Biomarkers of Creatine Metabolism in Humans: From Plasma to Saliva and Beyond
Abstract
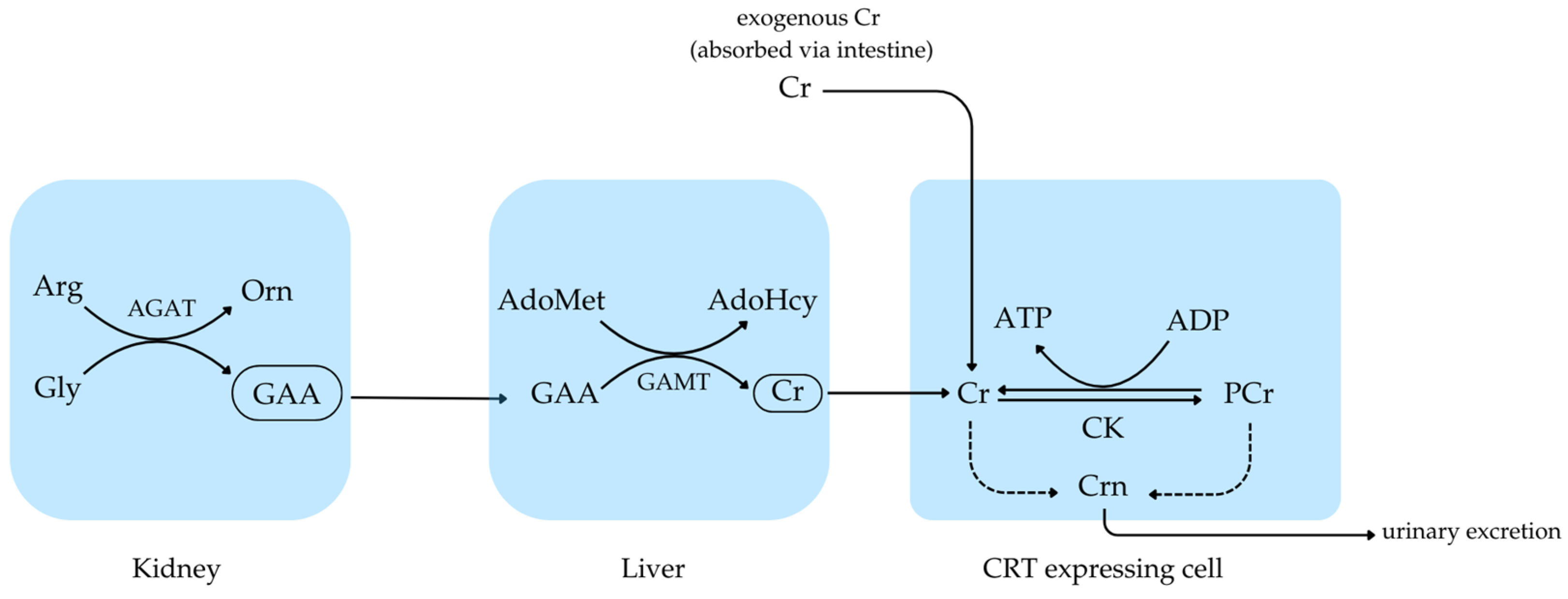
1. Introduction
2. Creatine Biomarkers in Blood
3. Creatine Biomarkers in Urine
4. Creatine Biomarkers in CSF
5. Creatine Biomarkers in Saliva
6. Creatine Biomarkers in Semen
7. Creatine Biomarkers in Human Milk
8. Analytical Assessment of Creatine Biomarkers
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Cohen, P. Creatine metabolism: Energy homeostasis, immunity and cancer biology. Nat. Rev. Endocrinol. 2020, 16, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Béard, E.; Braissant, O. Synthesis and transport of creatine in the CNS: Importance for cerebral functions. J. Neurochem. 2010, 115, 297–313. [Google Scholar] [CrossRef]
- Moore, N.P. The distribution, metabolism and function of creatine in the male mammalian reproductive tract: A review. Int. J. Androl. 2000, 23, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; da Silva, R.P.; Brosnan, M.E. The metabolic burden of creatine synthesis. Amino Acids 2011, 40, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef]
- Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. [Google Scholar] [CrossRef]
- Almeida, L.S.; Salomons, G.S.; Hogenboom, F.; Jakobs, C.; Schoffelmeer, A.N. Exocytotic release of creatine in rat brain. Synapse 2006, 60, 118–123. [Google Scholar] [CrossRef]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct Antioxidant Properties of Creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef]
- Young, J.F.; Larsen, L.B.; Malmendal, A.; Nielsen, N.C.; Straadt, I.K.; Oksbjerg, N.; Bertram, H.C. RCesreearachtairnticele-induced activation of antioxidative defence in myotube cultures revealed by explorative NMR-based metabonomics and proteomics. J. Int. Soc. Sports Nutr. 2010, 7, 9. [Google Scholar] [CrossRef]
- Wallimann, T.; Tokarska-Schlattner, M.; Kay, L.; Schlattner, U. Role of creatine and creatine kinase in UCP1-independent adipocyte thermogenesis. Am. J. Physiol. Metab. 2020, 319, E944–E946. [Google Scholar] [CrossRef]
- Hespel, P.; Eijnde, B.O.; Van Leemputte, M.; Ursø, B.; Greenhaff, P.L.; Labarque, V.; Dymarkowski, S.; Van Hecke, P.; Richter, E.A. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J. Physiol. 2001, 536, 625–633. [Google Scholar] [CrossRef]
- Louis, M.; Van Beneden, R.; Dehoux, M.; Thissen, J.P.; Francaux, M. Creatine increases IGF-I and myogenic regulatory factor mRNA in C2C12 cells. FEBS Lett. 2004, 557, 243–247. [Google Scholar] [CrossRef]
- Peral, M.; Carretero, V.; Ilundain, A. Na+/Cl−/creatine transporter activity and expression in rat brain synaptosomes. Neuroscience 2010, 165, 53–60. [Google Scholar] [CrossRef]
- Stöckler, S.; Holzbach, U.; Hanefeld, F.; Marquardt, I.; Helms, G.; Requart, M.; Hänicke, W.; Frahm, J. Creatine Deficiency in the Brain: A New, Treatable Inborn Error of Metabolism. Pediatr. Res. 1994, 36, 409–413. [Google Scholar] [CrossRef]
- Schulze, A. Creatine deficiency syndromes. Mol. Cell. Biochem. 2003, 244, 143–150. [Google Scholar] [CrossRef]
