A Review of the Sustainability of Helium: An Assessment of Its Past, Present and a Zero-Carbon Future
Abstract
:1. Introduction
2. Review Methodology
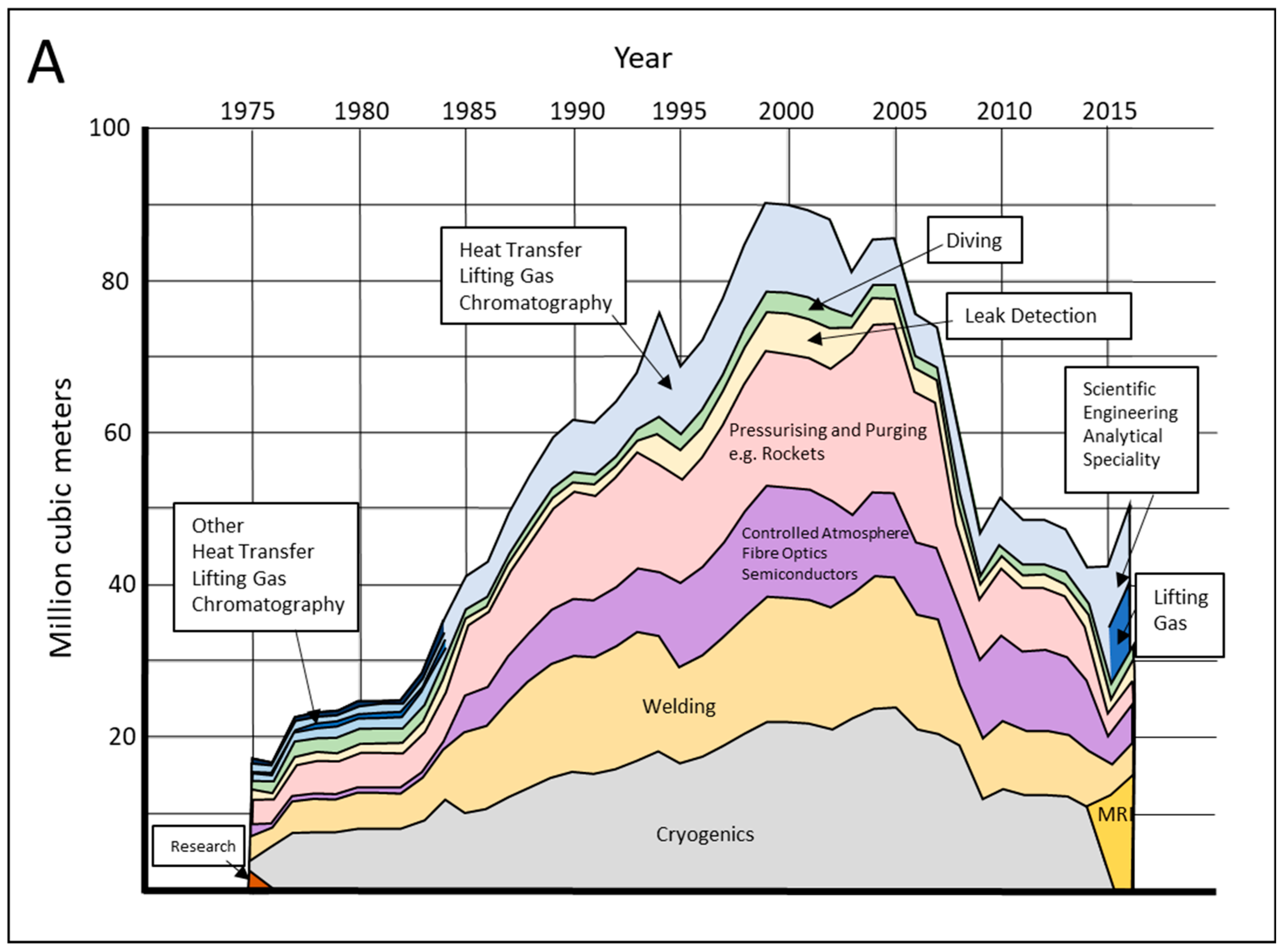
3. Uses of Helium
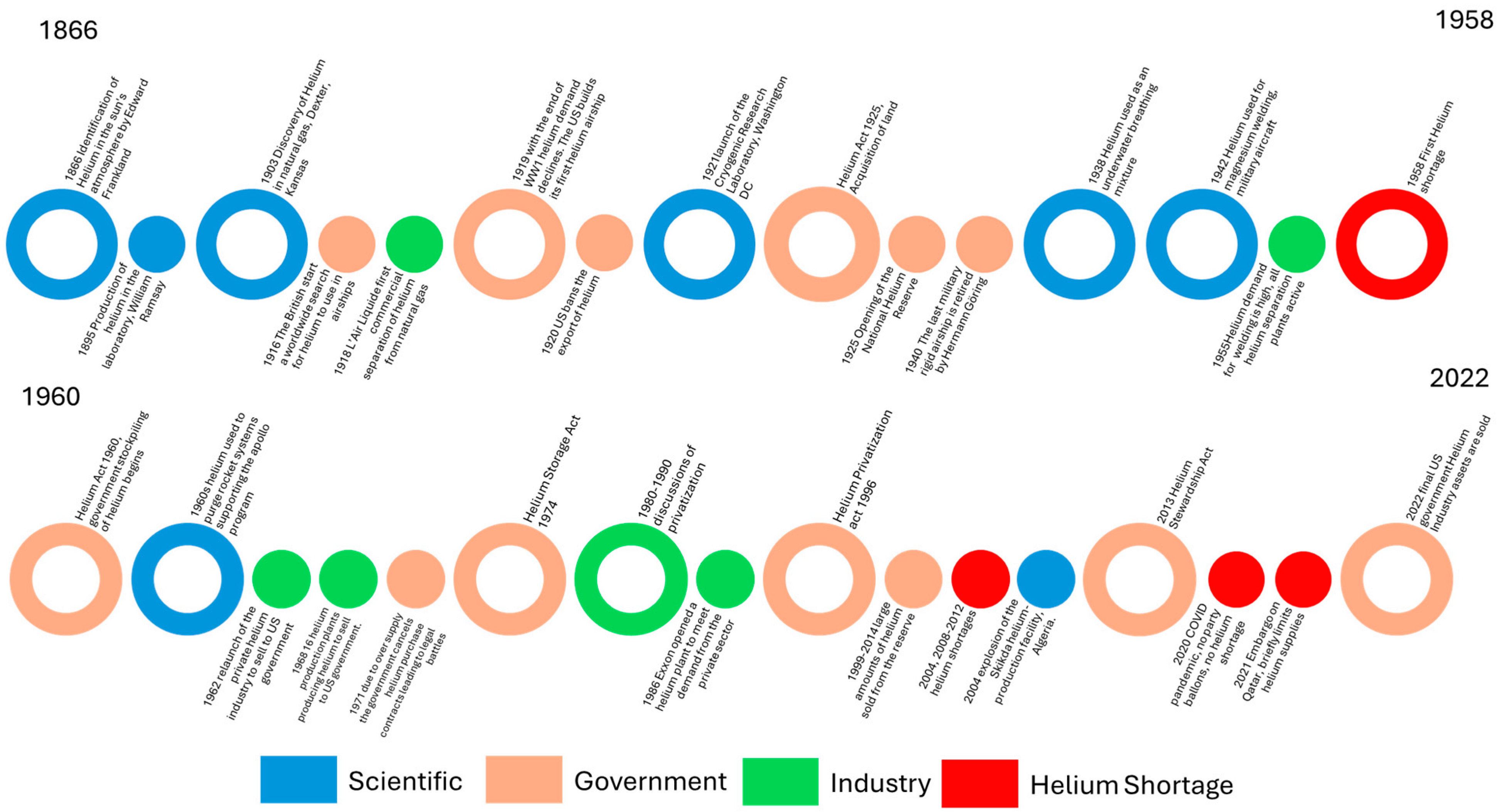
4. The History of the Helium Industry
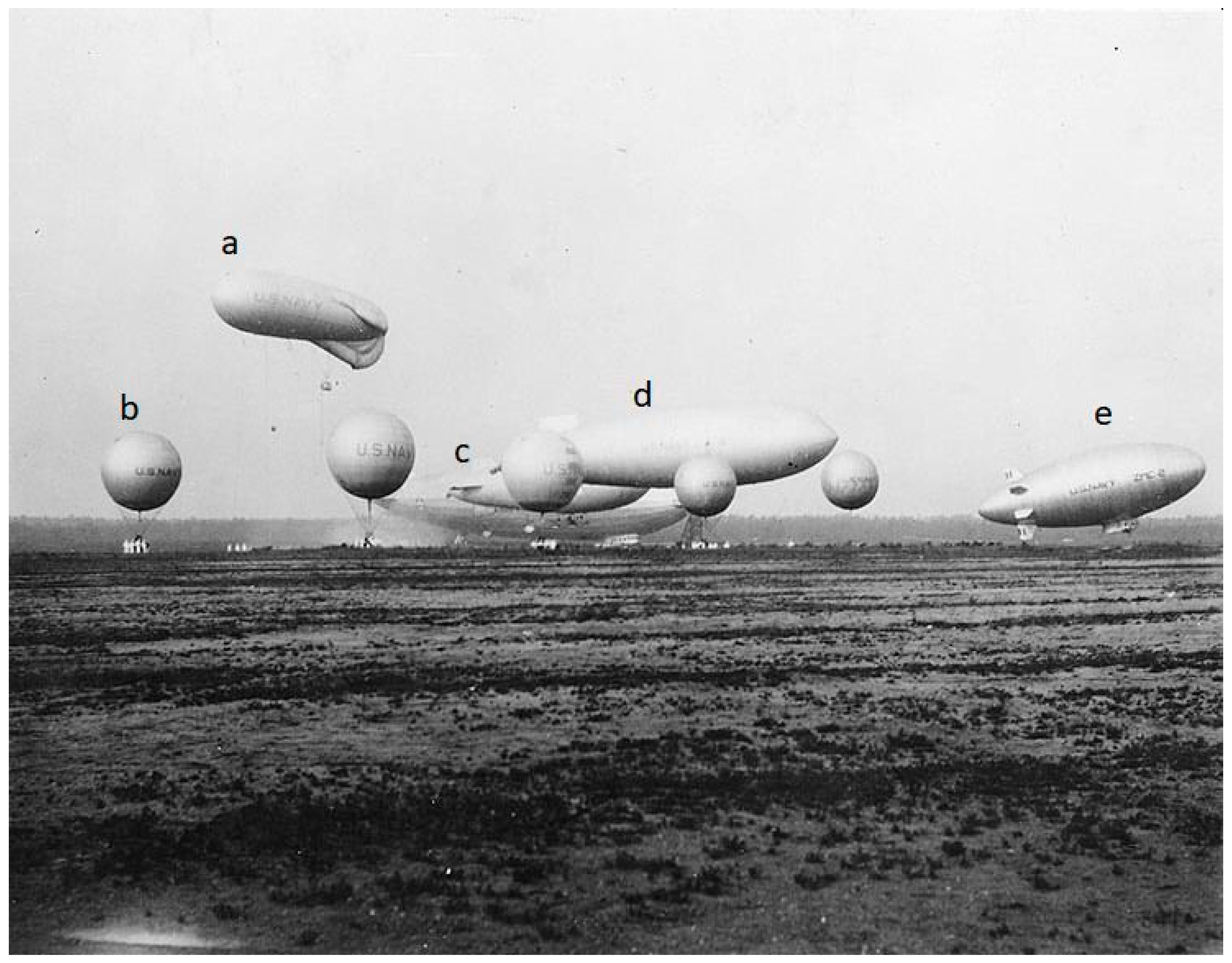
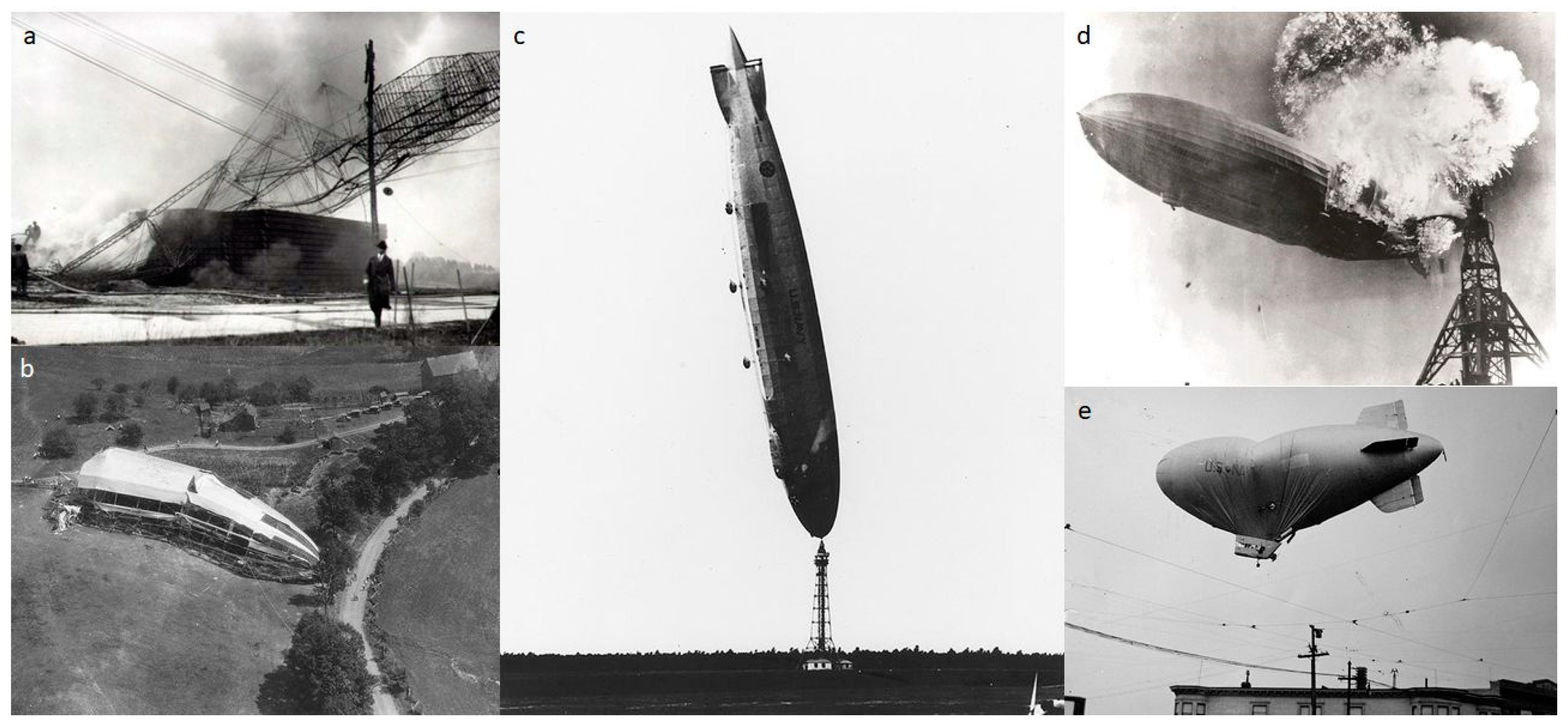
