Phenotypic and Phytochemical Variability Among Four Populations of Hedeoma multiflora Benth. (Tomillito de las Sierras) Native to the Province of Córdoba—In Situ Evaluation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Climatic and Edaphic Characterization
2.3. Plant Characterization
2.4. Statistical Analysis
3. Results
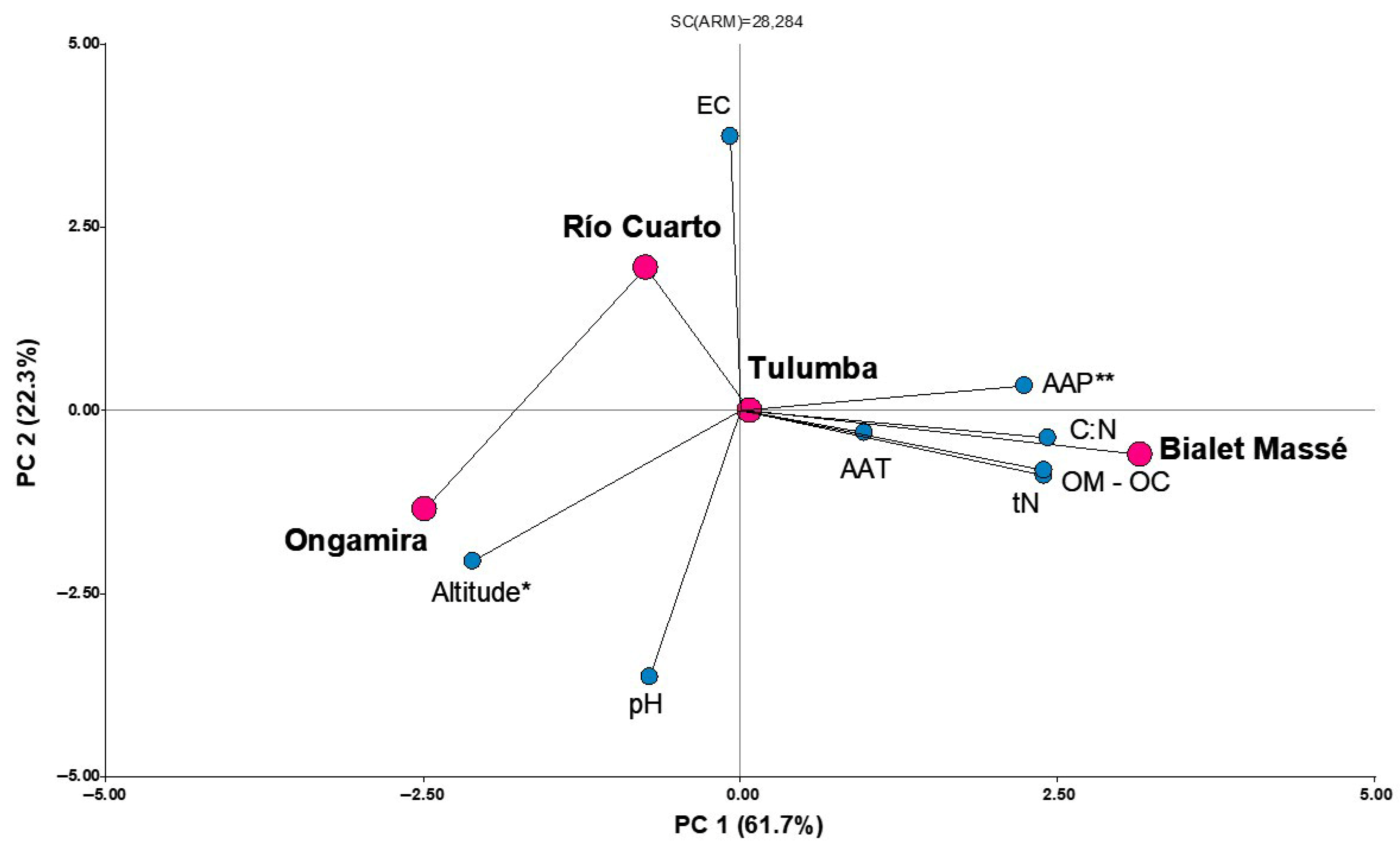
3.1. Climatic and Edaphic Characterization
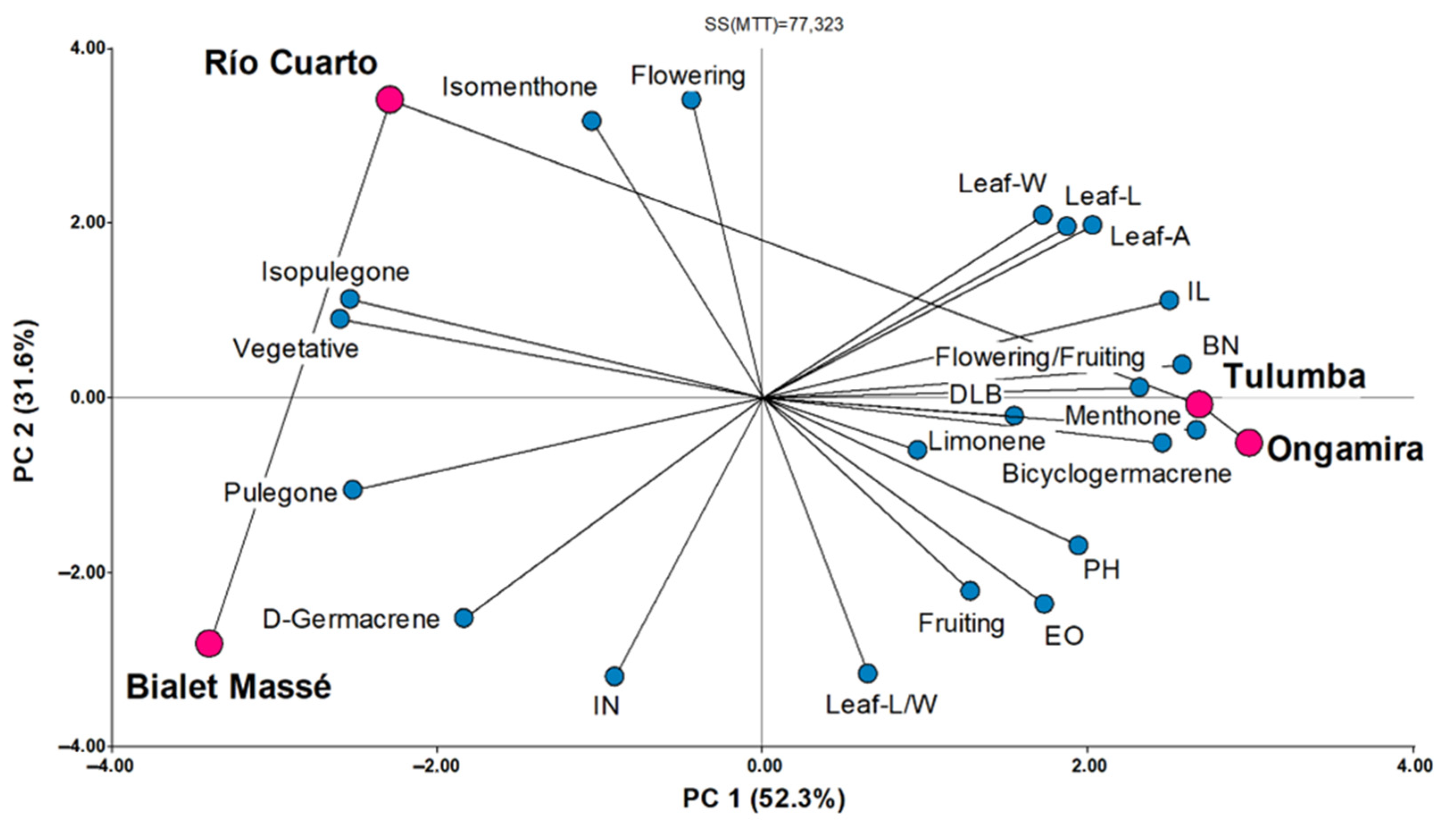
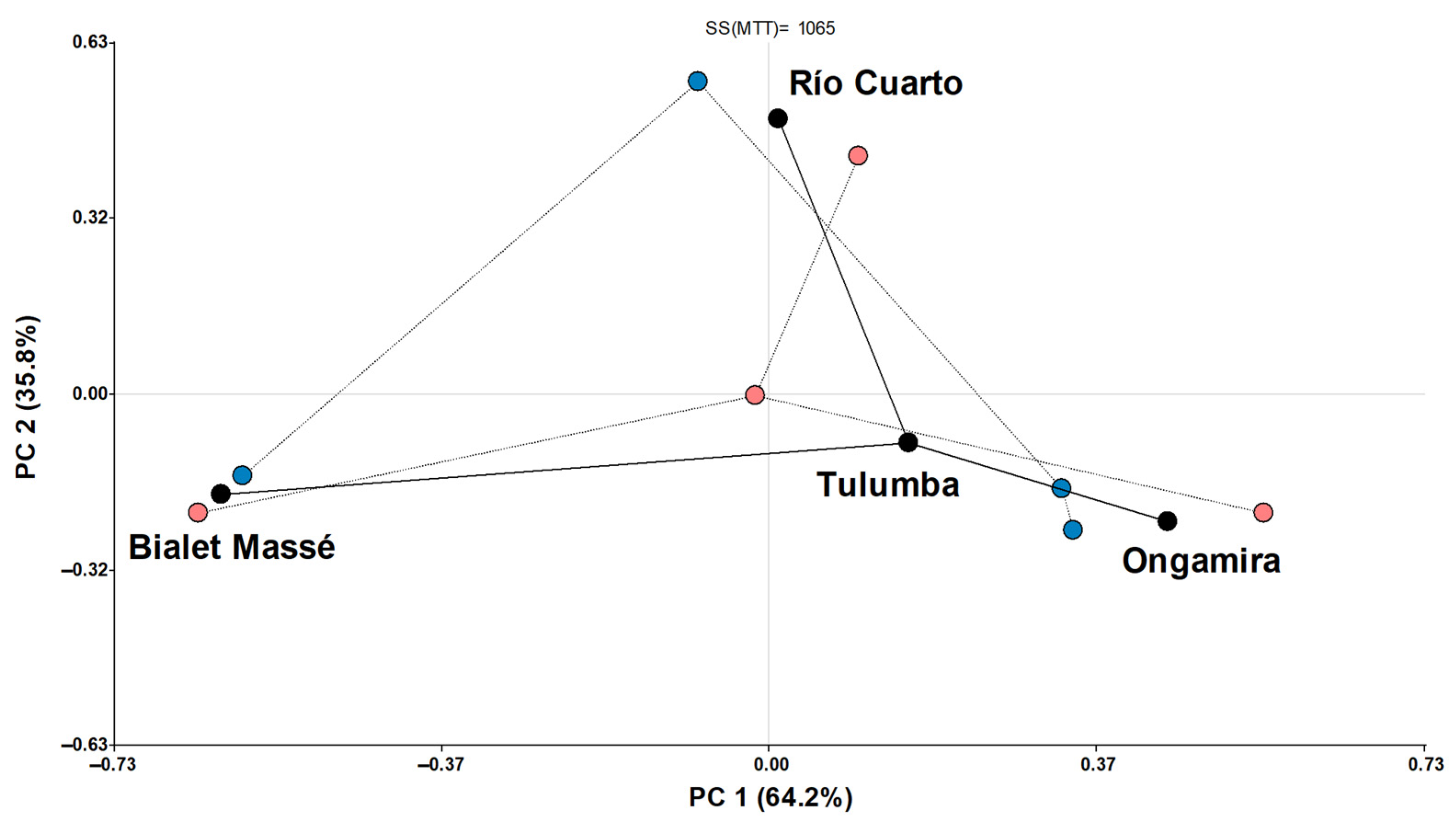
3.2. Plant Characterization
4. Discussion
4.1. Climatic and Edaphic Characterization
4.2. Plant Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| LABSA | Soil and Water Laboratory |
| ANOVA | Analysis of Variance |
| PCA | Principal Component Analysis |
| PC1 | First principal component |
| PC2 | Second principal component |
| GPA | Generalized Procrustes Analysis |
| Jan-Max | January average maximum temperature |
| Jan-Min | January average minimum temperature |
| Jul-Max | July average maximum temperature |
| Jul-Min | July average minimum temperature |
| AAT | Annual average temperature |
| AAP | Average annual precipitation |
| PH | Plant height |
| BN | Number of branches per plant |
| IN | Internode number |
| IL | Internode length |
| DLB | Diameter of base longest branch |
| Leaf-L | Leaf length |
| Leaf-W | Leaf width |
| Leaf-A | Leaf area |
| Leaf L/W | Leaf-L/Leaf-W ratio |
| EO | Essential oil content |
| OM | Organic matter |
| OC | Organic carbon |
| tN | Total nitrogen |
| C:N | Ratio: carbon-nitrogen ratio |
| EC | Electric conductivity |
| CV | Coefficient of variation |
References
- Elechosa, M.; Aguirre, E.; Bandoni, A.; Di Leo Lira, P.; Fernández, E.; Heit, C.; Juárez, M.; López, S.; Martínez, A.; Martínez, E.; et al. Manual de Recolección Sustentable de Plantas Aromáticas Nativas de la Región Central y Noroeste de la Argentina; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2009. [Google Scholar]
