Norwood, J.N.; Zhang, Q.; Card, D.; Craine, A.; Ryan, T.M.; Drew, P.J. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. eLife 2019, 8, e44278. [Google Scholar] [CrossRef]
- Silver, I.; Kim, C.; Mollanji, R.; Johnston, M. Cerebrospinal fluid outflow resistance in sheep: Impact of blocking cerebrospinal fluid transport through the cribriform plate. Neuropathol. Appl. Neurobiol. 2002, 28, 67–74. [Google Scholar] [CrossRef]
- Spera, I.; Cousin, N.; Ries, M.; Kedracka, A.; Castillo, A.; Aleandri, S.; Vladymyrov, M.; Mapunda, J.A.; Engelhardt, B.; Luciani, P.; et al. Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves. eBioMedicine 2023, 91, 104558. [Google Scholar] [CrossRef]
- Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal. Fluid Res. 2004, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Mollanji, R.; Bozanovic-Sosic, R.; Zakharov, A.; Makarian, L.; Johnston, M.G. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 282, R1593–R1599. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakamura, Y.; Yamada, K.; Igarashi, H.; Kasuga, K.; Yokoyama, Y.; Ikeuchi, T.; Nishizawa, M.; Kwee, I.L.; Nakada, T. Reduced CSF Water Influx in Alzheimer’s Disease Supporting the β-Amyloid Clearance Hypothesis. PLoS ONE 2015, 10, e0123708. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096.e10. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chandra, R.K.; Li, P.; Hull, B.P.; Turner, J.H. Olfactory and middle meatal cytokine levels correlate with olfactory function in chronic rhinosinusitis. Laryngoscope 2018, 128, E304–E310. [Google Scholar] [CrossRef]
- Amantea, D.; Petrelli, F.; Greco, R.; Tassorelli, C.; Corasaniti, M.T.; Tonin, P.; Bagetta, G. Azithromycin Affords Neuroprotection in Rat Undergone Transient Focal Cerebral Ischemia. Front. Neurosci. 2019, 13, 1256. [Google Scholar] [CrossRef]
- Pynnonen, M.A.; Venkatraman, G.; Davis, G.E. Macrolide Therapy for Chronic Rhinosinusitis. Otolaryngol. Neck Surg. 2013, 148, 366–373. [Google Scholar] [CrossRef]
- Balducci, C.; Santamaria, G.; La Vitola, P.; Brandi, E.; Grandi, F.; Viscomi, A.R.; Beeg, M.; Gobbi, M.; Salmona, M.; Ottonello, S.; et al. Doxycycline counteracts neuroinflammation restoring memory in Alzheimer’s disease mouse models. Neurobiol. Aging 2018, 70, 128–139. [Google Scholar] [CrossRef]
- Thomsen, T.R.; Galpern, W.R.; Asante, A.; Arenovich, T.; Fox, S.H. Ipratropium bromide spray as treatment for sialorrhea in Parkinson’s disease. Mov. Disord. 2007, 22, 2268–2273. [Google Scholar] [CrossRef]
- Vouri, S.M.; Chung, J.M.; Binder, E.F. Successful intervention to mitigate an acetylcholinesterase inhibitor-induced rhinorrhea prescribing cascade: A case report. J. Clin. Pharm. Ther. 2017, 42, 370–371. [Google Scholar] [CrossRef]
- Basiago, A.; Binder, D.K. Effects of Deep Brain Stimulation on Autonomic Function. Brain Sci. 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Lovick, T. Deep brain stimulation and autonomic control. Exp. Physiol. 2014, 99, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Maniyar, F.H.; Starr, P.; Goadsby, P.J. Paroxysmal sneezing after hypothalamic deep brain stimulation for cluster headache. Cephalalgia 2012, 32, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hou, Y.; Wang, X.; Li, Y.-X.; Li, F.; Zhang, C.; Li, W.-G. Impact of Subthalamic Deep Brain Stimulation on Hyposmia in Patients With Parkinson’s Disease Is Influenced by Constipation and Dysbiosis of Microbiota. Front. Neurol. 2021, 12, 653833. [Google Scholar] [CrossRef]