4.1. The Rise and Fall of the Helium Airship Industry (1903–1937)
“The adoption of this amendment will effect the conservation of helium, the non-combustible gas, most essential in the use of dirigibles”.
“Helium, would come under a proviso of this kind, and as helium is now being developed in certain large quantities on a commercial basis, and as that gas is used largely in the Army and Navy dirigible work”.
“This is an industry which is needed in times of peace and war. Helium tends to afford, in peace, safety from accident; in war, safety from attack. Both in peace and war, therefore, many lives will be saved because of the use of it”.
“Provided, that all right, title, and interest to all helium in the lands or deposits subject to disposition under this act are hereby expressly reserved and shall remain in the Government of the United States”.
4.2. A Vision of a Technological Future for Helium (1937–1970)
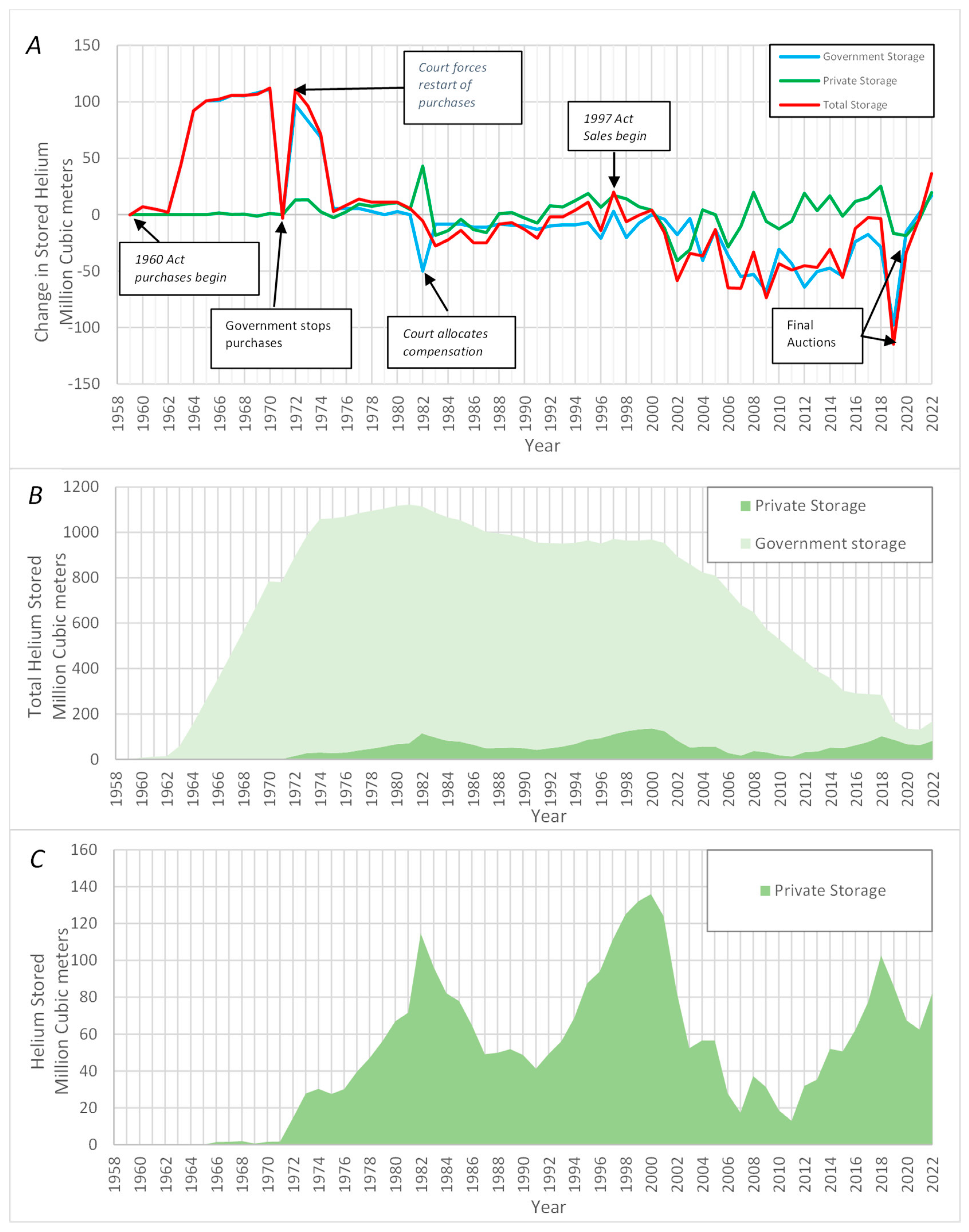
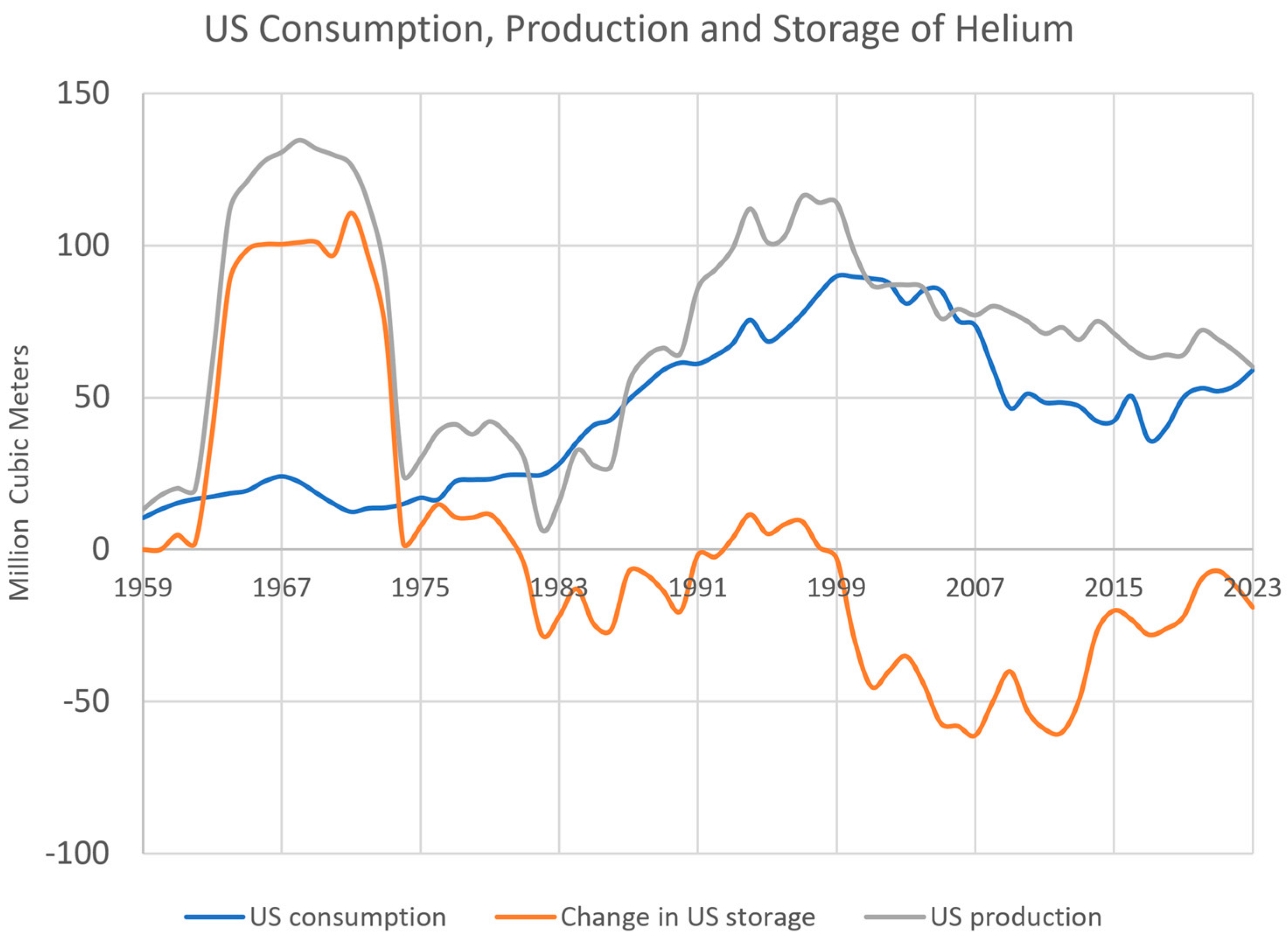
4.3. The Decline of the Helium Industry (1970–1990)
“If natural gases were not now being used for fuel, there would be no controversy over helium conservation. The helium would simply remain stored in the earth”.