- Roig, F. Flora Medicinal Mendocina: Las Plantas Medicinales y Aromáticas de la Provincia de Mendoza (Argentina); Universidad Nacional de Cuyo: Mendoza, Argentina, 2001; p. 303. [Google Scholar]
- Ojeda, M. Caracterización de Poblaciones y Avances en la Domesticación de Peperina Minthostachys mollis (Kunth) Griseb. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2004. [Google Scholar]
- Martínez, G.; Planchuelo, A.; Fuentes, E.; Ojeda, M. A numeric index to establish conservation priorities for medicinal plants in the Paravachasca Valley, Córdoba, Argentina. Biodivers. Conserv. 2006, 15, 2457–2475. [Google Scholar] [CrossRef]
- Bustos, J. Caracterización Poblacional y de Hábitat de la Peperina (Minthostachys mollis (Kunth) Griseb.) en el Noroeste de Córdoba. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2009. [Google Scholar]
- Brunetti, P. Estudios en Lippia integrifolia INCAYUYO Orientados a su Domesticación y Mejoramiento Genético. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2017. [Google Scholar]
- Peralta, P.; Guariniello, J.; Escandón, A. Review of the situation of Hedeoma multiflora Benth. (Peperina de las Lomas): An aromatic-medicinal Argentine species at risk. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2020, 19, 1–14. [Google Scholar]
- Brunetti, P.; Leiva, R.; Zapata, R.; Torres, L.; Chaves, A.; Juliani, H.; Ojeda, M. Inter and intra population phenotypic variability of Lippia integrifolia (Verbenaceae) and its natural situation in the west-center of Argentina. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2022, 21, 242–255. [Google Scholar] [CrossRef]
