Determination of Gamma-Glutamylcysteine Ethyl Ester Efficacy via Enzymatic Analysis in Moderate Traumatic Brain Injury
Abstract
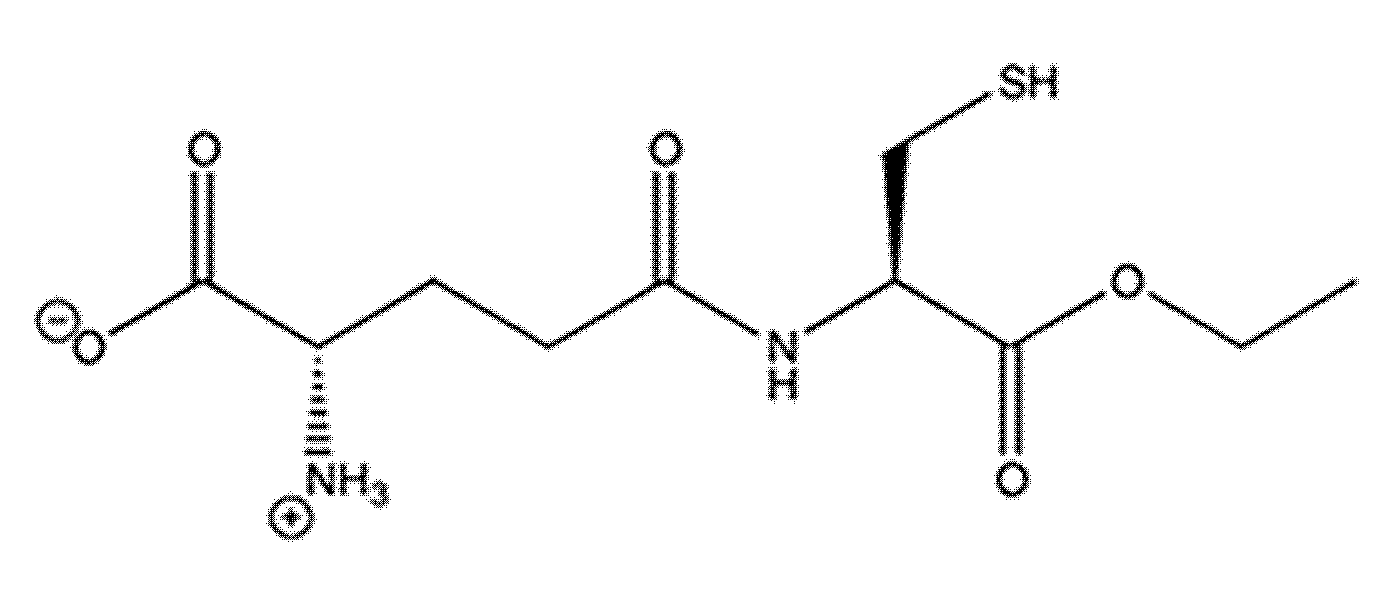
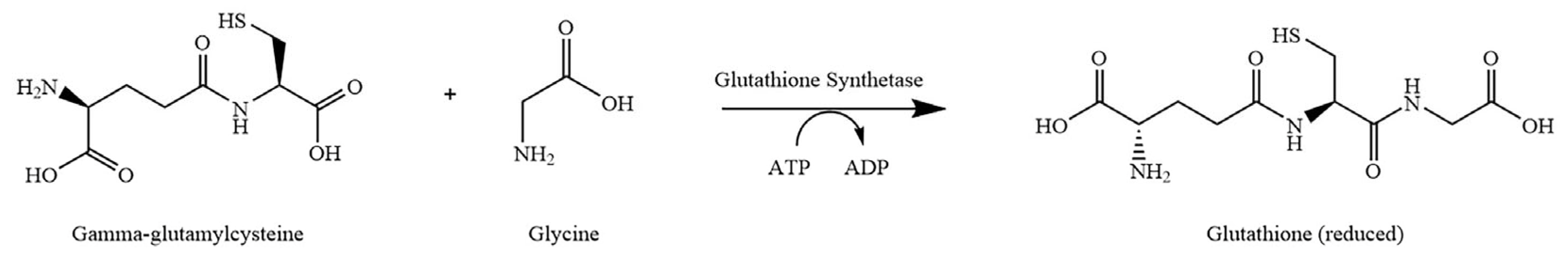
1. Introduction
2. Materials and Methods
2.1. Surgical Methods
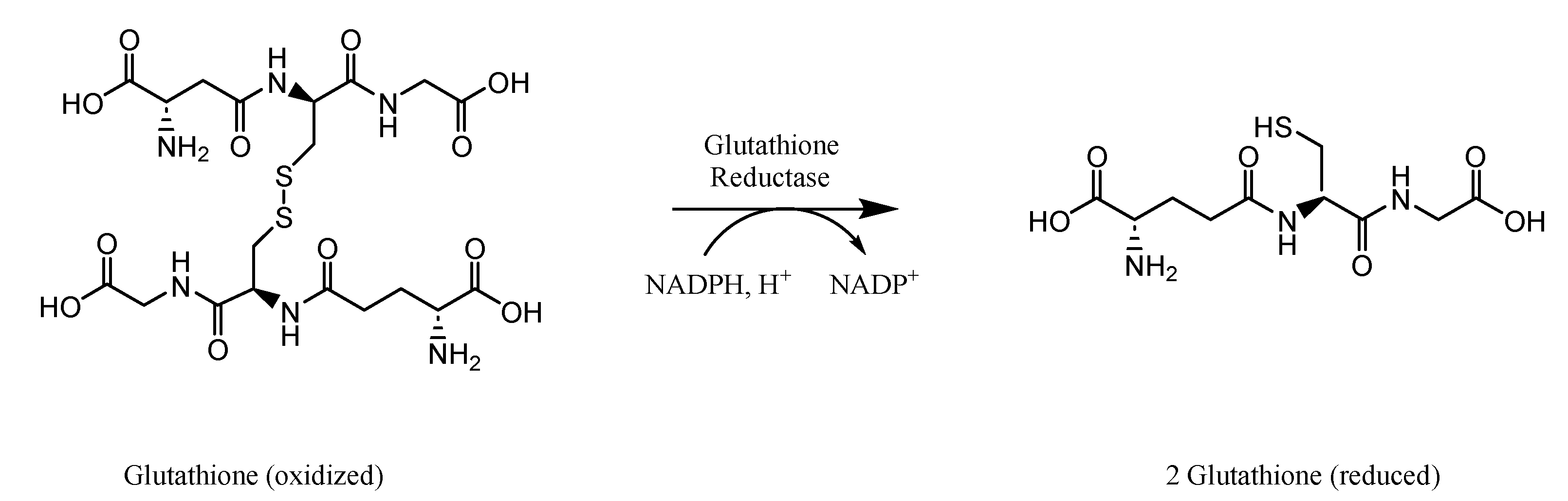
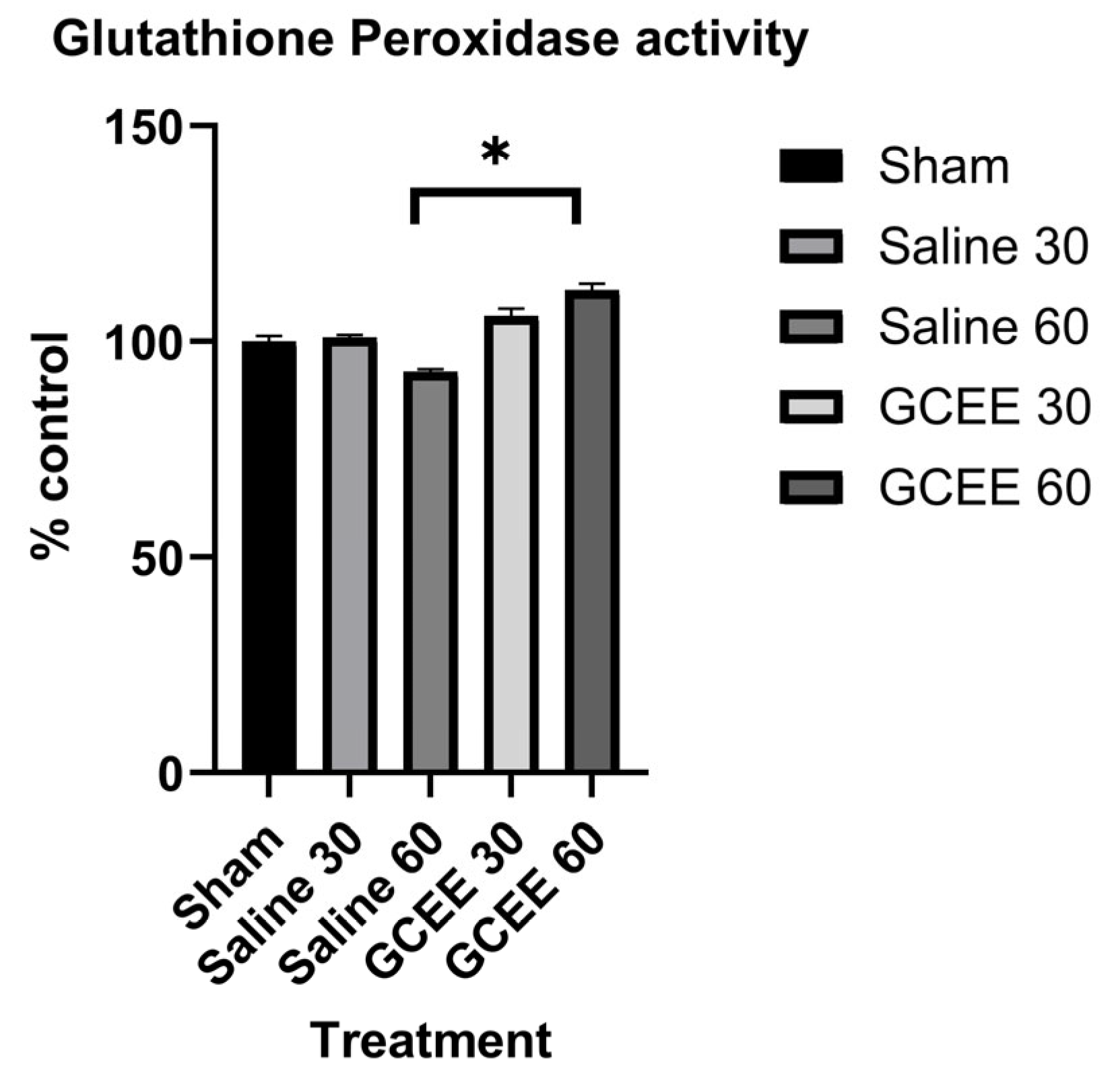
2.2. Analysis of Glutathione Peroxidase Activity
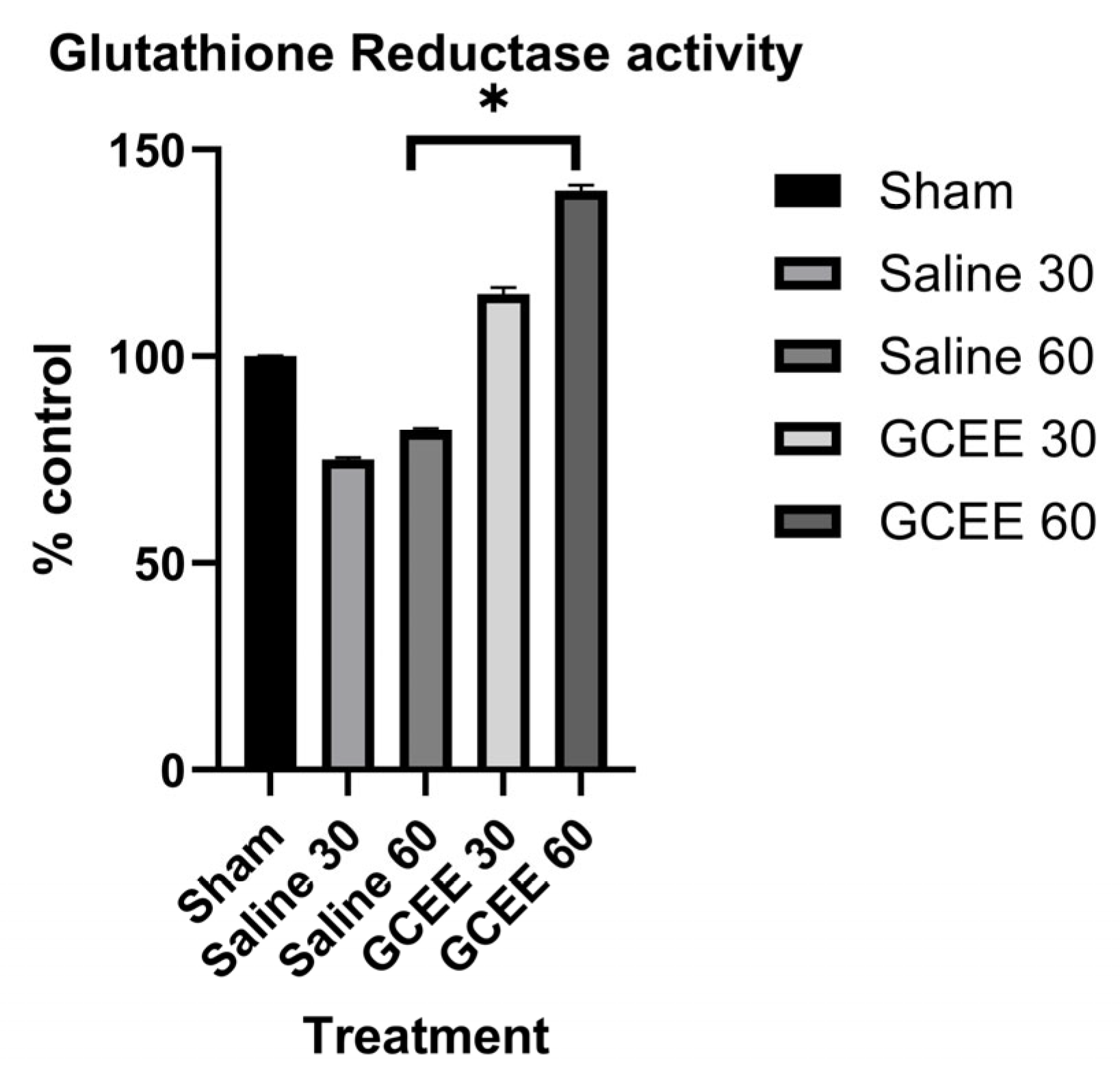
2.3. Analysis of Glutathione Reductase Activity
2.4. Gene Expression Analysis Sample Preparation
2.5. RNA Purity and Concentration Determination
2.6. Converting RNA to cDNA
2.7. TaqMan PCR (qRT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Enzymatic Analysis
3.2. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GCEE | gamma-glutamylcysteine ethyl ester |
| GPx | glutathione peroxidase |
| GPX1 | glutathione peroxidase 1 |
| GPX4 | glutathione peroxidase 4 |
| GR/GSR | glutathione reductase |
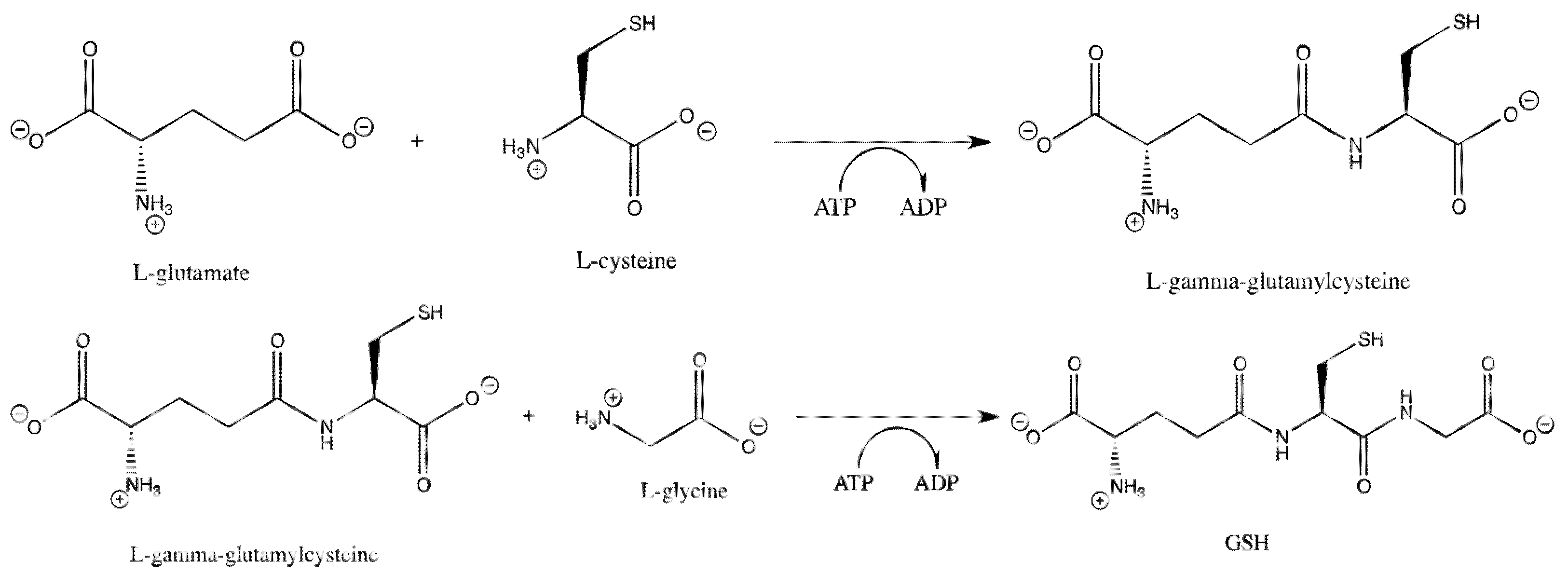
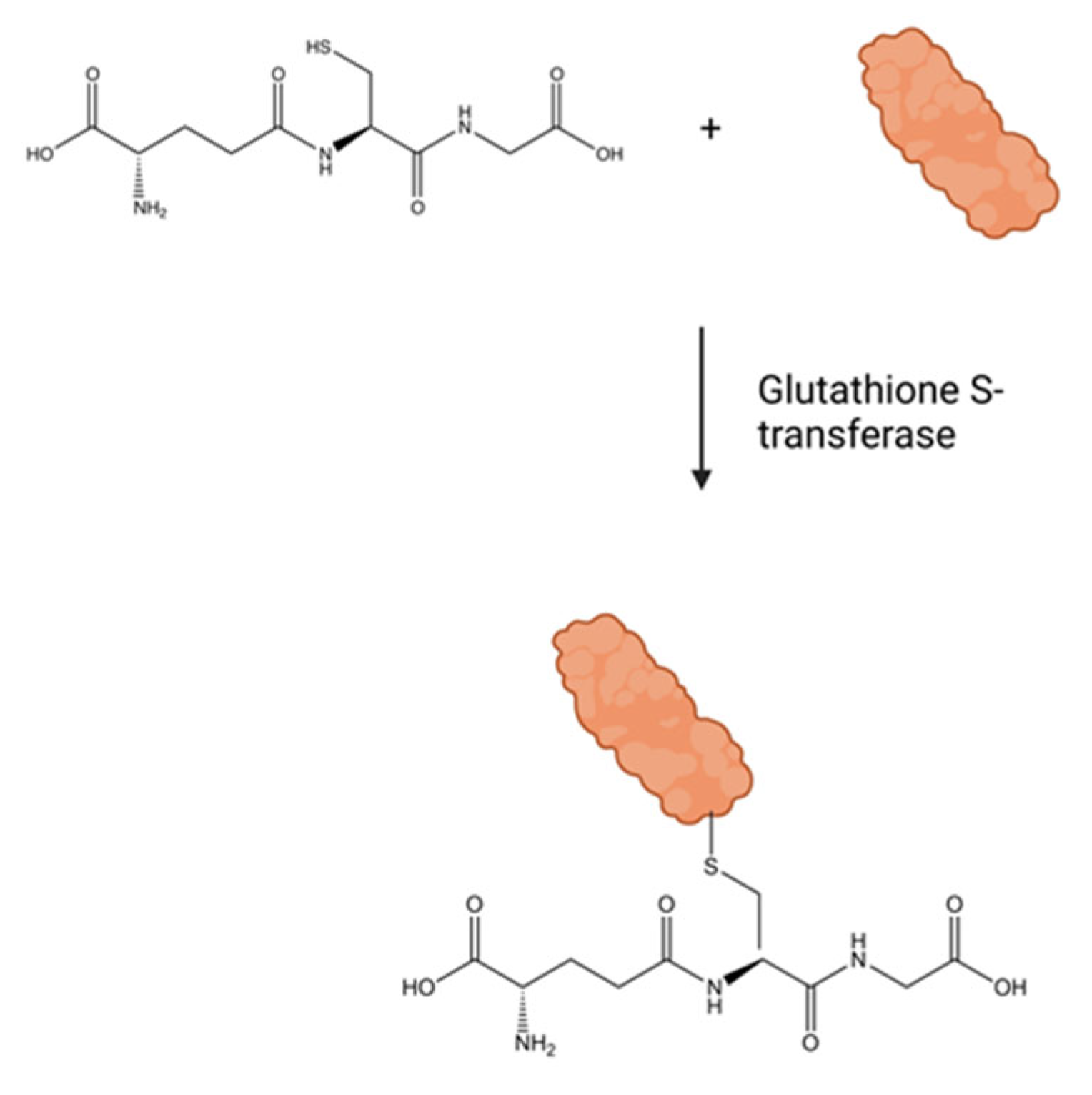
| GSH | glutathione |
| GSSG | glutathione disulfide |
| GSTT1 | glutathione S-transferase 1 |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| TBI | traumatic brain injury |
References
- Sellers, Z.P.; Williams, R.A.; Overbay, J.W.; Cho, J.; Henderson, M.; Reed, T.T. Current Therapeutic Modalities, Enzyme Kinetics, and Redox Proteomics in Traumatic Brain Injury. In Traumatic Brain Injury; InTechOpen: London, UK, 2014; pp. 39–77. [Google Scholar]
- Stelfa, G.; Svalbe, B.; Vavers, E.; Duritis, I.; Dambrova, M.; Zvejniece, L. Moderate traumatic brain injury triggers long-term risks for the development of peripheral pain sensitivity and depressive-like behavior in mice. Front. Neurol. 2022, 13, 985895. [Google Scholar] [CrossRef] [PubMed]