- Almeida, L.S.; Verhoeven, N.M.; Roos, B.; Valongo, C.; Cardoso, M.L.; Vilarinho, L.; Salomons, G.S.; Jakobs, C. Creatine and guanidinoacetate: Diagnostic markers for inborn errors in creatine biosynthesis and transport. Mol. Genet. Metab. 2004, 82, 214–219. [Google Scholar] [CrossRef]
- Ostojic, S.M. Low Tissue Creatine: A Therapeutic Target in Clinical Nutrition. Nutrients 2022, 14, 1230. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Forbes, S.C. Perspective: Creatine, a Conditionally Essential Nutrient: Building the Case. Adv. Nutr. Int. Rev. J. 2022, 13, 34–37. [Google Scholar] [CrossRef]
- See, H.H.; Schmidt-Marzinkowski, J.; Pormsila, W.; Morand, R.; Krähenbühl, S.; Hauser, P.C. Determination of creatine and phosphocreatine in muscle biopsy samples by capillary electrophoresis with contactless conductivity detection. Anal. Chim. Acta 2012, 727, 78–82. [Google Scholar] [CrossRef]
- Ostojic, J.; Kozic, D.; Ostojic, S.M. N-Acetylaspartate-to-creatine ratio in twelve brain locations among healthy men and women with different levels of education. Neurosci. Lett. 2019, 692, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Drid, P.; Ostojic, J. Guanidinoacetic acid increases skeletal muscle creatine stores in healthy men. Nutrition 2016, 32, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Ostojic, J.; Drid, P.; Vranes, M.; Jovanov, P. Dietary guanidinoacetic acid increases brain creatine levels in healthy men. Nutrition 2017, 33, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Agren, H.; Niklasson, F. Creatinine and creatine in CSF: Indices of brain energy metabolism in depression. J. Neural Transm. 1988, 74, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J. Cachex Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef]
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum Creatinine: Not So Simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef]
- Mihatsch, W.A.; Stahl, B.; Braun, U. The Umbilical Cord Creatine Flux and Time Course of Human Milk Creatine across Lactation. Nutrients 2024, 16, 345. [Google Scholar] [CrossRef]
- Martínez, L.D.; Bezard, M.; Brunotto, M.; De Kremer, R.D. Creatine metabolism: Detection of creatine and guanidinoacetate in saliva of healthy subjects. Acta Odontol. Latinoam. 2016, 29, 49–53. [Google Scholar]
- Nasrallah, F.; Hammami, M.; Omar, S.; Aribia, H.; Sanhaji, H.; Feki, M. Semen Creatine and Creatine Kinase Activity as an Indicator of Sperm Quality. Clin. Lab. 2020, 66, 1751–1757. [Google Scholar] [CrossRef]
- Al-Ghamdi, F.; Darras, B.T.; Ghosh, P.S. Spectrum of Neuromuscular Disorders With HyperCKemia From a Tertiary Care Pediatric Neuromuscular Center. J. Child Neurol. 2018, 33, 389–396. [Google Scholar] [CrossRef]
- Nardin, R.A.; Zarrin, A.R.; Horowitz, G.L.; Tarulli, A.W. Effect of newly proposed CK reference limits on neuromuscular diagnosis. Muscle Nerve 2009, 39, 494–497. [Google Scholar] [CrossRef]
- Cabaniss, C.D. Creatine Kinase. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Oxford, UK, 1990. Available online: http://www.ncbi.nlm.nih.gov/books/NBK352/ (accessed on 3 June 2024).
