Precision Nutrition for Dementia: Exploring the Potential in Mitigating Dementia Progression
Abstract
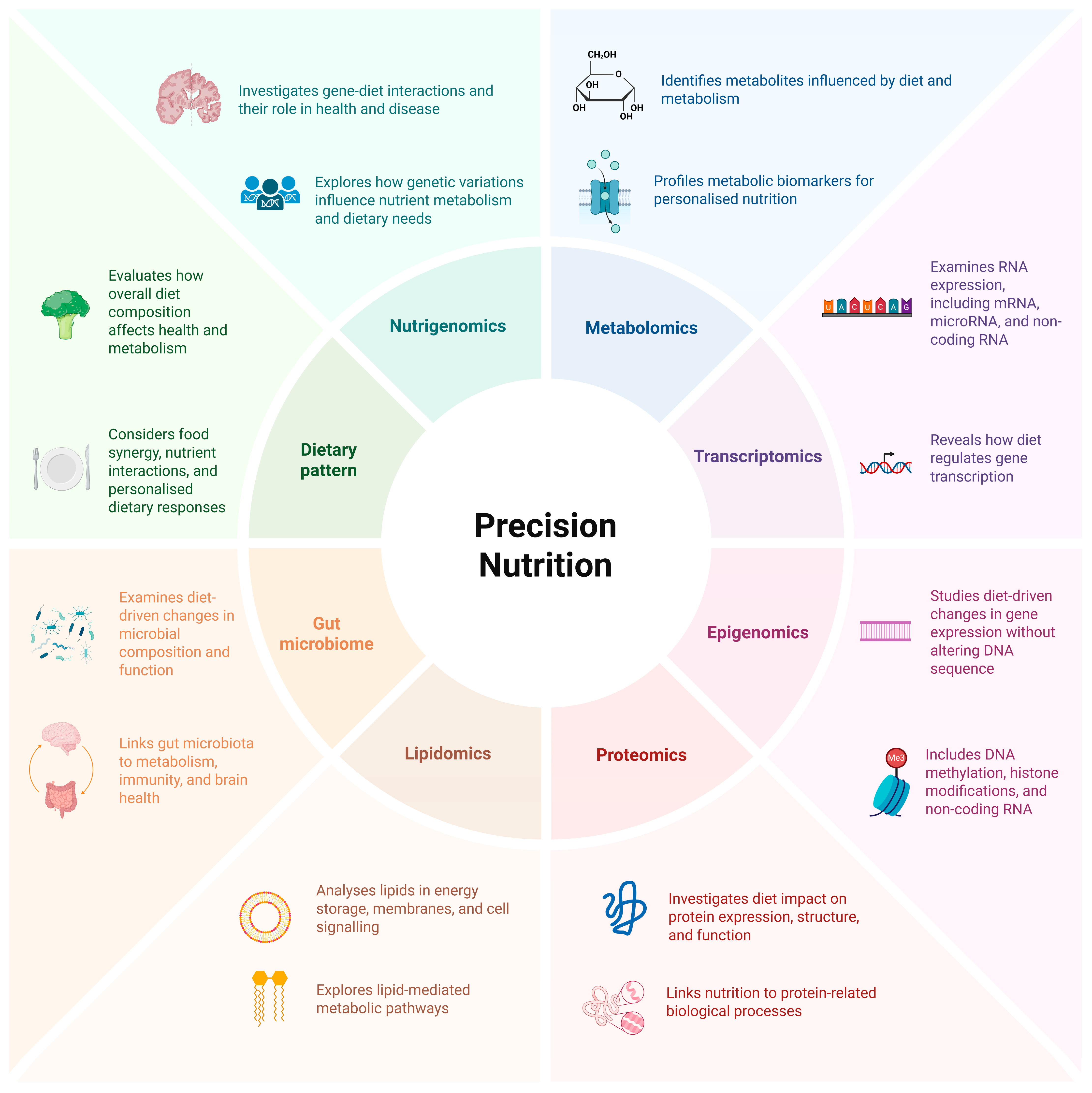
1. Introduction
2. Methods
3. Current Evidence
3.1. Dietary Patterns, ApoE4, the Gut Microbiome and Dementia Risk
3.1.1. Mediterranean, DASH and MIND Diet
3.1.2. Ketogenic Diet
3.2. ApoE4 and Nutrition
3.3. The Role of the Gut Microbiome and Malnutrition in Precision Nutrition
4. Nutrient–Gene Interactions as Potential Targets for Precision Nutrition and Dementia
4.1. Omega-3 Polyunsaturated Fatty Acids
4.2. Vitamin D
4.3. B6, B9 and B12 Vitamins
5. Considerations for Precision Nutrition in Dementia
5.1. Pharmacological Considerations: Malnutrition, Gut Microbiome and Polypharmacy
5.2. Direct-to-Consumer Genetic Testing
6. Advancing Precision Nutrition in Dementia: Opportunities and Solutions
6.1. Insights from Precision Nutrition Trials in Other Chronic Diseases to Inform Precision Nutrition in Dementia
6.2. The Role of Healthcare Professionals and Care Partners in Supporting Precision Nutrition
7. Artificial Intelligence in Dementia
7.1. Conceptual Applications of Artificial Intelligence in Precision Nutrition for Dementia
7.2. Wearable Sensor Technology and Biomarkers for Early Detection of Dementia
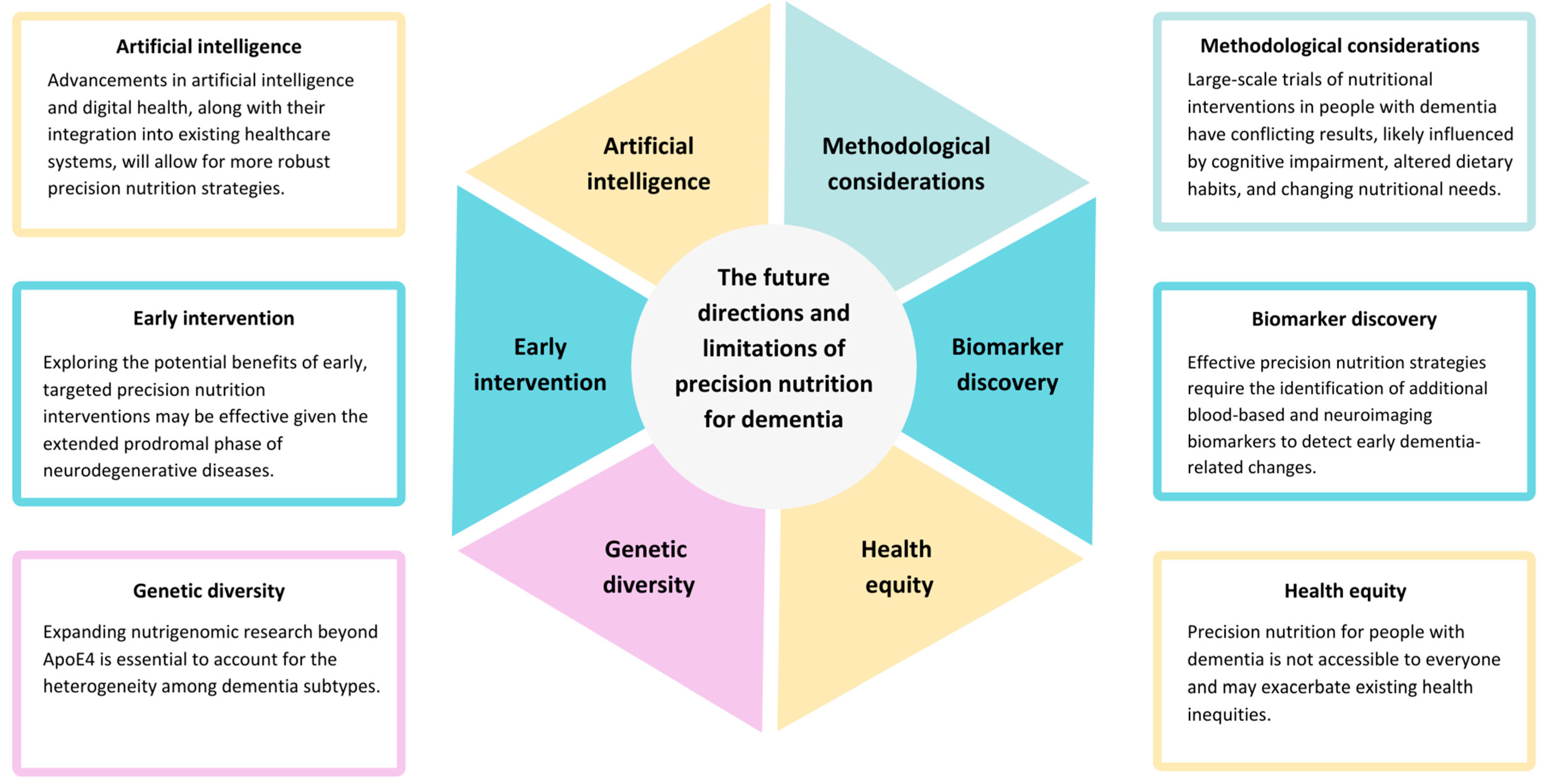
8. Future Directions and Limitations
8.1. Advances in Precision Nutrition for Dementia
8.2. Targeting Early Intervention, Genetic Diversity and Methodological Issues
8.3. Biomarker Discovery and Validation
8.4. Health Equity
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chin, K.S. Pathophysiology of dementia. Aust. J. Gen. Pract. 2023, 52, 516–521. [Google Scholar] [CrossRef]
- Word Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Dementia%20is%20a%20syndrome%20that,usual%20consequences%20of%20biological%20ageing (accessed on 18 January 2025).
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 18 January 2025).
- Samieri, C.; Yassine, H.N.; Melo van Lent, D.; Lefèvre-Arbogast, S.; van de Rest, O.; Bowman, G.L.; Scarmeas, N. Personalized nutrition for dementia prevention. Alzheimer’s Dement. 2022, 18, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Lagoumintzis, G.; Patrinos, G.P. Triangulating nutrigenomics, metabolomics and microbiomics toward personalised nutrition and healthy living. Hum. Genom. 2023, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Marcum, J.A. Nutrigenetics/nutrigenomics, personalized nutrition, and precision healthcare. Curr. Nutr. Rep. 2020, 9, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, M.K. Chapter Six—Nutrigenetics and nutrigenomics—A personalized approach to nutrition. In Advances in Genetics; Kumar, D., Ed.; Academic Press: New York, NY, USA, 2021; Volume 108, pp. 277–340. [Google Scholar]
- Bettencourt, C.; Skene, N.; Bandres-Ciga, S.; Anderson, E.; Winchester, L.M.; Foote, I.F.; Schwartzentruber, J.; Botia, J.A.; Nalls, M.; Singleton, A.; et al. Artificial intelligence for dementia genetics and omics. Alzheimer’s Dement. 2023, 19, 5905–5921. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; de Roos, B. Nutrigenomics: Lessons learned and future perspectives. Am. J. Clin. Nutr. 2021, 113, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D.; Pandita, A. Omics technology for the promotion of nutraceuticals and functional foods. Front. Physiol. 2022, 13, 817247. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, B.J.; Reis, B.Z.; Duarte, G.B.S.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of microRNAs and nutrition in modulating inflammation and chronic diseases. Nutrients 2017, 9, 1168. [Google Scholar] [CrossRef]
- Abinaya, B.; Waseem, M.; Kashif, M.; Srinivasan, H. Lipidomics: An excellent tool for chronic disease detection. Curr. Res. Transl. Med. 2022, 70, 103346. [Google Scholar] [CrossRef]
- Lyall, D.M.; Kormilitzin, A.; Lancaster, C.; Sousa, J.; Petermann-Rocha, F.; Buckley, C.; Harshfield, E.L.; Iveson, M.H.; Madan, C.R.; McArdle, R.; et al. Artificial intelligence for dementia-Applied models and digital health. Alzheimer’s Dement. 2023, 19, 5872–5884. [Google Scholar] [CrossRef]