- Chaves, A.; Turco, F.; Lingua, G.; de Blas, F.; Brunetti, P.; Costero, B.; Londero, W.; Konigheim, B.; Torre, L. Variability evaluation of the native medicinal species Baccharis crispa Spreng. for its domestication and conservation in the province of Córdoba, Argentina. In Proceedings of the 6th Global Enviromental Health Summit, Amsterdam, The Netherlands, 14–15 April 2025. [Google Scholar]
- Cantero, J.; Núñez, C.; Bernardello, G.; Amuchástegui, A.; Mulko, J.; Brandolín, P.; Palchetti, M.; Iparraguirre, J.; Virginil, N.; Espinar, L.A. Las Plantas de Interés Económico de Argentina; UniRío editora (Universidad Nacional de Río Cuarto): Córdoba, Argentina, 2019. [Google Scholar]
- Irving, R. The Systematics of Hedeoma (Labiatae). SIDA Contrib. Bot. 1980, 8, 219–261. [Google Scholar]
- Barboza, G.; Cantero, J.; Núñez, C.; Espinar, L.A. Flora Medicinal de la Provincia de Córdoba (Argentina) Editorial Museo Botánico; Universidad Nacional de Córdoba: Córdoba, Argentina, 2006. [Google Scholar]
- Barboza, G.; Bonzani, N.; Filipa, E.; Lujan, M.; Morero, R.; Bugatti, M.; Decolatti, N.; Espinar, L.A. Atlas Histo-Morfológico de Plantas de Interés Medicinal de Uso Corriente en Argentina; Editorial Museo Botánico Córdoba: Córdoba, Argentina, 2001. [Google Scholar]
- Brunetti, P. Propagación In Vitro de Hedeoma multiflorum Benth. y Composición de aceites Esenciales. Master’s Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2007. [Google Scholar]
- van Baren, C.; Sanguinetti, S.; Di Leo Lira, P.; Bandoni, A.; Juárez, M.; Elechosa, M.; Martínez, E. El aceite esencial de Hedeoma multiflora Benth. (Lamiaceae) de poblaciones naturales en la provincia de San Luis, Argentina. Estudio comparativo. Dominguezia 2010, 26, 13–20. [Google Scholar]