- Cánovas, R.; Cuartero, M.; Crespo, G.A. Modern creatinine (Bio)sensing: Challenges of point-of-care platforms. Biosens. Bioelectron. 2019, 130, 110–124. [Google Scholar] [CrossRef]
- Sharer, J.D.; Bodamer, O.; Longo, N.; Tortorelli, S.; Wamelink, M.M.; Young, S.; Workgroup of the ACMG Laboratory Quality Assurance Committee. Assurance Committee Laboratory diagnosis of creatine deficiency syndromes: A technical standard and guideline of the American College of Medical Genetics and Genomics. Anesth. Analg. 2017, 19, 256–263. [Google Scholar] [CrossRef]
- Burke, D.G.; MacLean, P.G.; Walker, R.A.; Dewar, P.J.; Smith-Palmer, T. Analysis of creatine and creatinine in urine by capillary electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1999, 732, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Almada, A.L.; Mitchell, T.L. High-Performance Capillary Electrophoresis-Pure Creatine Monohydrate Reduces Blood Lipids in Men and Women. Clin. Sci. 1996, 91, 113–118. [Google Scholar] [CrossRef]
- Zinellu, A.; Caria, M.A.; Tavera, C.; Sotgia, S.; Chessa, R.; Deiana, L.; Carru, C. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal. Biochem. 2005, 342, 186–193. [Google Scholar] [CrossRef]
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Möller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between Human Plasma and Serum Metabolite Profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef]
- Curt, M.J.-C.; Cheillan, D.; Briand, G.; Salomons, G.S.; Mention-Mulliez, K.; Dobbelaere, D.; Cuisset, J.-M.; Lion-François, L.; Portes, V.D.; Chabli, A.; et al. Creatine and guanidinoacetate reference values in a French population. Mol. Genet. Metab. 2013, 110, 263–267. [Google Scholar] [CrossRef]
- Bodamer, O.A.; Bloesch, S.M.; Gregg, A.R.; Stockler-Ipsiroglu, S.; O’Brien, W.E. Analysis of guanidinoacetate and creatine by isotope dilution electrospray tandem mass spectrometry. Clin. Chim. Acta 2001, 308, 173–178. [Google Scholar] [CrossRef]
- Marescau, B.; Deshmukh, D.R.; Kockx, M.; Possemiers, I.; Qureshi, I.A.; Wiechert, P.; De Deyn, P.P. Guanidino compounds in serum, urine, liver, kidney, and brain of man and some ureotelic animals. Metabolism 1992, 41, 526–532. [Google Scholar] [CrossRef]
- Arias, A.; Garcia-Villoria, J.; Ribes, A. Guanidinoacetate and creatine/creatinine levels in controls and patients with urea cycle defects. Mol. Genet. Metab. 2004, 82, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Ormazabal, A.; Moreno, J.; González, B.; Vilaseca, M.A.; García-Villoria, J.; Pàmpols, T.; Briones, P.; Artuch, R.; Ribes, A. Methods for the diagnosis of creatine deficiency syndromes: A comparative study. J. Neurosci. Methods 2006, 156, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Stea, T.H.; Engeset, D. Creatine as a Promising Component of Paternal Preconception Diet. Nutrients 2022, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Stajer, V.; Vranes, M.; Ostojic, S.M. Correlation between biomarkers of creatine metabolism and serum indicators of peripheral muscle fatigue during exhaustive exercise in active men. Res. Sports Med. 2020, 28, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, N.M.; Salomons, G.S.; Jakobs, C. Laboratory diagnosis of defects of creatine biosynthesis and transport. Clin. Chim. Acta 2005, 361, 1–9. [Google Scholar] [CrossRef]
- Carducci, C.; Birarelli, M.; Leuzzi, V.; Carducci, C.; Battini, R.; Cioni, G.; Antonozzi, I. Guanidinoacetate and Creatine plus Creatinine Assessment in Physiologic Fluids: An Effective Diagnostic Tool for the Biochemical Diagnosis of Arginine: Glycine Amidinotransferase and Guanidinoacetate Methyltransferase Deficiencies. Clin. Chem. 2002, 48, 1772–1778. [Google Scholar] [CrossRef]
- Threlfall, C.J.; Maxwell, A.R.; Stoner, H.B. Post-traumatic creatinuria. J. Trauma Acute Care Surg. 1984, 24, 516–523. [Google Scholar] [CrossRef]
- Struys, E.; Jansen, E.; Brink, H.T.; Verhoeven, N.; van der Knaap, M.; Jakobs, C. An accurate stable isotope dilution gas chromatographic–mass spectrometric approach to the diagnosis of guanidinoacetate methyltransferase deficiency. J. Pharm. Biomed. Anal. 1998, 18, 659–665. [Google Scholar] [CrossRef]