- Lannsjo, M.; Borg, J.; Lewen, A.; von Seth, C.; Enblad, P.; Abu Hamdeh, S. Long-Term Outcomes of Moderate to Severe Diffuse Axonal Traumatic Brain Injury: A Prospective Study. J. Rehabil. Med. Clin. Commun. 2025, 8, 42299. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.; Johnson, L.; Ashpole, R.; Mamo, A.; Chagpar, S.; Williams, G.; Clanchy, K.; Waters, N.; Vassallo, G.; Scheinberg, A.; et al. The long road back to physical activity: The experience of people with moderate-to-severe traumatic brain injury. Disabil. Rehabil. 2025, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bigler, E.D. Distinguished Neuropsychologist Award Lecture 1999. The lesion(s) in traumatic brain injury: Implications for clinical neuropsychology. Arch. Clin. Neuropsychol. 2001, 16, 95–131. [Google Scholar] [CrossRef]
- Weber, J.T. Altered calcium signaling following traumatic brain injury. Front. Pharmacol. 2012, 3, 60. [Google Scholar] [CrossRef]
- Surgucheva, I.; He, S.; Rich, M.C.; Sharma, R.; Ninkina, N.N.; Stahel, P.F.; Surguchov, A. Role of synucleins in traumatic brain injury—An experimental in vitro and in vivo study in mice. Mol. Cell Neurosci. 2014, 63, 114–123. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Sultana, R. Identification of 3-Nitrotyosine Modified Brain Proteins byRedox Proteomics. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 440, pp. 295–308. [Google Scholar]
- Dai, W.; Cheng, H.L.; Huang, R.Q.; Zhuang, Z.; Shi, J.X. Quantitative detection of the expression of mitochondrial cytochrome c oxidase subunits mRNA in the cerebral cortex after experimental traumatic brain injury. Brain Res. 2009, 1251, 287–295. [Google Scholar] [CrossRef]
- Hall, E.D.; Vaishnav, R.A.; Mustafa, A.G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics 2010, 7, 51–61. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol. Res. Health 2003, 27, 277–284. [Google Scholar]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free. Radic. Biol. Med. 2008, 45, 443–452. [Google Scholar] [CrossRef]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Pocernich, C.B.; Cardin, A.L.; Racine, C.L.; Lauderback, C.M.; Butterfield, D.A. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: Relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem. Int. 2001, 39, 141–149. [Google Scholar] [CrossRef]
- Joshi, G.; Aluise, C.D.; Cole, M.P.; Sultana, R.; Pierce, W.M.; Vore, M.; St Clair, D.K.; Butterfield, D.A. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: Implications for oxidative stress-mediated chemobrain. Neuroscience 2010, 166, 796–807. [Google Scholar] [CrossRef]
- Lok, J.; Leung, W.; Zhao, S.; Pallast, S.; van Leyen, K.; Guo, S.; Wang, X.; Yalcin, A.; Lo, E.H. gamma-glutamylcysteine ethyl ester protects cerebral endothelial cells during injury and decreases blood-brain barrier permeability after experimental brain trauma. J. Neurochem. 2011, 118, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Satou, T.; Nishida, S.; Tsubaki, M.; Hashimoto, S.; Ito, H. The novel free radical scavenger, edaravone, increases neural stem cell number around the area of damage following rat traumatic brain injury. Neurotox. Res. 2009, 16, 378–389. [Google Scholar] [CrossRef]
- Singh, I.N.; Sullivan, P.G.; Hall, E.D. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res. 2007, 85, 2216–2223. [Google Scholar] [CrossRef]
- Mohan, M.C.; Anjana, A.S.; Hilmi Jaufer, T.A.; Deepti, A.; Krishnakumar, I.M.; Baby Chakrapani, P.S. Co-delivery of curcumin-resveratrol-carnosic acid complex promotes neurogenesis and cognitive recovery in a rodent model of repeated mild traumatic brain injury. Biomed. Pharmacother. 2025, 183, 117818. [Google Scholar] [CrossRef]
- Park, M.K.; Choi, B.Y.; Kho, A.R.; Lee, S.H.; Hong, D.K.; Kang, B.S.; Lee, C.J.; Yang, H.W.; Woo, S.Y.; Park, S.W.; et al. L-theanine ameliorates traumatic-brain-injury-induced hippocampal neuronal death in rats. Phytomedicine 2025, 139, 156457. [Google Scholar] [CrossRef]