- Lee, B.Y.; Ordovás, J.M.; Parks, E.J.; Anderson, C.A.M.; Barabási, A.L.; Clinton, S.K.; de la Haye, K.; Duffy, V.B.; Franks, P.W.; Ginexi, E.M.; et al. Research gaps and opportunities in precision nutrition: An NIH workshop report. Am. J. Clin. Nutr. 2022, 116, 1877–1900. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Parnell, L.D.; Magadmi, R.; Zwanger, S.; Shukitt-Hale, B.; Lai, C.Q.; Ordovás, J.M. Dietary responses of dementia-related genes encoding metabolic enzymes. Nutrients 2023, 15, 644. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Duan, S.; Wang, Z.; Li, X.; Zhou, Y.; Zhang, X.; Zhang, Y.W.; Xu, H.; Zheng, H. Insights into the role of CSF1R in the central nervous system and neurological disorders. Front. Aging Neurosci. 2021, 13, 789834. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Wang, L.; Xu, J.; Chen, X.; Bao, Y.; Hu, Y.; Jin, S.; Tian, R.; Bai, W.; et al. Alzheimer’s disease rs11767557 variant regulates EPHA1 gene expression specifically in human whole blood. J. Alzheimer’s Dis. 2018, 61, 1077–1088. [Google Scholar] [CrossRef]
- 19 Del Río, C.; Segura-Carretero, A. Neuroprotection with bioactive compounds. Nutrients 2023, 15, 4612. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. Epigenetics, Nutrition, and the vrain: Improving mental health through diet. Int. J. Mol. Sci. 2024, 25, 4036. [Google Scholar] [CrossRef]
- Tripathi, A.; Pandey, V.K.; Sharma, G.; Sharma, A.R.; Taufeeq, A.; Jha, A.K.; Kim, J.C. Genomic insights into dementia: Precision medicine and the impact of gene-environment interaction. Aging Dis. 2024, 15, 2113–2135. [Google Scholar] [CrossRef] [PubMed]
- Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Mackay, C.E.; Cuthbertson, D.J. High intake of ultra-processed food is associated with dementia in adults: A systematic review and meta-analysis of observational studies. J. Neurol. 2024, 271, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Gunstad, J.; Gustafson, D.R.; Heymsfield, S.B.; Kral, J.G.; Launer, L.J.; Penninger, J.; Phillips, D.I.; Scarmeas, N. The paradox of overnutrition in aging and cognition. Ann. N. Y. Acad. Sci. 2013, 1287, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Yerstein, O.; Mendez, M.F. Dietary recommendations for patients with dementia. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12011. [Google Scholar] [CrossRef]
- Passos, L.; Tavares, J.; Batchelor, M.; Figueiredo, D. Interventions to address mealtime support needs in dementia: A scoping review. PLoS ONE 2024, 19, e0300987. [Google Scholar] [CrossRef]
- Arifin, H.; Chen, R.; Banda, K.J.; Kustanti, C.Y.; Chang, C.-Y.; Lin, H.-C.; Liu, D.; Lee, T.-Y.; Chou, K.-R. Meta-analysis and moderator analysis of the prevalence of malnutrition and malnutrition risk among older adults with dementia. Int. J. Nurs. Stud. 2024, 150, 104648. [Google Scholar] [CrossRef] [PubMed]
- Kishino, Y.; Sugimoto, T.; Kimura, A.; Kuroda, Y.; Uchida, K.; Matsumoto, N.; Saji, N.; Niida, S.; Sakurai, T. Longitudinal association between nutritional status and behavioral and psychological symptoms of dementia in older women with mild cognitive impairment and early-stage Alzheimer’s disease. Clin. Nutr. 2022, 41, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Sugimoto, T.; Kitamori, K.; Saji, N.; Niida, S.; Toba, K.; Sakurai, T. Malnutrition is associated with behavioral and psychiatric symptoms of dementia in older women with mild cognitive impairment and early-stage Alzheimer’s disease. Nutrients 2019, 11, 1951. [Google Scholar] [CrossRef]
- Kauzor, K.; Drewel, M.; Gonzalez, H.; Rattinger, G.B.; Hammond, A.G.; Wengreen, H.; Lyketsos, C.G.; Tschanz, J.T. Malnutrition and neuropsychiatric symptoms in dementia: The cache county dementia progression study. Int. Psychogeriatr. 2023, 35, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.; Csipke, E.; Moniz-Cook, E.; Leung, P.; Walton, H.; Charlesworth, G.; Spector, A.; Hogervorst, E.; Mountain, G.; Orrell, M. The development of the promoting independence in dementia (PRIDE) intervention to enhance independence in dementia. Clin. Interv. Aging 2019, 14, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.; Done, N.; Desai, U.; Georgieva, M.; Gomez-Lievano, A.; Ye, W.; Zhao, A.; Eid, D.; Hilts, A.; Kirson, N.; et al. Potential implications of slowing disease progression in amyloid-positive early Alzheimer’s disease: Estimates from real-world data. J. Prev. Alzheimer’s Dis. 2024, 11, 310–319. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Faxén-Irving, G.; Frühwald, T.; Hooper, L.; Keller, H.; Porter, J.; Rothenberg, E.; Suominen, M.; Wirth, R.; et al. ESPEN guideline on nutrition and hydration in dementia–Update 2024. Clin. Nutr. 2024, 43, 1599–1626. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- Cammann, D.; Lu, Y.; Cummings, M.J.; Zhang, M.L.; Cue, J.M.; Do, J.; Ebersole, J.; Chen, X.; Oh, E.C.; Cummings, J.L.; et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci. Rep. 2023, 13, 5258. [Google Scholar] [CrossRef]