- Fingerhut, R. Stable isotope dilution method for the determination of guanidinoacetic acid by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 717–722. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Ratgeber, L.; Olah, A.; Betlehem, J.; Acs, P. Guanidinoacetic acid deficiency: A new entity in clinical medicine? Int. J. Med Sci. 2020, 17, 2544–2550. [Google Scholar] [CrossRef]
- Sawynok, J.; Dawborn, J.K. Plasma concentration and urinary excretion of guanidine derivatives in normal subjects and patients with renal failure. Clin. Exp. Pharmacol. Physiol. 1975, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shiraga, H.; Watanabe, Y.; Mori, A. Guanidino compound levels in the serum of healthy adults and epileptic patients. Epilepsy Res. 1991, 8, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, I.; Simell, O.; Arjomaa, P. Gyrate atrophy of the choroid and retina with hyperornithinemia. Deficient formation of guanidinoacetic acid from arginine. J. Clin. Investig. 1980, 66, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Junge, W.; Wilke, B.; Halabi, A.; Klein, G. Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified Jaffé method. Clin. Chim. Acta 2004, 344, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Finney, H.; Newman, D.J.; Price, C.P. Adult Reference Ranges for Serum Cystatin C, Creatinine and Predicted Creatinine Clearance. Ann. Clin. Biochem. 2000, 37, 49–59. [Google Scholar] [CrossRef]
- Huang, M.; Yang, J.-J.; Tang, A.-G. Reference intervals for serum creatinine levels in the healthy geriatric population. Clin. Biochem. 2013, 46, 1419–1422. [Google Scholar] [CrossRef]
- Temilola, D.O.; Bezuidenhout, K.; Erasmus, R.T.; Stephen, L.; Davids, M.R.; Holmes, H. Salivary creatinine as a diagnostic tool for evaluating patients with chronic kidney disease. BMC Nephrol. 2019, 20, 387. [Google Scholar] [CrossRef]
- García-Delgado, M.; Peral, M.J.; Cano, M.; Calonge, M.L.; Ilundáin, A.A. Creatine Transport in Brush-Border Membrane Vesicles Isolated from Rat Kidney Cortex. J. Am. Soc. Nephrol. 2001, 12, 1819–1825. [Google Scholar] [CrossRef]
- Guimbal, C.; Kilimann, M. A Na(+)-dependent creatine transporter in rabbit brain, muscle, heart, and kidney. cDNA cloning and functional expression. J. Biol. Chem. 1993, 268, 8418–8421. [Google Scholar] [CrossRef]
- Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 15 April 2024).
- Bader, M.; Messerer, P.; Will, W. Urinary creatinine concentrations in an industrial workforce and comparison with reference values of the general population. Int. Arch. Occup. Environ. Health 2012, 86, 673–680. [Google Scholar] [CrossRef]
- Cognat, S.; Cheillan, D.; Piraud, M.; Roos, B.; Jakobs, C.; Vianey-Saban, C. Determination of Guanidinoacetate and Creatine in Urine and Plasma by Liquid Chromatography–Tandem Mass Spectrometry. Clin. Chem. 2004, 50, 1459–1461. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, F.; Feki, M.; Briand, G.; Kaabachi, N. GC/MS determination of guanidinoacetate and creatine in urine: A routine method for creatine deficiency syndrome diagnosis. Clin. Biochem. 2010, 43, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Urakami, Y.; Kimura, N.; Okuda, M.; Inui, K.-I. Creatinine Transport by Basolateral Organic Cation Transporter hOCT2 in the Human Kidney. Pharm. Res. 2004, 21, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- Cocker, J.; Mason, H.J.; Warren, N.D.; Cotton, R.J. Creatinine adjustment of biological monitoring results. Occup. Med. 2011, 61, 349–353. [Google Scholar] [CrossRef]
- Tachikawa, M.; Fujinawa, J.; Takahashi, M.; Kasai, Y.; Fukaya, M.; Sakai, K.; Yamazaki, M.; Tomi, M.; Watanabe, M.; Sakimura, K.; et al. Expression and possible role of creatine transporter in the brain and at the blood-cerebrospinal fluid barrier as a transporting protein of guanidinoacetate, an endogenous convulsant. J. Neurochem. 2008, 107, 768–778. [Google Scholar] [CrossRef]
- Braissant, O.; Henry, H. AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: A review. J. Inherit. Metab. Dis. 2008, 31, 230–239. [Google Scholar] [CrossRef]