- Liébana, C.; Torres, L.; Ordóñez, A. Cytogenetic Characterization of Three Populations of Hedeoma multiflorum Benth. (Lamiaceae) Native from Córdoba, Argentina. Cytologia 2017, 82, 297–301. [Google Scholar] [CrossRef]
- Garro, M.; Sosa, A.; Fusco, M.; María, A.; Pelzer, L. Evaluation of the gastrointestinal activity of Hedeoma multiflora in rats. Biocell 2006, 30, 231. [Google Scholar]
- Goleniowski, M.; Bongiovanni, G.; Palacio, L.; Núñez, C.; Cantero, J. Medicinal plants from the Sierra de Comechingones, Argentina. J. Ethnopharmacol. 2006, 107, 324–341. [Google Scholar] [CrossRef]
- Dadé, M.; Fioravanti, D.; Schinella, G.; Tournier, H.A. Total antioxidant capacity and polyphenol content of 21 aqueous extracts obtained from native plants of Traslasierra valley (Argentina). Boletín Latinoam. Caribe Plantas Med. Aromáticas 2009, 8, 529–539. [Google Scholar]
- Dadé, M.; Schinella, G.; Fioravanti, D.; Tournier, H.A. Antioxidant and cytotoxic properties of an aqueous extract from the Argentinean plant Hedeoma multiflora. Pharm. Biol. 2011, 49, 633–639. [Google Scholar] [CrossRef]
- Martínez, G. Recolección y Comercialización de Plantas Medicinales en el Departamento Santa María, Provincia de Córdoba, Argentina. Acta Farm. Bonaerense 2005, 24, 575–584. [Google Scholar]
- Galli, C. Los yuyeros del noreste de San Luis: Actores sociales en torno a la recolección de plantas con usos aromáticos y/o medicinales. In Proceedings of the V Congreso Argentino y Latinoamericano de Antropología Rural, Santa Rosa, Argentina, 11–15 March 2013. [Google Scholar]
- Peralta, P. Evaluación del Grado de Vulnerabilidad y Estrategia de Conservación ex situ para y Peperina de las Lomas (Hedeoma multiflora Benth.); Universidad Nacional de Quilmes: Bernal, Argentina, 2022; Available online: http://ridaa.unq.edu.ar/handle/20.500.11807/3560 (accessed on 15 April 2024).