- World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Shoaib, S.; Ansari, M.A.; Fatease, A.A.; Safhi, A.Y.; Hani, U.; Jahan, R.; Alomary, M.N.; Ansari, M.N.; Ahmed, N.; Wahab, S.; et al. Plant-derived bioactive compounds in the management of neurodegenerative disorders: Challenges, future directions and molecular mechanisms involved in neuroprotection. Pharmaceutics 2023, 15, 749. [Google Scholar] [CrossRef]
- Lourida, I.; Soni, M.; Thompson-Coon, J.; Purandare, N.; Lang, I.A.; Ukoumunne, O.C.; Llewellyn, D.J. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 2013, 24, 479–489. [Google Scholar] [CrossRef]
- Charisis, S.; Ntanasi, E.; Yannakoulia, M.; Anastasiou, C.A.; Kosmidis, M.H.; Dardiotis, E.; Gargalionis, A.N.; Patas, K.; Chatzipanagiotou, S.; Mourtzinos, I.; et al. Diet inflammatory index and dementia incidence: A population-based study. Neurology 2021, 97, e2381–e2391. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Sommariva, A.; Degoni, L.M.; Gallo, G.; Mancarella, M.; Natarelli, F.; Savoia, A.; Catalini, A.; Ferranti, R.; Pregliasco, F.E.; et al. Association between Mediterranean diet and dementia and Alzheimer disease: A systematic review with meta-analysis. Aging Clin. Exp. Res. 2024, 36, 77. [Google Scholar] [CrossRef]
- Radd-Vagenas, S.; Duffy, S.L.; Naismith, S.L.; Brew, B.J.; Flood, V.M.; Fiatarone Singh, M.A. Effect of the Mediterranean diet on cognition and brain morphology and function: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2018, 107, 389–404. [Google Scholar] [CrossRef]
- Barnes, L.L.; Dhana, K.; Liu, X.; Carey, V.J.; Ventrelle, J.; Johnson, K.; Hollings, C.S.; Bishop, L.; Laranjo, N.; Stubbs, B.J.; et al. Trial of the MIND diet for prevention of cognitive decline in older persons. N. Engl. J. Med. 2023, 389, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; Agarwal, P.; Holland, T.M.; van Dam, R.M. MIND dietary pattern and its association with cognition and incident dementia in the UK Biobank. Nutrients 2022, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; James, B.D.; Agarwal, P.; Aggarwal, N.T.; Cherian, L.J.; Leurgans, S.E.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A. MIND diet, common brain pathologies, and cognition in community-dwelling older adults. J. Alzheimer’s Dis. 2021, 83, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, Á.; Lehoczki, A.; Mózes, N.; Grosso, G.; Godos, J.; et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: A meta-analysis. GeroScience 2025, 47, 3111–3130. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; Rajan, K.; Aggarwal, N.; Arfanakis, K.; Carey, V.; Sacks, F.; Barnes, L. Dietary intervention, biomarkers of brain pathology and neurodegeneration, and cognition: The MIND trial study. Alzheimer’s Dement. 2025, 20, e087564. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging 2012, 33, 425.e419–425.e427. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Saif, N.; Ariza, I.E.; Isaacson, R.S. Precision Nutrition for Alzheimer’s Prevention in ApoE4 Carriers. Nutrients 2021, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Theodoridis, X.; Gkouskou, K.K.; Evangeliou, A.; Dardiotis, E.; Bogdanos, D.P. To keto or not to keto? A systematic review of randomized controlled trials assessing the effects of ketogenic therapy on Alzheimer disease. Adv. Nutr. 2020, 11, 1583–1602. [Google Scholar] [CrossRef]
- Devranis, P.; Vassilopoulou, Ε.; Tsironis, V.; Sotiriadis, P.M.; Chourdakis, M.; Aivaliotis, M.; Tsolaki, M. Mediterranean diet, Ketogenic diet or MIND diet for aging populations with cognitive decline: A systematic review. Life 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Akiyama, T.; Saigusa, D.; Hishinuma, E.; Matsukawa, N.; Shibata, T.; Tsuchiya, H.; Mori, A.; Fujii, Y.; Mogami, Y.; et al. Comprehensive study of metabolic changes induced by a ketogenic diet therapy using GC/MS- and LC/MS-based metabolomics. Seizure: Eur. J. Epilepsy 2023, 107, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Huynh, K.; Giles, C.; Wang, T.; Mellett, N.A.; Duong, T.; Blach, C.; Schimmel, L.; Register, T.C.; Blennow, K.; et al. Consuming a modified Mediterranean ketogenic diet reverses the peripheral lipid signature of Alzheimer’s disease in humans. Commun. Med. 2025, 5, 11. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Saddiki, H.; Fayosse, A.; Cognat, E.; Sabia, S.; Engelborghs, S.; Wallon, D.; Alexopoulos, P.; Blennow, K.; Zetterberg, H.; Parnetti, L.; et al. Age and the association between apolipoprotein E genotype and Alzheimer disease: A cerebrospinal fluid biomarker-based case-control study. PLoS Med. 2020, 17, e1003289. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.W.; Akay, L.A.; Davila-Velderrain, J.; von Maydell, D.; Mathys, H.; Davidson, S.M.; Effenberger, A.; Chen, C.-Y.; Maner-Smith, K.; Hajjar, I.; et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 2022, 611, 769–779. [Google Scholar] [CrossRef]