- Braissant, O. Creatine and guanidinoacetate transport at blood-brain and blood-cerebrospinal fluid barriers. J. Inherit. Metab. Dis. 2012, 35, 655–664. [Google Scholar] [CrossRef]
- Tachikawa, M.; Hosoya, K.-I. Transport characteristics of guanidino compounds at the blood-brain barrier and blood-cerebrospinal fluid barrier: Relevance to neural disorders. Fluids Barriers CNS 2011, 8, 13. [Google Scholar] [CrossRef]
- Braissant, O.; Henry, H.; Loup, M.; Eilers, B.; Bachmann, C. Endogenous synthesis and transport of creatine in the rat brain: An in situ hybridization study. Mol. Brain Res. 2001, 86, 193–201. [Google Scholar] [CrossRef]
- Ensenauer, R.; Thiel, T.; Schwab, K.; Tacke, U.; Stöckler-Ipsiroglu, S.; Schulze, A.; Hennig, J.; Lehnert, W. Guanidinoacetate methyltransferase deficiency: Differences of creatine uptake in human brain and muscle. Mol. Genet. Metab. 2004, 82, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Bachert, P.; Schlemmer, H.; Harting, I.; Polster, T.; Salomons, G.S.; Verhoeven, N.M.; Jakobs, C.; Fowler, B.; Hoffmann, G.F.; et al. Lack of creatine in muscle and brain in an adult with GAMT deficiency. Ann. Neurol. 2003, 53, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Battini, R.; Leuzzi, V.; Carducci, C.; Tosetti, M.; Bianchi, M.C.; Item, C.B.; Stöckler-Ipsiroglu, S.; Cioni, G. Creatine depletion in a new case with AGAT deficiency: Clinical and genetic study in a large pedigree. Mol. Genet. Metab. 2002, 77, 326–331. [Google Scholar] [CrossRef] [PubMed]
- deGrauw, T.J.; Salomons, G.S.; Cecil, K.M.; Chuck, G.; Newmeyer, A.; Schapiro, M.B.; Jakobs, C. Congenital Creatine Transporter Deficiency. Neuropediatrics 2002, 33, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Cecil, K.M.; Salomons, G.S.; Ball, W.S.; Wong, B.; Chuck, G.; Verhoeven, N.M.; Jakobs, C.; DeGrauw, T.J. Irreversible brain creatine deficiency with elevated serum and urine creatine: A creatine transporter defect? Ann. Neurol. 2001, 49, 401–404. [Google Scholar] [CrossRef]
- Salomons, G.S.; van Dooren, S.J.; Verhoeven, N.M.; Cecil, K.M.; Ball, W.S.; Degrauw, T.J.; Jakobs, C. X-Linked Creatine-Transporter Gene (SLC6A8) Defect: A New Creatine-Deficiency Syndrome. Am. J. Hum. Genet. 2001, 68, 1497–1500. [Google Scholar] [CrossRef]
- Tachikawa, M.; Kasai, Y.; Yokoyama, R.; Fujinawa, J.; Ganapathy, V.; Terasaki, T.; Hosoya, K. The blood–brain barrier transport and cerebral distribution of guanidinoacetate in rats: Involvement of creatine and taurine transporters. J. Neurochem. 2009, 111, 499–509. [Google Scholar] [CrossRef]
- Keep, R.F.; Xiang, J. Choroid plexus taurine transport. Brain Res. 1996, 715, 17–24. [Google Scholar] [CrossRef]
- Sijens, P.; Verbruggen, K.; Oudkerk, M.; van Spronsen, F.; Soorani-Lunsing, R. 1H MR spectroscopy of the brain in Cr transporter defect. Mol. Genet. Metab. 2005, 86, 421–422. [Google Scholar] [CrossRef]
- van de Kamp, J.M.; Pouwels, P.J.W.; Aarsen, F.K.; Hoopen, L.W.T.; Knol, D.L.; de Klerk, J.B.; de Coo, I.F.; Huijmans, J.G.M.; Jakobs, C.; van der Knaap, M.S.; et al. Long-term follow-up and treatment in nine boys with X-linked creatine transporter defect. J. Inherit. Metab. Dis. 2012, 35, 141–149. [Google Scholar] [CrossRef]
- van de Kamp, J.M.; Betsalel, O.T.; Mercimek-Mahmutoglu, S.; Abulhoul, L.; Grünewald, S.; Anselm, I.; Azzouz, H.; Bratkovic, D.; de Brouwer, A.; Hamel, B.; et al. Phenotype and genotype in 101 males with X-linked creatine transporter deficiency. J. Med. Genet. 2013, 50, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, M.; Kasai, Y.; Takahashi, M.; Fujinawa, J.; Kitaichi, K.; Terasaki, T.; Hosoya, K. The blood-cerebrospinal fluid barrier is a major pathway of cerebral creatinine clearance: Involvement of transporter-mediated process. J. Neurochem. 2008, 107, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.H.; Miller, D.S.; Pritchard, J.B. Ventricular Choline Transport. J. Biol. Chem. 2001, 276, 41611–41619. [Google Scholar] [CrossRef] [PubMed]
- De Deyn, P.P.; Vanholder, R.; Eloot, S.; Glorieux, G. PROGRESS IN UREMIC TOXIN RESEARCH: Guanidino Compounds as Uremic (Neuro)Toxins. Semin. Dial. 2009, 22, 340–345. [Google Scholar] [CrossRef]