- Lung, M.A. The Role of the Autonomic Nerves in the Control of Nasal Circulation. Neurosignals 1995, 4, 179–185. [Google Scholar] [CrossRef]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1282–1294. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Fang, J.; Dai, W.; Zhou, J.; Wang, X.; Zhou, M. SS-31 Provides Neuroprotection by Reversing Mitochondrial Dysfunction after Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2018, 2018, 4783602. [Google Scholar] [CrossRef]
- James, A.M.; Sharpley, M.S.; Manas, A.-R.B.; Frerman, F.E.; Hirst, J.; Smith, R.A.J.; Murphy, M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007, 282, 14708–14718. [Google Scholar] [CrossRef]
- Matthew, J.R.; Jessica, R.S.-P.; Chelsea, A.C.S.; Nina, Z.B.; Lauren, M.C.; Hannah, L.R.; Kayla, A.; Chonchol, M.W.; Rachel, A.G.-R.; Michael, P.M.; et al. Chronic Supplementation with a Mitochondrial Antioxidant (MitoQ) Improves Vascular Func-tion in Healthy Older Adults. Hypertens. Dallas Tex 1979 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The mitochondrial-targeted compound SS-31 Re-energizes ischemic mitochondria by interacting with cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Zuo, Q.-N.; Wang, T.; Xu, D.; Lian, L.; Gao, L.-J.; Wan, C.; Chen, L.; Wen, F.-Q.; Shen, Y.-C. Mitochondrial-Targeting Antioxidant SS-31 Suppresses Airway Inflammation and Oxidative Stress Induced by Cigarette Smoke. Oxidative Med. Cell. Longev. 2021, 2021, 6644238. [Google Scholar] [CrossRef]
- Mårtensson, A.; Cervin-Hoberg, C.; Huygens, F.; Lindstedt, M.; Sakellariou, C.; Greiff, L.; Cervin, A. Upper airway microbiome transplantation for patients with chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2023, 13, 979–988. [Google Scholar] [CrossRef]
- Junca, H.; Pieper, D.H.; Medina, E. The emerging potential of microbiome transplantation on human health interventions. Comput. Struct. Biotechnol. J. 2022, 20, 615–627. [Google Scholar] [CrossRef]
- Qian, W.; Liu, D.; Liu, J.; Liu, M.; Ji, Q.; Zhang, B.; Yang, Z.; Cheng, Y.; Zhou, S. The Mitochondria-Targeted Micelle Inhibits Alzheimer’s Disease Progression by Alleviating Neuronal Mitochondrial Dysfunction and Neuroinflammation. Small 2025, 21, e2408581. [Google Scholar] [CrossRef]


| Author, Year | Title of Study | Study Design | Sample Size (n) | Key Findings | p-Value | Study Quality (Newcastle–Ottawa Scale) |
|---|---|---|---|---|---|---|
| Song et al., 2025 [10] | Nasal microbiome in relation to olfactory dysfunction and cognitive decline in older adults | Cross-sectional | 510 |
| 0.008 | High |
| Gao et al., 2025 [11] | Chronic rhinosinusitis and cognition: a systematic review and meta-analysis | Review | 1149 |
| 0.05 | Moderate |
| Kim et al., 2024 [12] | A longitudinal study investigating whether chronic rhinosinusitis influences the subsequent risk of developing dementia | Longitudinal | 10,630 |
| NA | High |
| Liu et al., 2023 [13] | Intrinsic brain abnormalities in chronic rhinosinusitis associated with mood and cognitive function | Observational | 64 |
| 0.05 | Moderate |
| Chen et al., 2022 [14] | Prevalence of rhinorrhea in Parkinson’s disease: a systematic review and meta-analysis | Review | 451 |
| 0.001 | High |
| Jafari et al., 2021 [15] | Association of sinonasal inflammation with functional brain connectivity | Case-control | 44 |
| 0.05 | Moderate |
| Jung et al., 2021 [16] | Chronic rhinosinusitis and progression of cognitive impairment in dementia or mild cognitive impairment | Retrospective | 661 |