- Tanhai, G.; Chahardehi, A.M.; Sohrabi, M.A.; Afshoon, M.; Saberian, P.; Pourshams, M.; Ghasemi, D.; Motaghi, S.M.; Arefnezhad, R.; Niknam, Z. Ameliorative properties of quercetin in the treatment of traumatic brain injury: A mechanistic review based on underlying mechanisms. Mol. Biol. Rep. 2024, 51, 695. [Google Scholar] [CrossRef]
- Dash, P.K.; Orsi, S.A.; Zhang, M.; Grill, R.J.; Pati, S.; Zhao, J.; Moore, A.N. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS ONE 2010, 5, e11383. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tong, J.; Zhang, J.; Farahvar, A.; Wang, E.; Yang, J.; Samadani, U.; Smith, D.H.; Huang, J.H. Imipramine treatment improves cognitive outcome associated with enhanced hippocampal neurogenesis after traumatic brain injury in mice. J. Neurotrauma 2011, 28, 995–1007. [Google Scholar] [CrossRef]
- Ochalski, P.G.; Fellows-Mayle, W.; Hsieh, L.B.; Srinivas, R.; Okonkwo, D.O.; Dixon, C.E.; Adelson, P.D. Flumazenil administration attenuates cognitive impairment in immature rats after controlled cortical impact. J. Neurotrauma 2010, 27, 647–651. [Google Scholar] [CrossRef]
- Tu, Y.; Han, D.; Liu, Y.; Hong, D.; Chen, R. Nicorandil attenuates cognitive impairment after traumatic brain injury via inhibiting oxidative stress and inflammation: Involvement of BDNF and NGF. Brain Behav. 2024, 14, e3356. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhu, H.Y.; Wang, X.L.; Lu, X.W.; Pan, C.L.; Xu, L.; Liu, X.; Xu, N.; Zhang, Z.Y. Oridonin Ameliorates Traumatic Brain Injury-Induced Neurological Damage by Improving Mitochondrial Function and Antioxidant Capacity and Suppressing Neuroinflammation through the Nrf2 Pathway. J. Neurotrauma 2022, 39, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Keller, J.N.; Bussen, W.L.; Scheff, S.W. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res. 2002, 949, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Djebaili, M.; Guo, Q.; Pettus, E.H.; Hoffman, S.W.; Stein, D.G. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma 2005, 22, 106–118. [Google Scholar] [CrossRef]
- Joshi, G.; Hardas, S.; Sultana, R.; St Clair, D.K.; Vore, M.; Butterfield, D.A. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. J. Neurosci. Res. 2007, 85, 497–503. [Google Scholar] [CrossRef]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J. Neurotrauma 2008, 25, 513–526. [Google Scholar] [CrossRef]
- Goss, J.R.; Taffe, K.M.; Kochanek, P.M.; DeKosky, S.T. The antioxidant enzymes glutathione peroxidase and catalase increase following traumatic brain injury in the rat. Exp. Neurol. 1997, 146, 291–294. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Yuan, Q.; Yu, J.; Hu, J. GPX3 as a Novel and Potential Therapeutic Target in the Shared Molecular Mechanisms of Traumatic Brain Injury and Parkinson’s Disease. J. Inflamm. Res. 2025, 18, 1911–1928. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.; Rice, B.; Sebastian, A.; Sullivan, P.G.; King, C.; Robinson, R.A.; Reed, T.T. Neuroproteomic study of nitrated proteins in moderate traumatic brain injured rats treated with gamma glutamyl cysteine ethyl ester administration post injury: Insight into the role of glutathione elevation in nitrosative stress. Proteom. Clin. Appl. 2016, 10, 1218–1224. [Google Scholar] [CrossRef]
- Reed, T.T.; Owen, J.; Pierce, W.M.; Sebastian, A.; Sullivan, P.G.; Butterfield, D.A. Proteomic identification of nitrated brain proteins in traumatic brain-injured rats treated postinjury with gamma-glutamylcysteine ethyl ester: Insights into the role of elevation of glutathione as a potential therapeutic strategy for traumatic brain injury. J. Neurosci. Res. 2009, 87, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Matney, C.; Bowman, K.; Berwick, D. (Eds.) Traumatic Brain Injury: A Roadmap for Accelerating Progress; The National Academies Press: Washington, DC, USA, 2022. [Google Scholar]