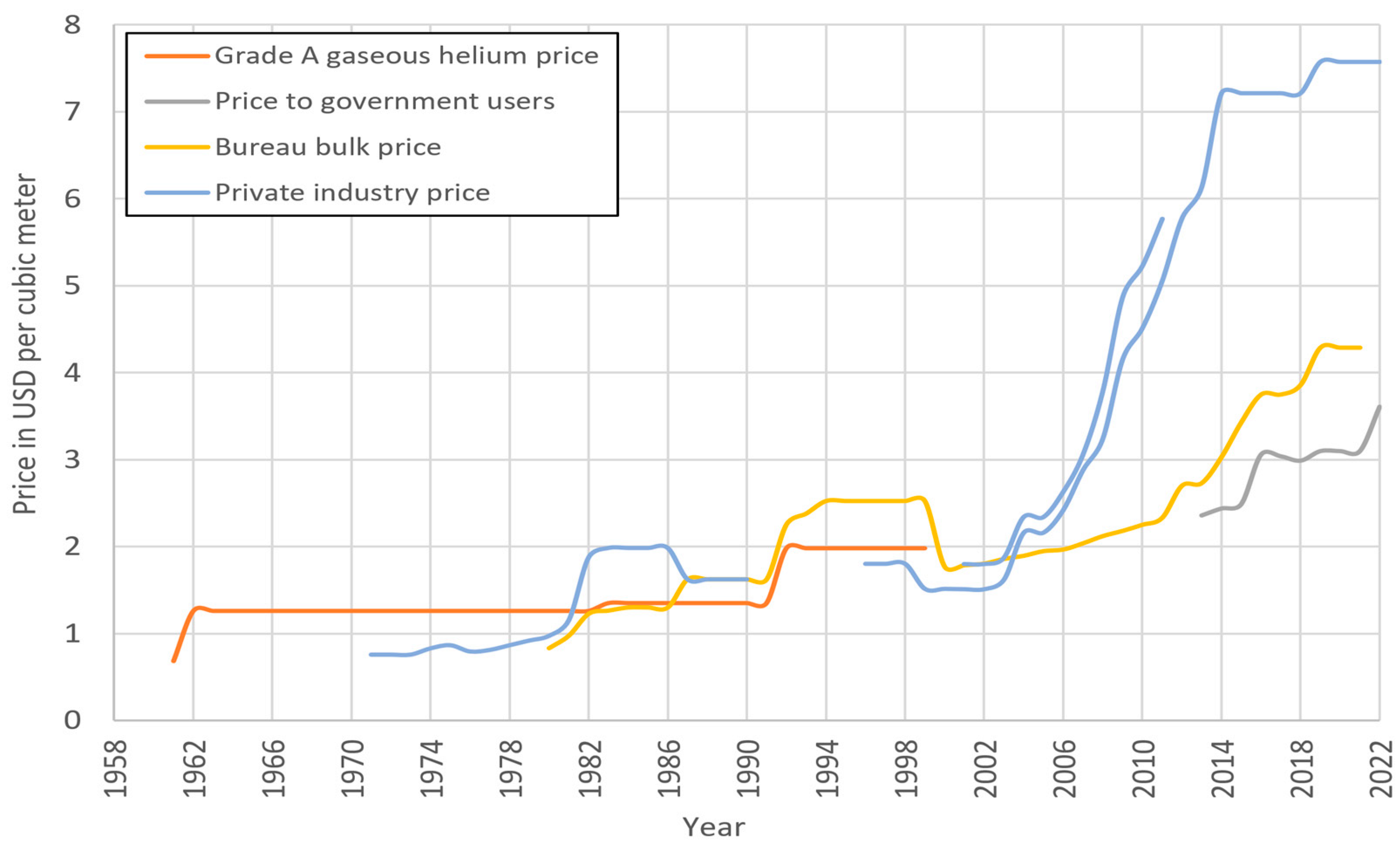
4.4. Helium Shortages, Challenges for Scientists (1990–2012)
“In the course of its deliberations, members of the committee, scientists and non-scientists alike, were struck by the inordinate impact that increases in helium prices and its periodic scarcity are having on the small-scale science community. Unless structural changes are adopted … continued price increases and scarcities may result in these programs losing significant research capability”.
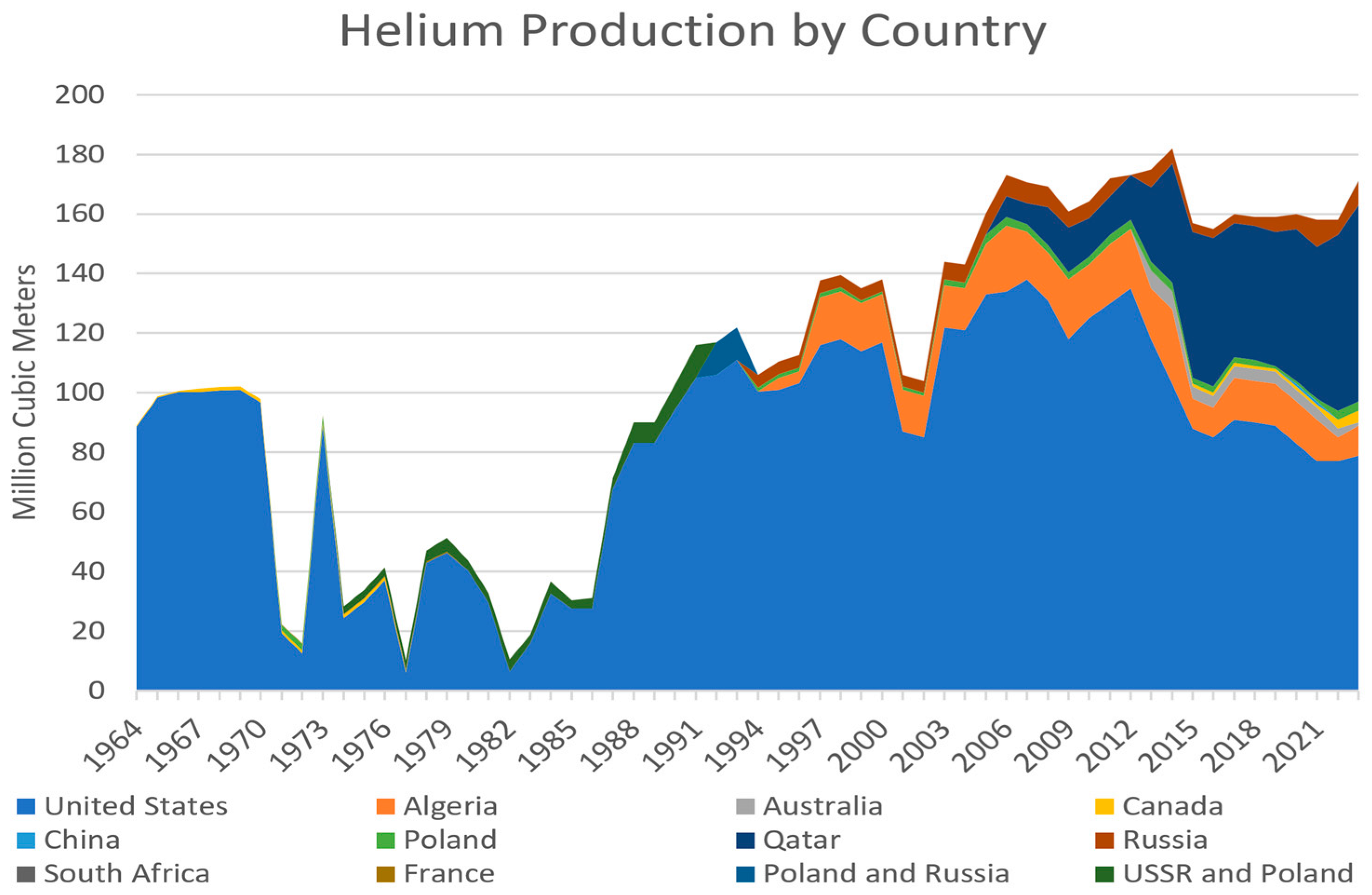
4.5. Final Closure of the Public Helium Industry and the Rise of the International Helium Market (2013–2022)
“In general, existing models of helium either do not account for an oligopoly controlling supply, or they do not evaluate potential helium extraction and storage programs based on an intertemporal maximization of the value of the resource. Such models could be of very limited use to decision-makers”.