- Swahn, C.-G.; Sedvall, G. CSF creatinine in schizophrenia. Biol. Psychiatry 1988, 23, 586–594. [Google Scholar] [CrossRef]
- Chiappin, S.; Antonelli, G.; Gatti, R.; Elio, F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241–248. [Google Scholar]
- Suzuki, M.; Furuhashi, M.; Sesoko, S.; Kosuge, K.; Maeda, T.; Todoroki, K.; Inoue, K.; Min, J.Z.; Toyo’Oka, T. Determination of creatinine-related molecules in saliva by reversed-phase liquid chromatography with tandem mass spectrometry and the evaluation of hemodialysis in chronic kidney disease patients. Anal. Chim. Acta 2016, 911, 92–99. [Google Scholar] [CrossRef]
- Moore, N.P.; Gray, T.J.B.; Timbrell, J.A. Creatine metabolism in the seminiferous epithelium of rats. I. Creatine synthesis by isolated and cultured cells. Reproduction 1998, 112, 325–330. [Google Scholar] [CrossRef]
- Srivastava, A.; Chopra, S.; Dasgupta, P. Biochemical Analysis of Human Seminal Plasma II. Protein, Non-Protein Nitrogen, Urea, Uric Acid and Creatine. Andrologia 1984, 16, 265–268. [Google Scholar] [CrossRef]
- Allahkarami, S.; Atabakhsh, M.; Moradi, M.N.; Ghasemi, H.; Bahmanzadeh, M.; Tayebinia, H. Correlation of uric acid, urea, ammonia and creatinine of seminal plasma with semen parameters and fertilization rate of infertile couples. Avicenna J. Med. Biochem. 2017, 5, 76–80. [Google Scholar] [CrossRef]
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk—A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896. [Google Scholar] [CrossRef] [PubMed]
- Edison, E.E.; Brosnan, M.E.; Aziz, K.; Brosnan, J.T. Creatine and guanidinoacetate content of human milk and infant formulas: Implications for creatine deficiency syndromes and amino acid metabolism. Br. J. Nutr. 2013, 110, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Hülsemann, J.; Manz, F.; Wember, T.; Schöch, G. Die Zufuhr von Kreatin und Kreatinin mit Frauenmilch und Säuglingsmilchpräparaten. ISCAYAHL 2020 1987, 199, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Spevacek, A.R.; Smilowitz, J.T.; Chin, E.L.; A Underwood, M.; German, J.B.; Slupsky, C.M. Infant Maturity at Birth Reveals Minor Differences in the Maternal Milk Metabolome in the First Month of Lactation. J. Nutr. 2015, 145, 1698–1708. [Google Scholar] [CrossRef]
- Wu, J.; Domellöf, M.; Zivkovic, A.M.; Larsson, G.; Öhman, A.; Nording, M.L. NMR-based metabolite profiling of human milk: A pilot study of methods for investigating compositional changes during lactation. Biochem. Biophys. Res. Commun. 2016, 469, 626–632. [Google Scholar] [CrossRef]
- Peral, M.; Gálvez, M.; Soria, M.; Ilundáin, A. Developmental decrease in rat small intestinal creatine uptake. Mech. Ageing Dev. 2005, 126, 523–530. [Google Scholar] [CrossRef]
- Lempert, C. The Chemistry Of The Glycocyamidines. Chem. Rev. 1959, 59, 667–736. [Google Scholar] [CrossRef]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum Creatinine as an Index of Renal Function: New Insights into Old Concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [CrossRef]
- Cheng, R.H.C.; Majlessi, M.R.; Cheng, S.-C. Utilization of Barritt color reaction for studying synaptosomal creatine phosphokinase. Neurochem. Res. 1984, 9, 571–576. [Google Scholar] [CrossRef]
- Schulze, A.; Mayatepek, E.; Rating, D.; Bremer, H.J. Sakaguchi reaction: A useful method for screening guanidinoacetate-methyltransferase deficiency. J. Inherit. Metab. Dis. 1996, 19, 706. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Hess, T.; Wevers, R.; Mayatepek, E.; Bachert, P.; Marescau, B.; Knopp, M.V.; De Deyn, P.P.; Bremer, H.J.; Rating, D. Creatine deficiency syndrome caused by guanidinoacetate methyltransferase deficiency: Diagnostic tools for a new inborn error of metabolism. J. Pediatr. 1997, 131, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, M. Ueber den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Biol. Chem. 1886, 10, 391–400. [Google Scholar] [CrossRef]
- Moore, J.F.; Sharer, J.D. Methods for Quantitative Creatinine Determination. Curr. Protoc. Hum. Genet. 2017, 93, A.3O.1–A.3O.7. [Google Scholar] [CrossRef] [PubMed]