- Lagrottería, M.; Lozada, C. Medicinal and aromatic plants from Córdoba, Argentina. Their commercial and socio-cultural aspects. Acta Hortic. 1993, 330, 101–106. [Google Scholar] [CrossRef]
- Peralta, P.; Nores, M.; Bach, H.; Robbiati, F. Facing climate change: Range dynamics and chromosome diversity in Hedeoma multiflora Benth., a South American aromatic-medicinal plant at risk. Flora 2024, 315, 152519. [Google Scholar] [CrossRef]
- Liébana, C. Caracterización Citogenética de Poblaciones de (Hedeoma multiflora Benth.) Tomillito de las Sierras; Universidad Nacional de Córdoba: Córdoba, Argentina, 2011. [Google Scholar]
- FAO. Informe Sobre el Estado de los Recursos Fitogenéticos en el Mundo; FAO: Rome, Italy, 1996. [Google Scholar]
- Jaramillo, S.; Baena, M. Conservación Ex Situ de Recursos Fitogenéticos. Material de Capacitación; International Plant Genetic Resources Institute: Rome, Italy, 2000. [Google Scholar]
- Chandran, K.; Pandya, S. Morphological characterization of Arachis species of section Arachis. Plant Genet. Resour. News 2000, 121, 38–41. [Google Scholar]
- Chaves, A.; Brunetti, P.; Massuh, Y.; Ocaño, S.; Torres, L.; Ojeda, M. Variabilidad entre poblaciones silvestres de Baccharis crispa Spreng. de la Provincia de Córdoba, Argentina. Phyton 2014, 83, 4–11. [Google Scholar] [CrossRef]
- Massuh, Y. Comparación entre poblaciones de Tagetes minuta de la Provincia de Córdoba, una especie aromática promisoria. Master’s Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2007. [Google Scholar]
- Cúneo, C.D. Baccharis trimera (Less.) DC. y Baccharis Crispa Spreng. Especies de Carquejas Utilizadas con Fines Medicinales, Caracterización y Análisis Genéticos de Poblaciones de Lavalleja, Uruguay; Universidad de la República: Montevideo, Uruguay, 2012. [Google Scholar]
- Brunetti, P.; Bruno, C.; Zapata, R.; Torres, L.; Barboza, G.; Ojeda, M.S. Sample size estimation for in situ morphological variability studies of Lippia integrifolia (Verbenaceae) in Parque Nacional Talampaya, La Rioja (Argentina). Bonplandia 2014, 23, 15–23. [Google Scholar] [CrossRef]
- Chaves, A. Caracterización Fenotípica de Poblaciones Silvestres de Carqueja (Baccharis crispa Spreng.) de la Zona de Sierras de la Provincia de Córdoba. Master’s Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2012. [Google Scholar]
- Torres, L.; Brunetti, P.; Baglio, C.; Bauzá, P.; Chaves, A.; Massuh, Y.; Ocaño, S.; Ojeda, M. Field evaluation of twelve clones of oregano grown in the main production areas of Argentina: Identification of quantitative trait with the highest discriminant value. ISRN Agron. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Massuh, Y. Estudio de Caracteres Morfológicos y Bioquímicos En Suico (Tagetes minuta L.). Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2014. [Google Scholar]
- Torres, C.; Domínguez, M.; Carbonari, J.; Sabini, M.; Sabini, L.; Zanon, S. Study of Antiviral and Virucidal Activities of Aqueous Extract of Baccharis articulata against Herpes suis virus. Nat. Prod. Commun. 2011, 6, 994. [Google Scholar] [CrossRef]
- Rasband, R. ImageJ: Image Processing and Analysis in Java, version 1.54p; National Institutes of Health, Estados Unidos: Bethesda, MD, USA, 2002. Available online: https://imagej.softonic.com/ (accessed on 17 April 2024).
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.; González, L.; Tablada, M.; Robledo, C. InfoStat, version 2020; Grupo InfoStat/FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. Available online: http://www.infostat.com.ar (accessed on 10 March 2025).