- Shatwan, I.M.; Weech, M.; Jackson, K.G.; Lovegrove, J.A.; Vimaleswaran, K.S. Apolipoprotein E gene polymorphism modifies fasting total cholesterol concentrations in response to replacement of dietary saturated with monounsaturated fatty acids in adults at moderate cardiovascular disease risk. Lipids Health Dis. 2017, 16, 222. [Google Scholar] [CrossRef]
- Carvalho-Wells, A.L.; Jackson, K.G.; Lockyer, S.; Lovegrove, J.A.; Minihane, A.M. APOE genotype influences triglyceride and C-reactive protein responses to altered dietary fat intake in UK adults. Am. J. Clin. Nutr. 2012, 96, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, H.; Zhao, Z.; Li, S.; Zhang, X.; Guo, R.; Liu, H.; Yuan, Y.; Li, W.; Song, Q.; et al. Type 3 diabetes and metabolic reprogramming of brain neurons: Causes and therapeutic strategies. Mol. Med. 2025, 31, 61. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.M.I. Insulin resistance as the molecular link between diabetes and Alzheimer’s disease. World J. Diabetes 2024, 15, 1430–1447. [Google Scholar] [CrossRef]
- Sarnowski, C.; Huan, T.; Ma, Y.; Joehanes, R.; Beiser, A.; DeCarli, C.S.; Heard-Costa, N.L.; Levy, D.; Lin, H.; Liu, C.-T.; et al. Multi-tissue epigenetic analysis identifies distinct associations underlying insulin resistance and Alzheimer’s disease at CPT1A locus. Clin. Epigenetics 2023, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lin, Y.; Li, X.; Driver, J.A.; Liang, L. Shared genetic architecture between metabolic traits and Alzheimer’s disease: A large-scale genome-wide cross-trait analysis. Hum. Genet. 2019, 138, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Rashtchian, A.; Etemadi, M.H.; Asadi, E.; Binaei, S.; Abbasi, M.; Bayani, M.; Izadi, E.; Sadat-Madani, S.-F.; Naziri, M.; Khoshravesh, S.; et al. Diabetes mellitus and risk of incident dementia in APOE ɛ4 carriers: An updated meta-analysis. BMC Neurosci. 2024, 25, 28. [Google Scholar] [CrossRef]
- Chawla, M.; Gupta, R.; Das, B. Gut microbiome dysbiosis in malnutrition. Prog. Mol. Biol. Transl. Sci. 2022, 192, 205–229. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Zhang, P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Łuc, M.; Misiak, B.; Pawłowski, M.; Stańczykiewicz, B.; Zabłocka, A.; Szcześniak, D.; Pałęga, A.; Rymaszewska, J. Gut microbiota in dementia. Critical review of novel findings and their potential application. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110039. [Google Scholar] [CrossRef] [PubMed]
- Ayten, Ş.; Bilici, S. Modulation of gut microbiota through dietary intervention in neuroinflammation and Alzheimer’s and Parkinson’s diseases. Curr. Nutr. Rep. 2024, 13, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Son, S.J.; Lee, D.Y.; Roh, H.W.; Ly, M.; Kolobaric, A.; Aizenstein, H.; Andreescu, C.; Jašarević, E.; Pascoal, T.A.; Ferreira, P.C.L.; et al. Brain age mediates gut microbiome dysbiosis-related cognition in older adults. Alzheimer’s Res. Ther. 2025, 17, 52. [Google Scholar] [CrossRef]
- Lukiw, W.J. Gastrointestinal (GI) tract microbiome-derived neurotoxins—Potent neuro-inflammatory signals from the GI tract via the systemic circulation into the brain. Front. Cell. Infect. Microbiol. 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Stamova, B.; Jin, L.W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; D’Angelo, S. Gut microbiota modulation through Mediterranean diet foods: Implications for human health. Nutrients 2025, 17, 948. [Google Scholar] [CrossRef]
- Khavandegar, A.; Heidarzadeh, A.; Angoorani, P.; Hasani-Ranjbar, S.; Ejtahed, H.-S.; Larijani, B.; Qorbani, M. Adherence to the Mediterranean diet can beneficially affect the gut microbiota composition: A systematic review. BMC Med. Genom. 2024, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.H.R.; Chappell, H.F.; Zulyniak, M.A. Dietary and supplemental long-chain omega-3 fatty acids as moderators of cognitive impairment and Alzheimer’s disease. Eur. J. Nutr. 2022, 61, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 205–211. [Google Scholar] [CrossRef]