- Syme, N.R.; Stevens, K.; Stirling, C.; McMillan, D.C.; Talwar, D. Clinical and Analytical Impact of Moving from Jaffe to Enzymatic Serum Creatinine Methodology. J. Appl. Lab. Med. 2020, 5, 631–642. [Google Scholar] [CrossRef]
- Guder, W.G.; Hoffmann, G.F.; Price, C.P. Multicentre evaluation of an Enzymatic Methodfor creatinine Determination Using a Sensitive Colour Reagent. J. Clin. Chem. Clin. Biochem. 1986, 24, 889–902. [Google Scholar] [CrossRef]
- Bonvicini, P.; Ceriotti, G.; de’Besi, T. Kinetic Enzymatic Determination of Creatinine by a Rapid Semiautomated Procedure. CCLM 1982, 20, 185–190. [Google Scholar] [CrossRef]
- Beyer, C.; Hoenderdos, A.; Mairuhu, W.; van Eps, L.; Ende, A.v.D. Evaluative and comparative study of an enzymatic method using creatine kinase for the determination of urinary creatine. Clin. Chim. Acta 1984, 136, 263–270. [Google Scholar] [CrossRef]
- Schoenmakers, C.H.H.; Kuller, T.; Lindemans, J.; Blijenberg, B.G. Automated Enzymatic Methods for Creatinine Measurement with Special Attention to Bilirubin Interference. Clin. Chem. Lab. Med. 1993, 31, 861–868. [Google Scholar] [CrossRef]
- Knapp, M.L.; Mayne, P.D. Development of an automated kinetic Jaffé method designed to minimise bilirubin interference in plasma creatinine assays. Clin. Chim. Acta 1987, 168, 239–246. [Google Scholar] [CrossRef]
- Hunneman, D.H.; Hanefeld, F. GC-MS determination of guanidinoacetate in urine and plasma. J. Inherit. Metab. Dis. 1997, 20, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Valongo, C.; Cardoso, M.L.; Domingues, P.; Almeida, L.; Verhoeven, N.; Salomons, G.; Jakobs, C.; Vilarinho, L. Age related reference values for urine creatine and guanidinoacetic acid concentration in children and adolescents by gas chromatography–mass spectrometry. Clin. Chim. Acta 2004, 348, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.A.; Andrade, F.; Martín, S.; Sanjurjo, P.; Elorz, J.; Aldámiz-Echevarría, L. Determination of creatine and guanidinoacetate by GC-MS: Study of their stability in urine at different temperatures. Clin. Biochem. 2009, 42, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Wolf, A.; Mitschke, A.; Gutzki, F.-M.; Will, W.; Bader, M. GC–MS determination of creatinine in human biological fluids as pentafluorobenzyl derivative in clinical studies and biomonitoring: Inter-laboratory comparison in urine with Jaffé, HPLC and enzymatic assays. J. Chromatogr. B 2010, 878, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, Y.; Kinoshita, T. Post-column derivatization of guanidino compounds in high-performance liquid chromatography using ninhydrin. J. Chromatogr. B Biomed. Sci. Appl. 1981, 226, 43–51. [Google Scholar] [CrossRef]
- Marescaua, B.; Qureshi, I.; De Deyn, P.; Letarte, J.; Ryba, R.; Lowenthal, A. Guanidino compounds in plasma, urine and cerebrospinal fluid of hyperargininemic patients during therapy. Clin. Chim. Acta 1985, 146, 21–27. [Google Scholar] [CrossRef]
- Young, S.; Struys, E.; Wood, T. Quantification of Creatine and Guanidinoacetate Using GC-MS and LC-MS/MS for the Detection of Cerebral Creatine Deficiency Syndromes. Curr. Protoc. Hum. Genet. 2007, 54, 17.3.1–17.3.18. [Google Scholar] [CrossRef]
- Carling, R.S.; Hogg, S.L.; Wood, T.C.; Calvin, J. Simultaneous determination of guanidinoacetate, creatine and creatinine in urine and plasma by un-derivatized liquid chromatography-tandem mass spectrometry. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2008, 45, 575–584. [Google Scholar] [CrossRef]
- Boenzi, S.; Rizzo, C.; Di Ciommo, V.M.; Martinelli, D.; Goffredo, B.M.; la Marca, G.; Dionisi-Vici, C. Simultaneous determination of creatine and guanidinoacetate in plasma by liquid chromatography–tandem mass spectrometry (LC–MS/MS). J. Pharm. Biomed. Anal. 2011, 56, 792–798. [Google Scholar] [CrossRef]
- Carducci, C.; Birarelli, M.; Santagata, P.; Leuzzi, V.; Carducci, C.; Antonozzi, I. Automated high-performance liquid chromatographic method for the determination of guanidinoacetic acid in dried blood spots: A tool for early diagnosis of guanidinoacetate methyltransferase deficiency. J. Chromatogr. B Biomed. Sci. Appl. 2001, 755, 343–348. [Google Scholar] [CrossRef]