- Di Rienzo, J.; Guzmán, W.; Casanoves, F.D.G.C. Test de Comparación de Medias. In InfoStat, version 1.1/Profesional; Grupo InfoStat, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba: Córdoba, Argentina, 2001. [Google Scholar]
- Franco, T.; Hidalgo, R. Análisis estadístico de datos de caracterización morfológica de recursos fitogenéticos. In Boletín Técnico No. 8; Instituto Internacional de Recursos Fitogenéticos (IPGRI): Cali, Colombia, 2003. [Google Scholar]
- Torres, L. Caracterización y Evaluación de Genotipos de Orégano Cultivados en las Principales Zonas de Producción de Argentina. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2011. [Google Scholar]
- Hang, S.; Negro, G.; Becerra, A.; Rampoldi, A. Suelos de Córdoba: Variabilidad de las Propiedades del Horizonte Superficial; Editorial Facultad de Ciencias Agropecuarias Universidad Nacional de Córdoba: Córdoba, Argentina, 2015. [Google Scholar]
- Carreira, D. Cuantificación de la Materia Orgánica del suelo. Método de WALKLEY & BLACK. In Jornadas de Actualización: Gestión de la Calidad en los Laboratorios de Análisis de Suelos Agropecuarios; SAMLA-PROINSA; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2011. [Google Scholar]
- Ritas, J.L.; Melida, J.L. El Diagnóstico de Suelos y Plantas (Métodos de Campo y Laboratorio); Mundi Prensa: Madrid, Spain, 1990. [Google Scholar]
- Taleisnik, E.; Grunberg, K.; Maria, G.S. La Salinización de Suelos en la Argentina; Editorial EDUCC: Córdoba, Argentina, 2007. [Google Scholar]
- Instituto Nacional de Tecnología Agropecuaria. Informe Técnico Mapa de Cartas de Suelos de la Provincia de Córdoba; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2020. [Google Scholar]
- Gutiérrez, E.; Seguy, S.; Denegri, A.; Reynoso, L. Caracterización de germoplasma de Hedeoma multiflora Benth. de la zona serrana de Córdoba y San Luis. Estudios preliminaries. In Proceedings of the Jornadas de Jóvenes Investigadores AUGM, Mendoza, Argentina, 17–19 October 2018. [Google Scholar]
- Koroch, A.; Juliani, H.; Trippi, V.; Juliani, H. Chemical constituents of the essential oil of Hedeoma multiflora Benth. (Lamiaceae). J. Essent Oil Res. 1999, 11, 165–166. [Google Scholar] [CrossRef]
- Fernández, E.; Martínez, E.; Juárez, M.; Elechosa, M.; Molina, A.; van Baren, C.; Di Leo Lira, P.; Bandoni, A. Estudio del aceite esencial de Hedeoma multiflora Benth. (Lamiaceae) peperina de las lomas obtenido de poblaciones naturales en la provincia de San Luis. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2007, 6, 246–247. [Google Scholar]