- Larrieu, T.; Layé, S. Food for mood: Relevance of nutritional omega-3 fatty acids for depression and anxiety. Front. Physiol. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Wu, W.C.; Wu, P.Y.; Chan, C.Y.; Lee, M.F.; Huang, C.Y. Effect of FADS1 rs174556 genotype on polyunsaturated fatty acid status: A systematic review and meta-analysis. Adv. Nutr. 2023, 14, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Xhaard, C.; Rouget, R.; Thuillier, Q.; Plunde, O.; Larsson, S.C.; Girerd, N.; Ferreira, J.P.; Boivin, J.-M.; Bozec, E.; et al. Fatty acid desaturase genetic variations and dietary omega-3 fatty acid intake associate with arterial stiffness. Eur. Heart J. Open 2022, 2, oeac016. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Pérez, S.D.; González-Becerra, K.; Barrón-Cabrera, E.; Muñoz-Valle, J.F.; Armendáriz-Borunda, J.; Martínez-López, E. FADS1 genetic variant and omega-3 supplementation are associated with changes in fatty acid composition in red blood cells of subjects with obesity. Nutrients 2024, 16, 3522. [Google Scholar] [CrossRef] [PubMed]
- Brain, J.; Greene, L.; Tang, E.Y.H.; Louise, J.; Salter, A.; Beach, S.; Turnbull, D.; Siervo, M.; Stephan, B.C.M.; Tully, P.J. Cardiovascular disease, associated risk factors, and risk of dementia: An umbrella review of meta-analyses. Front. Epidemiol. 2023, 3, 1095236. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cordero, J.A.; Pérez-Pérez, A.; Jiménez-Cortegana, C.; Alba, G.; Flores-Barragán, A.; Sánchez-Margalet, V. Obesity as a risk factor for dementia and Alzheimer’s disease: The role of leptin. Int. J. Mol. Sci. 2022, 23, 5202. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, L.; Qi, H.; Hu, C.; Jia, X.; Lin, H.; Wang, S.; Lin, L.; Zhang, Y.; Zheng, R.; et al. Brain tissue- and cell type-specific eQTL mendelian randomization reveals efficacy of FADS1 and FADS2 on cognitive function. Transl. Psychiatry 2024, 14, 77. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Zhao, Y.; Meng, X.; Su, J.; Zhi, S.; Song, D.; Gao, S.; Sun, J.; Sun, J. How to manage comorbidities in people with dementia: A scoping review. Ageing Res. Rev. 2023, 88, 101937. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.N.M.; West, A.L.; Ostermann, A.I.; Schebb, N.H.; Calder, P.C.; Minihane, A.M. APOE genotype modifies the plasma oxylipin response to omega-3 polyunsaturated fatty acid supplementation in healthy individuals. Front. Nutr. 2021, 8, 723813. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Georgousopoulou, E.N.; Dadigamuwage, L.; Kellett, J.; Panagiotakos, D.B.; Thomas, J.; McKune, A.J.; Mellor, D.D.; Naumovski, N. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: A 10-year systematic review of randomised controlled trials. Br. J. Nutr. 2018, 119, 280–298. [Google Scholar] [CrossRef]
- Ghahremani, M.; Smith, E.E.; Chen, H.-Y.; Creese, B.; Goodarzi, Z.; Ismail, Z. Vitamin D supplementation and incident dementia: Effects of sex, APOE, and baseline cognitive status. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12404. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin d supplementation improves cognitive function through reducing oxidative stress regulated by telomere length in older adults with mild cognitive impairment: A 12-month randomized controlled trial. J. Alzheimer’s Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef]
- Shea, M.K.; Barger, K.; Dawson-Hughes, B.; Leurgans, S.E.; Fu, X.; James, B.D.; Holland, T.M.; Agarwal, P.; Wang, J.; Matuszek, G.; et al. Brain vitamin D forms, cognitive decline, and neuropathology in community-dwelling older adults. Alzheimer’s Dement. 2023, 19, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Han, X.; Gong, J.; Wang, P.; Sun, W.; Xu, C.; Shan, A.; Wang, X.; Luan, H.; Li, S.; et al. Nutrition: A non-negligible factor in the pathogenesis and treatment of Alzheimer’s disease. Alzheimer’s Dement. 2025, 21, e14547. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, T.; Ma, L.; Wei, W.; Li, Z.; Jiang, X.; Sun, J.; Pei, H.; Li, H. Vitamin D receptor gene polymorphisms and risk of Alzheimer disease and mild cognitive impairment: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 2255–2264. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; De Luis-Román, D.-A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.-L. Vitamin d receptor (VDR) gene polymorphisms modify the response to vitamin d supplementation: A systematic review and meta-analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef]
- Ando, K.; Ferlini, L.; Suain, V.; Yilmaz, Z.; Mansour, S.; Le Ber, I.; Bouchard, C.; Leroy, K.; Durr, A.; Clot, F.; et al. De novo MAPT mutation G335A causes severe brain atrophy, 3R and 4R PHF-tau pathology and early onset frontotemporal dementia. Acta Neuropathol. Commun. 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and dementia: An international consensus statement. J. Alzheimer’s Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef]