- Dismuke-Greer, C.E.; Esmaeili, A.; Pugh, M.J.; Pogoda, T.K.; Amuan, M.; Cifu, D.X. Association of Clinical TBI Severity and Military Factors With Veteran TBI Service-Connected Disability Ratings and Total Compensation: A Long-Term Impact of Military Brain Injury Consortium (LIMBIC) Study. J. Head Trauma Rehabil. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Clark, D.; Joannides, A.; Adeleye, A.O.; Bajamal, A.H.; Bashford, T.; Biluts, H.; Budohoski, K.; Ercole, A.; Fernandez-Mendez, R.; Figaji, A.; et al. Casemix, management, and mortality of patients rreseceiving emergency neurosurgery for traumatic brain injury in the Global Neurotrauma Outcomes Study: A prospective observational cohort study. Lancet Neurol. 2022, 21, 438–449. [Google Scholar] [CrossRef]
- Orman, J.A.; Geyer, D.; Jones, J.; Schneider, E.B.; Grafman, J.; Pugh, M.J.; Dubose, J. Epidemiology of moderate-to-severe penetrating versus closed traumatic brain injury in the Iraq and Afghanistan wars. J. Trauma Acute Care Surg. 2012, 73, S496–S502. [Google Scholar] [CrossRef]
- Rutland-Brown, W.; Langlois, J.A.; Thomas, K.E.; Xi, Y.L. Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 2006, 21, 544–548. [Google Scholar] [CrossRef]
- Dasic, D.; Morgan, L.; Panezai, A.; Syrmos, N.; Ligarotti, G.K.I.; Zaed, I.; Chibbaro, S.; Khan, T.; Prisco, L.; Ganau, M. A scoping review on the challenges, improvement programs, and relevant output metrics for neurotrauma services in major trauma centers. Surg. Neurol. Int. 2022, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Detloff, M.R.; Johnson, K.; Kupina, N.C. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma 2004, 21, 9–20. [Google Scholar] [CrossRef]