5. Discussion
5.1. Helium Consumption, Demand and Storage
5.2. Possible Alternatives to Helium
5.3. The Future of Helium Supplies
5.4. Helium and Net-Zero Carbon
6. Conclusions
- Helium will become more expensive to extract as it will no longer be extracted as a by-product;
- Helium will no longer be emitted into the atmosphere when natural gases are burned, so it will be preserved in subsurface reservoirs for future generations.
- Where can helium be replaced by other gases (e.g., hydrogen/argon)?
- Where can the process be changed to not require helium (e.g., high-temperature superconductors)?
- How can the loss of helium be prevented (e.g., cryostats and cryocoolers)?
- How can helium be more easily extracted from the atmosphere (e.g., membrane techniques)?
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, O.Q.F.; Medeiros, J.L.d. How is the transition away from fossil fuels doing, and how will the low-carbon future unfold? Clean Technol. Environ. Policy 2021, 23, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Manning, D.A.C. Where does all the helium that we use come from? Rapid Commun. Mass Spetrometry 2008, 22, 1640–1642. [Google Scholar] [CrossRef]
- Halperin, W.P. The impact of helium shortages on basic research. Nat. Phys. 2014, 10, 467–470. [Google Scholar] [CrossRef]
- Nuttall, W.J.; Clarke, R.H.; Glowacki, B.A. Stop squandering helium. Nature 2012, 485, 573–575. [Google Scholar] [CrossRef]
- Butler, D. Qatar blockade hits helium supply. Nature 2017, 547, 16. [Google Scholar] [CrossRef]
- Chaudhury, D.R. Russia Restricts Exports of Helium & Neon Sourced by Western & E Asian Microchip Firms. The Economic Times. 2 June 2022. Available online: https://economictimes.indiatimes.com/news/international/world-news/russia-restricts-exports-of-helium-neon-sourced-by-western-e-asian-microchip-firms/articleshow/91963658.cms (accessed on 20 April 2023).
- Bradshaw, A.M.; Hamacher, T. Nuclear fusion and the helium supply problem. Fusion Eng. Des. 2013, 88, 2694–2697. [Google Scholar] [CrossRef]
- Olafsdottir, A.H.; Sverdrup, H.U. Assessing the Past and Future Sustainability of Global Helium Resources, Extraction, Supply and Use, Using the Integrated Assessment Model WORLD7. Biophys. Econ. Sustain. 2020, 5, 6. [Google Scholar] [CrossRef]
- Cady, H.P.; McFarland, D.F. Helium in Natural Gas. Science 1906, 24, 344. [Google Scholar] [CrossRef]
- Liu, B.-c. Helium conservation and supply and demand projections in the USA. Energy Econ. 1983, 5, 58–64. [Google Scholar] [CrossRef]
- Nuttall, W.J.; Clarke, R.H.; Glowacki, B.A. The future of helium. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 307–312. [Google Scholar]
- Smith, D.M.; Goodwin, T.W.; Schillinger, J.A. Challenges to the Worldwide Supply of Helium in the Next Decade. In Proceedings of the American Institute of Physics Conference Proceedings, Anchorage, AK, USA, 22–26 September 2003; Volume 710, pp. 119–138. [Google Scholar]
- Cai, Z.; Clarke, R.H.; Nuttall, W.J. Helium Demand Application, prices and Substitution. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 134–156. [Google Scholar]
- Cook, E. The Helium Question. Science 1979, 206, 1141–1147. [Google Scholar] [CrossRef]
- Thomas, A. Medical imaging why helium prevails. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 190–202. [Google Scholar]
- Lebrun-Gallagher, F.R.; Johnson, N.I.; Akhtar, M.; Weidt, S.; Bretaud, D.; Hile, S.J.; Owens, A.; Bonus, F.; Hensinger, W.K. A scalable helium gas cooling system for trapped-ion applications. Quantum Sci. Technol. 2022, 7, 12. [Google Scholar] [CrossRef]
- Posen, S.; Cravatta, A.; Checchin, M.; Aderhold, S.; Adolphsen, C.; Arkan, T.; Bafia, D.; Benwell, A.; Bice, D.; Chase, B.; et al. High gradient performance and quench behavior of a verification cryomodule for a high energy continuous wave linear accelerator. Phys. Rev. Accel. Beams 2022, 25, 13. [Google Scholar]
- Nedaei, N.; Hamrang, F.; Farshi, L.G. Design and 3E analysis of a hybrid power plant integrated with a single-effect absorption chiller driven by a heliostat field: A case study for Doha, Qatar. Energy 2022, 239, 122415. [Google Scholar]
- Chahartaghi, M.; Sheykhi, M. Energy, environmental and economic evaluations of a CCHP system driven by Stirling engine with helium and hydrogen as working gases. Energy 2019, 174, 1251–1266. [Google Scholar] [CrossRef]
- Scholes, C.A. Helium: Is the party really over? Chem. Aust. 2011, 78, 20–22. [Google Scholar]
- Glowacki, B.A.; Nuttall, W.J.; Clarke, R.H. Beyond the Helium Conundrum. IEEE Trans. Appl. Supercond. 2013, 23, 0500113. [Google Scholar] [CrossRef]
- AlNouss, A.; Al-Shobi, S.A. Circular Economy Analysis of Helium Recovery from Sales Gas Product. Comput. Aided Chem. Eng. 2020, 48, 913–918. [Google Scholar]
- Abdul Quader, M.; Smart, S.; Rufford, T.E. Techno-economic evaluation of multistage membrane combinations using three different materials to recover helium from natural gas. Comput. Aided Chem. Eng. 2018, 18, 1201–1206. [Google Scholar]
- Haider, S.; Saeed, M.; Lindbrathen, A.; May-Britt, H. Techno-economic evaluation of helium recovery from natural gas; A comparison between inorganic and polymeric membrane technology. J. Membr. Sci. Res. 2019, 5, 126–136. [Google Scholar]
- Sears, B. A history of the helium industry. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 15–47. [Google Scholar]
- d’Orcy, L. The case for the airship. SAE Trans. 1919, 14, 521–533. [Google Scholar]
- McLennan, J.C. Report on Some Sources of Helium in the British Empire; Bulletin No 31; Canada Department of Mines: Ottawa, ON, Canada, 1920; 72p. [Google Scholar]
- Congressional Record-House. In Proceedings of the 66th Congress 1st Session 20 June 1919; Volume 58, Part 2, pp. 1485—1486.