- Gil Martínez, V.; Avedillo Salas, A.; Santander Ballestín, S. Vitamin supplementation and dementia: A systematic review. Nutrients 2022, 14, 1033. [Google Scholar] [CrossRef]
- Miyan, J.; Buttercase, C.; Beswick, E.; Miyan, S.; Moshkdanian, G.; Naz, N. Folate related pathway gene analysis reveals a novel metabolic variant associated with alzheimer’s disease with a change in metabolic profile. Metabolites 2022, 12, 475. [Google Scholar] [CrossRef]
- Roussotte, F.F.; Hua, X.; Narr, K.L.; Small, G.W.; Thompson, P.M. The C677T variant in MTHFR modulates associations between brain integrity, mood, and cognitive functioning in old age. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 280–288. [Google Scholar] [CrossRef]
- Péter, S.; Navis, G.; de Borst, M.H.; von Schacky, C.; van Orten-Luiten, A.C.B.; Zhernakova, A.; Witkamp, R.F.; Janse, A.; Weber, P.; Bakker, S.J.L.; et al. Public health relevance of drug–nutrition interactions. Eur. J. Nutr. 2017, 56, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Boullata, J.I.; Hudson, L.M. Drug–nutrient interactions: A broad view with implications for practice. J. Acad. Nutr. Diet. 2012, 112, 506–517. [Google Scholar] [CrossRef]
- Liyanage, S.I.; Vilekar, P.; Weaver, D.F. Nutrients in alzheimer’s disease: The interaction of diet, drugs and disease. Can. J. Neurol. Sci. 2019, 46, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.L.; Farlow, M.R.; Doody, R.S.; Mohs, R.; Friedhoff, L.T.; Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 1998, 50, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of Selegiline, Alpha-Tocopherol, or both as treatment for Alzheimer’s disease. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Corey-Bloom, J.; Anand, R.; Veach, J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int. J. Geriatr. Psychopharmacol. 1998, 1, 55–65. [Google Scholar]
- Raskind, M.A.; Peskind, E.R.; Wessel, T.; Yuan, W.; Galantamine USA-Study Group. Galantamine in AD. Neurology 2000, 54, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Isik, A.T.; Stubbs, B.; Solmi, M.; Volpe, M.; Luchini, C.; D’Onofrio, G.; Pilotto, A.; Manzato, E.; Sergi, G.; et al. Acetylcholinesterase inhibitors are associated with weight loss in older people with dementia: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Ericson, U.; Jönsson, D.; Miari, M.; Athanasiadis, P.; Baldanzi, G.; Brunkwall, L.; Hellstrand, S.; Klinge, B.; Melander, O.; et al. New connections of medication use and polypharmacy with the gut microbiota composition and functional potential in a large population. Sci. Rep. 2024, 14, 23723. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Nishijima, S.; Miyoshi-Akiyama, T.; Kojima, Y.; Kimura, M.; Aoki, R.; Ohsugi, M.; Ueki, K.; Miki, K.; Iwata, E.; et al. Population-level metagenomics uncovers distinct effects of multiple medications on the human gut microbiome. Gastroenterology 2022, 163, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, R.; Penhos, E. The gut microbiome and Alzheimer’s disease: Complex and bidirectional interactions. Neurosci. Biobehav. Rev. 2022, 141, 104814. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Ruhl, G.L.; Hazel, J.W.; Clayton, E.W.; Malin, B.A. Public attitudes toward direct to consumer genetic testing. AMIA Annu. Symp. Proc. 2019, 2019, 774–783. [Google Scholar]
- Guasch-Ferré, M.; Dashti, H.S.; Merino, J. Nutritional genomics and direct-to-consumer genetic testing: An overview. Adv. Nutr. 2018, 9, 128–135. [Google Scholar] [CrossRef]
- Nolan, J.J.; Ormondroyd, E. Direct-to-consumer genetic tests providing health risk information: A systematic review of consequences for consumers and health services. Clin. Genet. 2023, 104, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.A.; Guerrini, C.J.; McGuire, A.L. Direct-to-consumer genetic testing: Value and risk. Annu. Rev. Med. 2021, 72, 151–166. [Google Scholar] [CrossRef]
- Jiang, S.; Liberti, L.; Lebo, D. Direct-to-consumer genetic testing: A comprehensive review. Ther. Innov. Regul. Sci. 2023, 57, 1190–1198. [Google Scholar] [CrossRef]