- Sepúlveda-Jiménez, G.; Porta-Ducoing, H.; Rocha-Sosa, M. La participación de los metabolitos secundarios en la defensa de las plantas. Rev. Mex. Fitopatol. 2003, 21, 355–363. [Google Scholar]
- Sarmiento, M.S.; Algarra, R.B.; Paternina, D.G.; Terán, A.M.; Rivero, D.S. Productos Naturales: Metabolitos Secundarios y Aceites Esenciales; Fundación Universitaria Agraria de Colombia, UNIAGRARIA: Cundinamarca, Colombia, 2018. [Google Scholar]
- Nincevic, T.; Pljevljakus, D.; Runjic, M.; Grdiša, M.; Šatović, Z. Phenotypic plasticity vs. local genetic adaptation: Essential oil diversity of natural immortelle (Helichrysum italicum (Roth.) G.Don) populations along eastern Adriatic coast. Front. Plant Sci. 2025, 16, 1467421. [Google Scholar] [CrossRef] [PubMed]
- Juárez, M.; van Baren, C.; Rosselot, V.; Di Leo Lira, P.; Martinez, A.; Retta, D.; Elechosa, M.; Bandoni, A. Diferencias en la composición de los aceites esenciales de Minthostachys verticillata (Griseb.) Epling (peperina) y Hedeoma multiflora Benth. (peperina de las lomas), en poblaciones de San Luis. Dominguezia 2018, 34, 53. [Google Scholar]
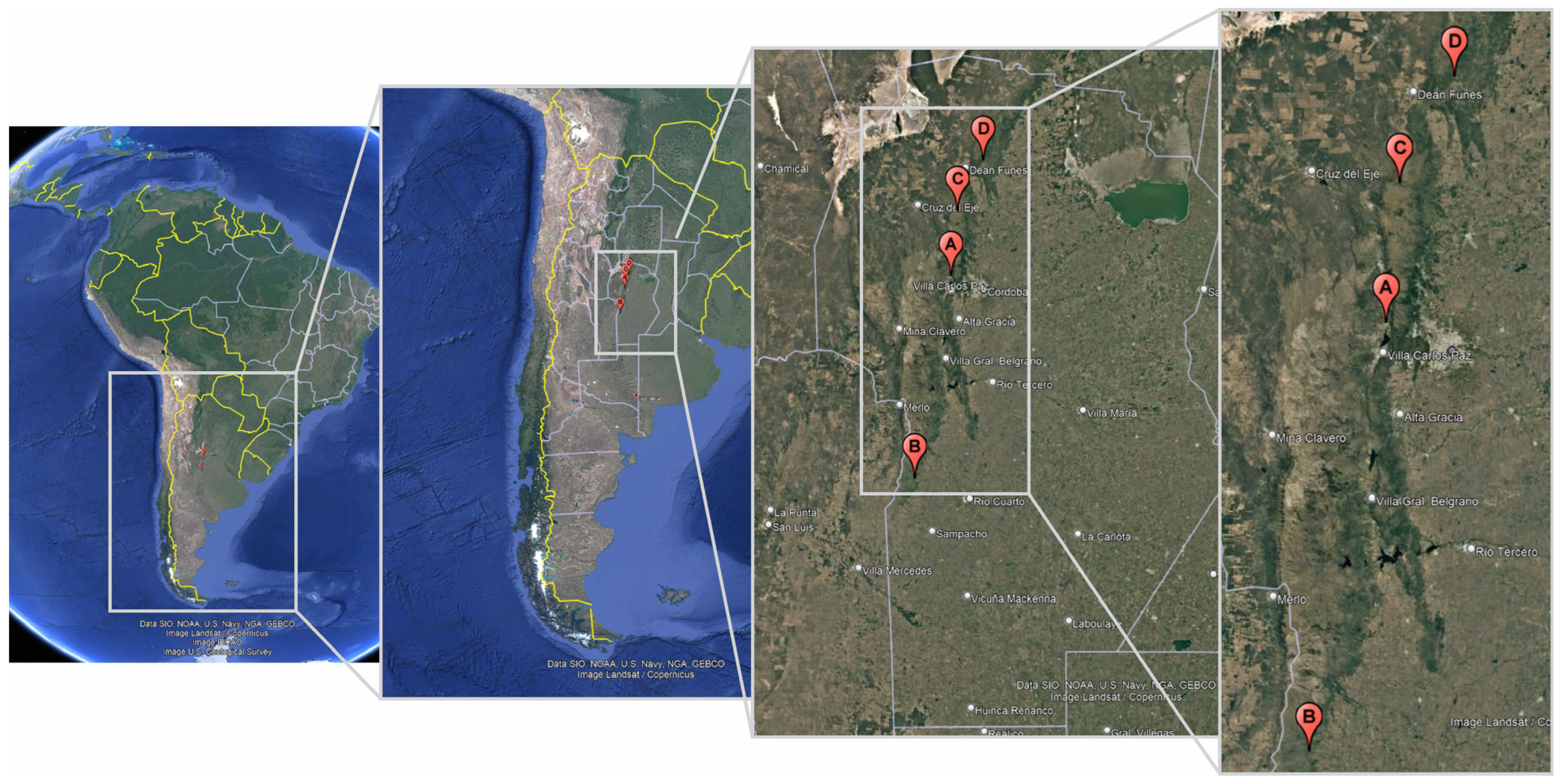



| Population | Department | Altitude * | Latitude | Longitude | Herbarium ID ** |
|---|---|---|---|---|---|
| A—Bialet Massé | Punilla | 688 | 31°18′26.7″ S | 64°28′00.2″ W | 2364 CS ACOR |
| B—Río Cuarto | Río Cuarto | 791 | 32°56′53.5″ S | 64°50′55.2″ W | 2366 CS ACOR |
| C—Ongamira | Ischilín | 1164 | 30°46′29.8″ S | 64°23′52.3″ W | 2367 CS ACOR |
| D—Tulumba | Tulumba | 809 | 30°22′01.4″ S | 64°08′56.6″ W | 2365 CS ACOR |
| Population | Jan-Max (°C) | Jan-Min (°C) | Jul-Max (°C) | Jul-Min (°C) | AAT (°C) | AAP * (mm) |
|---|---|---|---|---|---|---|
| Bialet Massé | 29.9 | 16.1 | 18.7 | 3.6 | 17.1 | 71.2 |
| Río Cuarto | 28.5 | 16.2 | 17.2 | 1.6 | 15.9 | 52.8 |
| Ongamira | 25.0 | 14.8 | 16.2 | 7.1 | 15.7 | 41.1 |
| Tulumba | 31.1 | 18.4 | 21.7 | 5.1 | 19.1 | 44.3 |
| Population | OM (%) | OC (%) | tN (%) | C:N ratio | pH | EC (dS/m) |
|---|---|---|---|---|---|---|
| Bialet Massé | 7.3 | 4.2 | 0.3 | 14.0 | 7.6 | 0.5 |
| Río Cuarto | 2.5 | 1.4 | 0.1 | 11.2 | 7.3 | 0.9 |
| Ongamira | 2.0 | 1.1 | 0.1 | 10.4 | 7.8 | 0.4 |
| Tulumba | 3.4 | 1.9 | 0.1 | 12.5 | 7.4 | 0.4 |
| Population | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bialet Massé | Río Cuarto | Ongamira | Tulumba | |||||||||
| Mean | CV * | Mean | CV * | Mean | CV * | Mean | CV * | |||||
| PH (cm) | 13.8 ± 0.5 | b | 25.1 | 10.4 ± 0.4 | a | 37.4 | 15.0 ± 0.7 | b | 30.4 | 18.5 ± 0.7 | c | 25.4 |
| BN (n◦. plant−1) | 2.5 ± 0.2 | a | 74.3 | 2.5 ± 0.2 | a | 87.1 | 5.0 ± 0.5 | b | 63.6 | 5.7 ± 0.6 | b | 70.1 |