- Congressional Record-Senate. 66th Congress 1st Session 23 June 1919; Volume 58, Part 2, pp. 1577—1579.
- Congressional Record-House. 66th Congress 1st Session 28 June 1919; Volume 58, Part 2, pp. 2041—2042.
- Congressional Record-House. 66th Congress 1st Session 25 October 1919, Volume 58, Part 8, p. 7527.
- Congressional Record-House. 66th Congress 2nd Session 24 January 1920, Volume 59, Part 2. p. 2022.
- Seibel, C.W. Helium, Child of the Sun; University Press of Kansas: Lawrence, KS, USA, 1968. [Google Scholar]
- Congressional Record-House. 67th Congress 2nd Session 3 March 1922; Volume 62, Part 4, p. 3365.
- Congressional Record-House. 67th Congress 1st Session 22 November 1921; Volume 61, Part 8, pp. 8149—8150.
- Congressional Record-House. 67th Congress 2nd Session 24 March 1922; Volume 62, Part 5, pp. 4523—4525.
- End, E. The Use of New Equipment and Helium Gas in a World Record Dive. J. Ind. Hyg. Toxicol. 1938, 20, 511. [Google Scholar]
- Winters, S.R. Helium Aids in Welding. Sci. Am. 1943, 168, 252–256. [Google Scholar] [CrossRef]
- Congressional Record-House. 85th Congress—2nd Session 22 July 1958; Volume 104, Part 11, pp. 14653—14660.
- The New York Times. NO PARADE BALLOONS. U. S. Bars Use of Helium for Macy’s Thanksgiving Fete. The New York Times, 17 October 1958; p. 10. [Google Scholar]
- Koelling, G.W. Helium. In Minerals Yearbook 1971, Volume 1, Metals Minerals and Fuels; Mines, B.O., Ed.; U.S. Government Printing Office: Washington, DC, USA, 1971; pp. 577–583. [Google Scholar]
- Epple, D.; Lave, L. Helium: Investments in the future. Bell J. Econ. 1980, 11, 617–630. [Google Scholar] [CrossRef]
- Hammel, E.F.; Krupka, M.C.; Williamson, J.D., Jr. Helium Policies. Science 1984, 225, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Hammel, E.F.; Krupka, M.C.; Williamson, K.D. The Continuing U.S. Helium Saga. Science 1984, 223, 789–792. [Google Scholar] [CrossRef]
- Krupka, M.C.; Harmnel, E.F. Energy Helium an the Future II. In Proceedings of the Third International Conference on Alternative Energy Sources, Miami Beach, FL, USA, 15–17 December 1980; p. 22. [Google Scholar]
- Crawford, M. Dismantling the Helium Empire. Science 1987, 237, 238–240. [Google Scholar] [CrossRef]
- Schumpeter, J.A. Capitalism, Socialism, and Democracy; Routledge: London, UK, 1942; p. 437. [Google Scholar]
- Vickers, J.; Yarrow, G. Economic Perspectives on Privatization. J. Econ. Perspect. 1991, 5, 111–132. [Google Scholar] [CrossRef]
- Birner, B.; Severinghaus, J.; Paplawsky, B.; Keeling, R.F. Increasing atmospheric helium due to fossil fuel exploitation. Nat. Geosci. 2022, 15, 346–348. [Google Scholar] [CrossRef]
- Bahl, S. Helium—Macro View; Edison Investment Research: London, UK, 2019; p. 21. [Google Scholar]
- National Research Council. Selling the Nation’s Helium Reserve; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Hooker, B. Helium in Russia. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 88–100. [Google Scholar]
- Reinoehl, B. Helium in Algeria. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 55–68. [Google Scholar]
- Yakutseni, V.P. The status of natural helium resources geopolitics of production and use. Chem. Pet. Eng. 1995, 31, 61–63. [Google Scholar] [CrossRef]
- US Bureau of Land Management. BLM Announces Disposal Process for Federal Helium System; US Bureau of Land Management: Washington, DC, USA, 2020. [Google Scholar]
- Bradshaw, T.W.; Miller, T. Closed-cycle refrigeration Minimizing helium demand in cryogenic applications. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 174–189. [Google Scholar]
- Anderson, S.T. Economics, Helium, and the U.S. Federal Helium Reserve: Summary and Outlook. Nat. Resour. Res. 2017, 27, 455–477. [Google Scholar]
- Petty, T.R. Final List of Critical Minerals 2018. Fed. Regist. 2018, 83, 23295. [Google Scholar]
- Nassar, N.T.; Fortier, S.M. Methodology and Technical Input for the 2021 Review and Revision of the U.S. Critical Minerals List; U.S. Geological Survey: Reston, VA, USA, 2021; p. 31. [Google Scholar]
- Applegate, J.D. 2021 Draft List of Critical Minerals. Fed. Regist. 2021, 86, 62199–62203. [Google Scholar]
- Kramer, D. Helium users are at the mercy of suppliers. Physics Today 2019, 72, 26–29. [Google Scholar] [CrossRef]
- Adetunji, J. Helium supplies at risk from plunging oil prices—Which is bad news for our coronavirus effort. The Conversation, 24 April 2020. Available online: https://theconversation.com/helium-supplies-at-risk-from-plunging-oil-prices-which-is-bad-news-for-our-coronavirus-effort-137106 (accessed on 19 June 2023).
- Kramer, D. Helium shortage has ended, at least for now. Phys. Today 2020. [Google Scholar] [CrossRef]
- Kramer, D. Helium is again in short supply. Phys. Today 2022. [Google Scholar] [CrossRef]
- Pavlova, A.C.a.U. Chipmakers Brace for More Trouble as Russia Limits Exports of Rare Gases. CNN Business. 17 June 2022. Available online: https://edition.cnn.com/2022/06/17/tech/russia-noble-gases-exports-semiconductors/index.html (accessed on 19 June 2023).