- Voruganti, V.S. Precision nutrition: Recent advances in obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Tuncay, C.; Ergoren, M.C. A systematic review of precision nutrition and mediterranean diet: A personalized nutrition approaches for prevention and management of obesity related disorders. Clin. Nutr. ESPEN 2020, 38, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary macronutrients and the gut microbiome: A precision nutrition approach to improve cardiometabolic health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Clarke, E.D.; Gómez-Martín, M.; Cross, V.; Collins, C.E.; Stanford, J. Do precision and personalised nutrition interventions improve risk factors in adults with prediabetes or metabolic syndrome? A systematic review of randomised controlled trials. Nutrients 2024, 16, 1479. [Google Scholar] [CrossRef]
- Lahiouel, A.; Kellett, J.; Isbel, S.; D’Cunha, N.M. An exploratory study of nutrition knowledge and challenges faced by informal carers of community-dwelling people with dementia: Online survey and thematic analysis. Geriatrics 2023, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Jinnette, R.; Narita, A.; Manning, B.; McNaughton, S.A.; Mathers, J.C.; Livingstone, K.M. Does personalized nutrition advice improve dietary intake in healthy adults? A systematic review of randomized controlled trials. Adv. Nutr. 2021, 12, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of personalized nutrition on health-related behaviour change: Evidence from the Food4Me European randomized controlled trial. Int. J. Epidemiol. 2016, 46, 578–588. [Google Scholar] [CrossRef]
- Rand, L.; Dunn, M.; Slade, I.; Upadhyaya, S.; Sheehan, M. Understanding and using patient experiences as evidence in healthcare priority setting. Cost. Eff. Resour. Alloc. 2019, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.; Gilliland, J.; O’Connor, C.; Seabrook, J.; Hannaberg, P.; Madill, J. The NOW trial: A pragmatic randomized controlled trial of personalized, genetic-based lifestyle advice. Ph.D. Thesis, Western University, London, ON, Canada, 12 March 2020. [Google Scholar]
- Leskinen, H.M.; Tringham, M.; Karjalainen, H.; Iso-Touru, T.K.; Hietaranta-Luoma, H.-L.; Marnila, P.J.; Pihlava, J.-M.; Hurme, T.; Kankaanpää, S.J.; Puolijoki, H.; et al. APOE genotype disclosure and lifestyle advice in a randomized intervention study with finnish participants. J. Nutr. 2021, 151, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.E.; Gooch, C.; Katz, A.; Wakelee, J.; Slavotinek, A.; Korf, B.R. Pitfalls and challenges in genetic test interpretation: An exploration of genetic professionals experience with interpretation of results. Clin. Genet. 2021, 99, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, M.; Frewer, L.J.; Bryant, E.; Stewart-Knox, B. Factors determining the integration of nutritional genomics into clinical practice by registered dietitians. Trends Food Sci. Technol. 2017, 59, 139–147. [Google Scholar] [CrossRef]
- Martins, M.F.; Murry, L.T.; Telford, L.; Moriarty, F. Direct-to-consumer genetic testing: An updated systematic review of healthcare professionals’ knowledge and views, and ethical and legal concerns. Eur. J. Hum. Genet. 2022, 30, 1331–1343. [Google Scholar] [CrossRef]
- Bensend, T.A.; Veach, P.M.; Niendorf, K.B. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J. Genet. Couns. 2014, 23, 48–63. [Google Scholar] [CrossRef]
- Mathew, M.R.; Medithi, S.; Muley, A. Dietitians’ and nutritionists’ knowledge of nutritional genomics and perception toward genetic testing for a personalized approach in noncommunicable diseases (NCDs) prevention and management in India: A cross-sectional survey. Int. J. Nutr. Pharmacol. Neurol. Dis. 2023, 13, 123–131. [Google Scholar] [CrossRef]
- Nacis, J.S.; Galang, M.R.; Labrador, J.P.H.; Gonzales, M.S.; Dablo, A.M.F.D.; Domalanta-Ronquillo, D.G.A.; Alfonso, V.F.J.; Glorioso, I.G.; Rodriguez, M.P. “Right diet for the right person”: A focus group study of nutritionist-dietitians’ perspectives on nutritional genomics and gene-based nutrition advice. J. Community Genet. 2022, 13, 49–57. [Google Scholar] [CrossRef]
- Greyvensteyn, D.; Walsh, C.M.; Nel, M.; Jordaan, E.M. Nutrigenomics: Perceptions of South African dietitians and general practitioners. Lifestyle Genom. 2022, 16, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Do Rosario, N.A.D.; Gokhale, D.; Gore, M. Exploring the future of nutrigenomics: Dietitians’ perceptions on integration in Indian practice. Genes Nutr. 2025, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Turbitt, E.; McEwen, A.; Atkins, L. Australasian genetic counselors’ perceptions of their role in supporting clients’ behavior change. J. Pers. Med. 2023, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Mayan, I.; Roth, H.; Ghosh, D.; Whitson, H.E.; Johnson, K.G. Genetic and biomarker disclosure process in a memory and aging study. J. Alzheimer’s Dis. 2025, 104, 312–318. [Google Scholar] [CrossRef]