| IN (n◦. plant−1) | 43.0 ± 1.3 | c | 22.2 | 26.1± 0.7 | a | 27.3 | 31.2 ± 0.9 | b | 19.2 | 32.9 ± 1.0 | b | 19.2 |
| IL (cm) | 0.3 ± 8 × 10−3 | a | 20.1 | 0.4 ± 8.7 × 10−3 | b | 24.0 | 0.4 ± 1.9 × 10−2 | c | 28.8 | 0.5 ± 1.7 × 10−2 | c | 22.5 |
| DLB (mm) | 0.7 ± 4.2 × 10−2 | a | 43.8 | 0.7 ± 3.8 × 10−2 | a | 54.3 | 0.9 ± 0.1 | b | 45.1 | 0.7 ± 0.1 | a | 46.7 |
| Leaf-L (cm) | 0.4 ± 9.4 × 10−3 | a | 17.6 | 0.5 ± 1.1 × 10−2 | b | 21.5 | 0.5 ± 2.0 × 10−2 | b | 26.8 | 0.6 ± 1.3 × 10−2 | c | 13.5 |
| Leaf-W (cm) | 0.1 ± 3.4 × 10−3 | a | 26.5 | 0.1 ± 4.1 × 10−3 | b | 32.1 | 0.1 ± 6.6 × 10−3 | b | 35.5 | 0.1 ± 5.0 × 10−3 | c | 21.2 |
| Leaf-A (cm2) | 2.4 × 10−2 ± 1.6 × 10−3 | a | 49.4 | 4.3 × 10−2 ± 2.2 × 10−3 | c | 53.0 | 3.7 × 10−2 ± 3.2 × 10−3 | b | 57.5 | 4.9 × 10−2 ± 1.6 × 10−3 | c | 20.0 |
| Leaf L/W | 4.4 ± 0.1 | a | 21.0 | 4.2 ± 0.1 | a | 18.9 | 4.3 ± 0.1 | a | 21.9 | 4.3 ± 0.2 | a | 25.5 |
| EO (%) | 1.6 ± 0.7 | a | 99.0 | 1.0 ± 0.4 | a | 70.2 | 2.1 ± 0.5 | a | 53.2 | 1.7 ± 0.5 | a | 72.9 |
| Population | ||||
|---|---|---|---|---|
| Bialet Massé | Río Cuarto | Ongamira | Tulumba | |
| Limonene | 0.8 | 0.6 | 2.8 | - |
| Menthone | 17.0 | 18.8 | 63.7 | 59.1 |
| Isomenthone | - | 27.4 | 2.2 | 2.10 |
| Isopulegone | 0.9 | 1.2 | - | - |
| Pulegone | 75.2 | 52.0 | 25.9 | 36.2 |
| D-Germacrene | 1.0 | - | - | - |
| Bicyclogermacrene | - | - | 5.3 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turco, F.R.; Chaves, A.G.; de Blas, F.J.; Torres, L.E. Phenotypic and Phytochemical Variability Among Four Populations of Hedeoma multiflora Benth. (Tomillito de las Sierras) Native to the Province of Córdoba—In Situ Evaluation. Wild 2025, 2, 33. https://doi.org/10.3390/wild2030033
Turco FR, Chaves AG, de Blas FJ, Torres LE. Phenotypic and Phytochemical Variability Among Four Populations of Hedeoma multiflora Benth. (Tomillito de las Sierras) Native to the Province of Córdoba—In Situ Evaluation. Wild. 2025; 2(3):33. https://doi.org/10.3390/wild2030033
Chicago/Turabian StyleTurco, Florencia R., Ana G. Chaves, Francisco J. de Blas, and Lorena E. Torres. 2025. "Phenotypic and Phytochemical Variability Among Four Populations of Hedeoma multiflora Benth. (Tomillito de las Sierras) Native to the Province of Córdoba—In Situ Evaluation" Wild 2, no. 3: 33. https://doi.org/10.3390/wild2030033
APA StyleTurco, F. R., Chaves, A. G., de Blas, F. J., & Torres, L. E. (2025). Phenotypic and Phytochemical Variability Among Four Populations of Hedeoma multiflora Benth. (Tomillito de las Sierras) Native to the Province of Córdoba—In Situ Evaluation. Wild, 2(3), 33. https://doi.org/10.3390/wild2030033