- Opii, W.O.; Nukala, V.N.; Sultana, R.; Pandya, J.D.; Day, K.M.; Merchant, M.L.; Klein, J.B.; Sullivan, P.G.; Butterfield, D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma 2007, 24, 772–789. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, X.; Pu, H.; Zhang, W.; An, C.; Hu, X.; Liou, A.K.; Leak, R.K.; Gao, Y.; Chen, J. Scriptaid, a Novel Histone Deacetylase Inhibitor, Protects Against Traumatic Brain Injury via Modulation of PTEN and AKT Pathway: Scriptaid Protects Against TBI via AKT. Neurotherapeutics 2013, 10, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Loetscher, P.D.; Rossaint, R.; Fahlenkamp, A.V.; Eberhardt, G.; Rex, S.; Weis, J.; Coburn, M. Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury. BMC Neurol. 2012, 12, 20. [Google Scholar] [CrossRef]
- Scheff, S.W.; Ansari, M.A.; Roberts, K.N. Neuroprotective effect of Pycnogenol(R) following traumatic brain injury. Exp. Neurol. 2013, 239, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Jiang, Z.L.; Li, Y.C.; Li, X.; Shi, H.; Gao, Y.Q.; Vosler, P.S.; Chen, J. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J. Neurotrauma 2011, 28, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Zhao, J.; Orsi, S.A.; Zhang, M.; Moore, A.N. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 2009, 460, 103–107. [Google Scholar] [CrossRef]
- Drake, J.; Kanski, J.; Varadarajan, S.; Tsoras, M.; Butterfield, D.A. Elevation of brain glutathione by gamma-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. J. Neurosci. Res. 2002, 68, 776–784. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Cornford, E.M.; Braun, L.D.; Crane, P.D.; Oldendorf, W.H. Blood-brain barrier restriction of peptides and the low uptake of enkephalins. Endocrinology 1978, 103, 1297–1303. [Google Scholar] [CrossRef]