- Dash, A.K.; Sawhney, A. A simple LC method with UV detection for the analysis of creatine and creatinine and its application to several creatine formulations. J. Pharm. Biomed. Anal. 2002, 29, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, N.M.; Elabd, N.E.; Selim, L.A.; Abdou, D.M.; Griffin, J.L. Creatine Deficiency Syndromes: Comparison of Screening Methods and Characterization of Four Novel Intronic Variants. Clin. Chim. Acta 2022, 536, 70–76. [Google Scholar] [CrossRef] [PubMed]


| Different Physiological Conditions | Biomarker Concentration in Blood |
|---|---|
| Creatine | |
| Normal | 62.0 ± 24.3 µM ♂ 63.1 ± 37.1 µM ♀ |
| AGAT deficiency | 0–7 µM |
| GAMT deficiency | 15–29 µM (combined with creatinine) |
| CRT deficiency | Not altered |
| UCD | Slightly decreased (28–100 µM) |
| Neuromuscular diseases | Increased * |
| Gyrate atrophy of the choroid and retina | Decreased * |
| GAA | |
| Normal | 1.5 ± 0.6 µM ♂ 1.4 ± 0.6 µM ♀ |
| AGAT deficiency | 0.01–0.04 µM |
| GAMT deficiency | 12–39 µM |
| CRT deficiency | Not altered |
| Uremia | Decreased * |
| Untreated epilepsy | Decreased * |
| UCD | Decreased * |
| Gyrate atrophy of the choroid and retina | Decreased (up to five-fold) * |
| Creatinine | |
| Normal | 63–107 µM ♂ 46–103 µM ♀ |
| AGAT deficiency | N/A |
| GAMT deficiency | N/A |
| CRT deficiency | N/A |
| CKD | Normal to greatly increased (depending on the stage) * |
| Different Physiological Conditions | Biomarker Concentration in Urine |
|---|---|
| Creatine | |
| Normal | 344.8 ± 350.5 mmol/mol Crn ♂ 371.8 ± 345.5 mmol/mol Crn ♀ |
| AGAT deficiency | 0–7 µM |
| GAMT deficiency | Normal to slightly decreased * |
| CRT deficiency | Greatly increased * |
| GAA | |
| Normal | 61.6 ± 37.8 mmol/mol Crn ♂ 73.1 ± 47.5 mmol/mol Crn ♀ |
| AGAT deficiency | 2.4–5.8 mmol/mol Crn |
| GAMT deficiency | 529–4368 mmol/mol Crn |
| CRT deficiency | Not altered |
| Different Physiological Conditions | Biomarker Concentration in CSF |
|---|---|
| Creatine | |
| Normal | 17–87 µM |
| AGAT deficiency | N/A |
| GAMT deficiency | <2 µM |
| CRT deficiency | Normal to slightly increased * |
| GAA | |
| Normal | 0.02–0.56 µM |
| AGAT deficiency | N/A |
| GAMT deficiency | ~14 µM |
| CRT deficiency | Normal to slightly increased |
| Creatinine | |
| Normal | 30–130 µM |
| AGAT deficiency | N/A |
| GAMT deficiency | 4.5 µM |
| CRT deficiency | N/A |
| Schizophrenia | Decreased * |
| Renal insufficiency | 168–521 µM |
| Different Physiological Conditions | Biomarker Concentration in Saliva |
|---|---|
| Creatine | |
| Normal | 9.24 ± 2.76 µM ♂ (aged 15+) 10.67 ± 5.07 µM ♀ (aged 15+) |
| AGAT deficiency | N/A |
| GAMT deficiency | N/A |
| CRT deficiency | N/A |
| CKD | 14.49 ± 9.15 µM |
| GAA | |
| Normal | 2.38 ± 1.44 µM ♂ (aged 15+) 2.69 ± 1.60 µM ♀ (aged 15+) |
| AGAT deficiency | N/A |
| GAMT deficiency | N/A |
| CRT deficiency | N/A |
| Creatinine | |
| Normal | 4.59 ± 4.33 µM |
| AGAT deficiency | N/A |
| GAMT deficiency | N/A |
| CRT deficiency | N/A |
| CKD | 64.53 ± 61.88 µM |
| Different Physiological Conditions | Biomarker Concentration in Semen |
|---|---|
| Creatine | |
| Normal (normozoospermia) | 791 ± 342 µM 1 1818 µM 2 |
| Asthenozoospermia | Normal 1 |
| Azoospermia | 1249 µM 2 |
| Vasectomy | 544 µM 2 |
| GAA | |
| Normal | N/A |
| Creatinine | |
| Normal | 316.48 ± 99.89 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedeljkovic, D.D.; Ostojic, S.M. Biomarkers of Creatine Metabolism in Humans: From Plasma to Saliva and Beyond. Clin. Bioenerg. 2025, 1, 2. https://doi.org/10.3390/clinbioenerg1010002
Nedeljkovic DD, Ostojic SM. Biomarkers of Creatine Metabolism in Humans: From Plasma to Saliva and Beyond. Clinical Bioenergetics. 2025; 1(1):2. https://doi.org/10.3390/clinbioenerg1010002
Chicago/Turabian StyleNedeljkovic, David D., and Sergej M. Ostojic. 2025. "Biomarkers of Creatine Metabolism in Humans: From Plasma to Saliva and Beyond" Clinical Bioenergetics 1, no. 1: 2. https://doi.org/10.3390/clinbioenerg1010002
APA StyleNedeljkovic, D. D., & Ostojic, S. M. (2025). Biomarkers of Creatine Metabolism in Humans: From Plasma to Saliva and Beyond. Clinical Bioenergetics, 1(1), 2. https://doi.org/10.3390/clinbioenerg1010002