| 0.034 | Moderate |
| Wee et al., 2020 [17] | Association between neurodegenerative dementia and chronic rhinosinusitis: a nested case-control study | Case-control | 88,170 |
| 0.653 | High |
| Rowan et al., 2019 [18] | The impact of medical therapy on cognitive dysfunction in chronic rhinosinusitis | Prospective | 27 |
| 0.046 | Moderate |
| Yoo et al., 2019 [19] | Effects of endoscopic sinus surgery on objective and subjective measures of cognitive dysfunction in chronic rhinosinusitis | Prospective | 33 |
| 0.001 | Moderate |
| Domellof et al., 2017 [20] | Olfactory dysfunction and dementia in newly diagnosed patients with Parkinson’s disease | Prospective | 125 |
| 0.005 | High |
| Hauser et al., 2017 [21] | Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss | Prospective | 69 |
| p < 0.05 | High |
| Alt et al., 2016 [22] | Endoscopic sinus surgery improves cognitive dysfunction in patients with chronic rhinosinusitis | Prospective | 247 |
| 0.012 | Moderate |
| Baba et al., 2012 [23] | Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3-year longitudinal study | Longitudinal | 44 |
| 0.001 | High |
| Chou et al., 2011 [24] | Rhinorrhea: a common nondopaminergic feature of Parkinson’s disease | Cross-sectional | 49 |
| 0.008 | Moderate |
| Peric et al., 2010 [25] | Correlation between cytokine levels in nasal fluid and eosinophil counts in nasal polyp tissue in asthmatic and non-asthmatic patients | Prospective | 30 |
| NA | Moderate |
| Author, Year | Title of Study | Study Design | Sample Size (n) | Key Findings | p-Value |
|---|---|---|---|---|---|
| Huang et al., 2024 [26] | Murine model of eosinophilic chronic rhinosinusitis with nasal polyposis inducing neuroinflammation and olfactory dysfunction | Experimental | 32 |
| NA |
| Pal et al., 2021 [27] | Deep nasal sinus cavity microbiota dysbiosis in Parkinson’s disease | Experimental | 41 |
| 0.05 |
| Yoon et al., 2020 [28] | Altered mitochondrial functions and morphologies in epithelial cells are associated with pathogenesis of chronic rhinosinusitis with nasal polyps | Experimental | 45 |
| 0.05 |
| Alt et al., 2014 [29] | Antisomnogenic cytokines, quality of life, and chronic rhinosinusitis: a pilot study | Experimental | 20 |
| p < 0.05 |
| Lane et al., 2010 [30] | A genetic model of chronic rhinosinusitis-associated olfactory inflammation | Experimental | NA |
| NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, N.Y.M.; Ang, L.F.; Loh, B.J.S.; Wang, J.D.J. Nasal Inflammation and Brain Bioenergetics: Does Chronic Rhinosinusitis Accelerate Neurodegeneration? Clin. Bioenerg. 2025, 1, 10. https://doi.org/10.3390/clinbioenerg1020010
Chua NYM, Ang LF, Loh BJS, Wang JDJ. Nasal Inflammation and Brain Bioenergetics: Does Chronic Rhinosinusitis Accelerate Neurodegeneration? Clinical Bioenergetics. 2025; 1(2):10. https://doi.org/10.3390/clinbioenerg1020010
Chicago/Turabian StyleChua, Nevin Yi Meng, Lee Fang Ang, Bo Jie Sean Loh, and Jia Dong James Wang. 2025. "Nasal Inflammation and Brain Bioenergetics: Does Chronic Rhinosinusitis Accelerate Neurodegeneration?" Clinical Bioenergetics 1, no. 2: 10. https://doi.org/10.3390/clinbioenerg1020010
APA StyleChua, N. Y. M., Ang, L. F., Loh, B. J. S., & Wang, J. D. J. (2025). Nasal Inflammation and Brain Bioenergetics: Does Chronic Rhinosinusitis Accelerate Neurodegeneration? Clinical Bioenergetics, 1(2), 10. https://doi.org/10.3390/clinbioenerg1020010