- Itoh, T.; Tabuchi, M.; Mizuguchi, N.; Imano, M.; Tsubaki, M.; Nishida, S.; Hashimoto, S.; Matsuo, K.; Nakayama, T.; Ito, A.; et al. Neuroprotective effect of (-)-epigallocatechin-3-gallate in rats when administered pre- or post-traumatic brain injury. J. Neural Transm. 2012, 120, 767–783. [Google Scholar] [CrossRef]


| Treatment | GPX1 | GSTT1 | GR |
|---|---|---|---|
| Saline to Sham 30 | 34.968 * | 43.008 | 7.198 * |
| GCEE to Sham 30 | 12.943 * | 21.279 | 3.479 |
| Saline to GCEE 30 | 2.702 * | 2.021 * | 2.069 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overbay, J.; Johnson, J.T.; Sellers, Z.P.; Williams, R.; Henderson, M.; Kalantar, A.; Sebastian, A.; Sullivan, P.G.; Reed, T.T. Determination of Gamma-Glutamylcysteine Ethyl Ester Efficacy via Enzymatic Analysis in Moderate Traumatic Brain Injury. Clin. Bioenerg. 2025, 1, 3. https://doi.org/10.3390/clinbioenerg1010003
Overbay J, Johnson JT, Sellers ZP, Williams R, Henderson M, Kalantar A, Sebastian A, Sullivan PG, Reed TT. Determination of Gamma-Glutamylcysteine Ethyl Ester Efficacy via Enzymatic Analysis in Moderate Traumatic Brain Injury. Clinical Bioenergetics. 2025; 1(1):3. https://doi.org/10.3390/clinbioenerg1010003
Chicago/Turabian StyleOverbay, Jonathan, Joseph T. Johnson, Zachariah P. Sellers, ReBecca Williams, Moses Henderson, Alborz Kalantar, Andrea Sebastian, Patrick G. Sullivan, and Tanea T. Reed. 2025. "Determination of Gamma-Glutamylcysteine Ethyl Ester Efficacy via Enzymatic Analysis in Moderate Traumatic Brain Injury" Clinical Bioenergetics 1, no. 1: 3. https://doi.org/10.3390/clinbioenerg1010003
APA StyleOverbay, J., Johnson, J. T., Sellers, Z. P., Williams, R., Henderson, M., Kalantar, A., Sebastian, A., Sullivan, P. G., & Reed, T. T. (2025). Determination of Gamma-Glutamylcysteine Ethyl Ester Efficacy via Enzymatic Analysis in Moderate Traumatic Brain Injury. Clinical Bioenergetics, 1(1), 3. https://doi.org/10.3390/clinbioenerg1010003