- Wietfeldt, F.E.; Biswas, R.; Haun, R.W.; Dewey, M.S.; Haddock, C.C.; Hoogerheide, S.F.; Mumm, H.P.; Nico, J.S.; Caylor, J.; Fomin, N.; et al. A Comment on “The possible explanation of neutron lifetime beam anomaly” by A. P. Serebrov; et al. arXiv 2020, arXiv:2004.01165. [Google Scholar]
- Lebrun, P. Superfluid Helium as a Technical Coolant; Large Hadron Collider Project Report No 125.; European Organization for Nuclear Research: Genève, Switzerland, 1997; p. 16. [Google Scholar]
- Dumas, A.; Trancossi, M.; Madonia, M. Hydrogen Airships: A Necessary Return Because of High Costs of Helium. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, IMECE2012, Huston, TX, USA, 9–15 November 2012; pp. 533–540. [Google Scholar]
- Glowacki, B.A. Substituting hydrogen for helium in cryogenic applications. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 265–295. [Google Scholar]
- Iida, K.; Qin, D.; Tarantini, C.; Hatano, T.; Wang, C.; Guo, Z.; Gao, H.; Saito, H.; Hata, S.; Naito, M.; et al. Approaching the ultimate superconducting properties of (Ba,K)Fe2As2 by naturally formed low-angle grain boundary networks. NPG Asia Mater. 2021, 13, 68. [Google Scholar] [CrossRef]
- Ayres, R.U. On the practical limits to substitution. Ecol. Econ. 2007, 61, 115–128. [Google Scholar] [CrossRef]
- Mohr, S.; Ward, J. Helium production and possible projection. Minerals 2014, 4, 130–144. [Google Scholar] [CrossRef]
- Scurlock, R.; Francis, A. Is there a helium problem? Ways forward. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 296–306. [Google Scholar]
- Clarke, R.H.; Cai, Z. Helium and nuclear fusion energy. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 235–264. [Google Scholar]
- Stainsby, R. Helium and nuclear fission energy. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 228–234. [Google Scholar]
- Kiswanta; Sumarno, E.; Santosa, K.; Indrakoesoema, K.; Handono, K. Electrical Design For Helium Purification and Supply System of RDE. J. Phys. Conf. Ser. 2019, 1198, 052001. [Google Scholar] [CrossRef]
- Das, N.K.; Bhandari, R.K.; Mallik, S.C. India Harnessing Helium from the Earth’s Interia in the Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 101–118. [Google Scholar]
- Brown, A.A. Formation of High Helium Gases: A Guide for Explorationists; American Association of Petroleum Geologists: Tulsa, OK, USA, 2010; p. 80115. [Google Scholar]
- Oil & Gas Journal Editors. Eastern Utah Harley Dome helium plant starts up. Oil Gas J. 2013. Available online: https://www.ogj.com/drilling-production/production-operations/article/17258977/eastern-utah-harley-dome-helium-plant-starts-up (accessed on 11 October 2024).
- D’Souza, M.R.; Otalvaro, D.M.; Singh, D.A. Harvesting Helium-3 from the Moon; Worcester Polytechnic Institute: Worcester, MA, USA, 2006. [Google Scholar]
- Clarke, R.H.; Nuttall, W.J.; Glowacki, B.A. Introduction. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 1–14. [Google Scholar]
- Stott, P.E. The feasibility of using D–3 He and D–D fusion fuels. Plasma Phys. Control. Fusion 2005, 47, 1305–1338. [Google Scholar] [CrossRef]
- Clarke, R.H.; Clare, R. Helium from the air, The backstop. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R., Glowacki, B., Eds.; Taylor & Francis Group: Abingdon, UK, 2012; pp. 158–172. [Google Scholar]
- Schrier, J. Helium Separation Using Porous Graphene Membranes. J. Phys. Chem. Lett. 2010, 1, 2284–2287. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; He, X.; Scholes, C.A.; Jiang, X.; Wang, B.; Guo, H.; Ma, Y.; Deng, L. Helium separation using membrane technology: Recent advances and perspectives. Sep. Purif. Technol. 2021, 274, 119044. [Google Scholar]
- Burgoyne, J.W.; Cuthbert, M.N. Rising to the challenges of constrained helium supply in cryogenic systems for the research market. In The Future of Helium as a Natural Resource; Nuttall, W.J., Clarke, R.H., Glowacki, B.A., Eds.; Routledge: London, UK, 2012; pp. 203–227. [Google Scholar]
- International Energy Agency. World Energy Outlook 2021; International Energy Agency: Paris, France, 2021; p. 383. [Google Scholar]
- bp. Statistical Review of World Energy; bp: London, UK, 2022; p. 57. [Google Scholar]
- Yadav, G.D. Carbon Dioxide Refineries, Hydrogen Economy, and the Net Zero Goal. In Climate Change and Sustainable Development; Fulekar, M.H., Dubey, R.S., Eds.; CRC Press: Boca Raton, FL, USA, 2023; p. 29. [Google Scholar]
- Masool, O.; Rifaat, O. Phasing out the U.S. Federal Helium Reserve: Policy insights from a world helium model. Resour. Energy Econ. 2018, 54, 186–211. [Google Scholar] [CrossRef]


| Country | Estimated Reserve (Million Cubic Meters) | Estimated Resources (Million Cubic Meters) |
|---|---|---|
| United States | 3900 | 20,600 |
| Algeria | 1800 | 8200 |
| Poland | 23 | - |
| Qatar | Large | 10,100 |
| Russia | 1700 | 6800 |
| Rest of the world | 6300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, S.; Gerth, F. A Review of the Sustainability of Helium: An Assessment of Its Past, Present and a Zero-Carbon Future. Reg. Sci. Environ. Econ. 2024, 1, 78-103. https://doi.org/10.3390/rsee1010006
Wilkinson S, Gerth F. A Review of the Sustainability of Helium: An Assessment of Its Past, Present and a Zero-Carbon Future. Regional Science and Environmental Economics. 2024; 1(1):78-103. https://doi.org/10.3390/rsee1010006
Chicago/Turabian StyleWilkinson, Stephen, and Florian Gerth. 2024. "A Review of the Sustainability of Helium: An Assessment of Its Past, Present and a Zero-Carbon Future" Regional Science and Environmental Economics 1, no. 1: 78-103. https://doi.org/10.3390/rsee1010006
APA StyleWilkinson, S., & Gerth, F. (2024). A Review of the Sustainability of Helium: An Assessment of Its Past, Present and a Zero-Carbon Future. Regional Science and Environmental Economics, 1(1), 78-103. https://doi.org/10.3390/rsee1010006