- Mitchelson, K.A.J.; MB, N.C.; Roche, H.M. Systems biology approaches to inform precision nutrition. Proc. Nutr. Soc. 2023, 82, 208–218. [Google Scholar] [CrossRef]
- Moore, J.B. From personalised nutrition to precision medicine: The rise of consumer genomics and digital health. Proc. Nutr. Soc. 2020, 79, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Soares Dias Portela, A.; Saxena, V.; Rosenn, E.; Wang, S.-H.; Masieri, S.; Palmieri, J.; Pasinetti, G.M. Role of artificial intelligence in multinomial decisions and preventative nutrition in Alzheimer’s disease. Mol. Nutr. Food Res. 2024, 68, 2300605. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Herzog, N.J.; Magoulas, G.D. Convolutional neural networks-based framework for early identification of dementia using MRI of brain asymmetry. Int. J. Neural Syst. 2022, 32, 2250053. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sui, H.; Jiao, R.; Zhang, M.; Zhao, X.; Wang, L.; Deng, W.; Liu, X. Random-forest-algorithm-based applications of the basic characteristics and serum and imaging biomarkers to diagnose mild cognitive impairment. Curr. Alzheimer Res. 2022, 19, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Petersen, M.; Johnson, L.; Hall, J.; O’Bryant, S.E. Recursive support vector machine biomarker selection for Alzheimer’s disease. J. Alzheimers Dis. 2021, 79, 1691–1700. [Google Scholar] [CrossRef]
- Shankar, R.; Bundele, A.; Mukhopadhyay, A. Natural language processing of electronic health records for early detection of cognitive decline: A systematic review. NPJ Digit. Med. 2025, 8, 133. [Google Scholar] [CrossRef]
- Bertagnolli, M.M. Advancing health through artificial intelligence/machine learning: The critical importance of multidisciplinary collaboration. PNAS Nexus 2023, 2, pgad356. [Google Scholar] [CrossRef]
- Kameyama, M.; Umeda-Kameyama, Y. Applications of artificial intelligence in dementia. Geriatr. Gerontol. Int. 2024, 24, 25–30. [Google Scholar] [CrossRef]
- Babu, M.; Lautman, Z.; Lin, X.; Sobota, M.H.B.; Snyder, M.P. Wearable devices: Implications for precision medicine and the future of health care. Annu. Rev. Med. 2024, 75, 401–415. [Google Scholar] [CrossRef]
- Soldevila-Domenech, N.; Ayala-Garcia, A.; Barbera, M.; Lehtisalo, J.; Forcano, L.; Diaz-Ponce, A.; Zwan, M.; van der Flier, W.M.; Ngandu, T.; Kivipelto, M.; et al. Adherence and intensity in multimodal lifestyle-based interventions for cognitive decline prevention: State-of-the-art and future directions. Alzheimer’s Res. Ther. 2025, 17, 61. [Google Scholar] [CrossRef]
- Licher, S.; Ahmad, S.; Karamujić-Čomić, H.; Voortman, T.; Leening, M.J.G.; Ikram, M.A.; Ikram, M.K. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat. Med. 2019, 25, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Greaves, C.V.; Rohrer, J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; Piguet, O.; Mummery, C.J.; Naismith, S.L.; Irish, M. The holy grail: Highlighting the need for equitable access to dementia treatments and clinical trials. Lancet Reg. Health West. Pac. 2025, 55, 101492. [Google Scholar] [CrossRef]
- Machado, B.F.H.; König Klever, E.; Libânio, C.d.S. Design for people with dementia: A scoping review on the perspective of inclusion, accessibility, and equity in healthcare. HERD-Health Environ. Res. 2025, 18, 176–193. [Google Scholar] [CrossRef]
- Andreeva, V.A.; Kesse-Guyot, E.; Barberger-Gateau, P.; Fezeu, L.; Hercberg, S.; Galan, P. Cognitive function after supplementation with B vitamins and long-chain omega-3 fatty acids: Ancillary findings from the SU.FOL.OM3 randomized trial. Am. J. Clin. Nutr. 2011, 94, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Vyas, C.M.; Okereke, O.I.; Ogata, S.; Albert, M.; Lee, I.M.; D’Agostino, D.; Buring, J.E.; Cook, N.R.; Grodstein, F.; et al. Marine n-3 fatty acids and cognitive change among older adults in the VITAL randomized trial. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12288. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.N.; Dantoine, T.; Dartigues, J.F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Duplantier, S.C.; Gardner, C.D. A critical review of the study of neuroprotective diets to reduce cognitive decline. Nutrients 2021, 13, 2264. [Google Scholar] [CrossRef] [PubMed]
- Rollo, M.E.; Williams, R.L.; Burrows, T.; Kirkpatrick, S.I.; Bucher, T.; Collins, C.E. What are they really eating? A review on new approaches to dietary intake assessment and validation. Curr. Nutr. Rep. 2016, 5, 307–314. [Google Scholar] [CrossRef]
- Maheshwari, S.; Singh, A.; Ansari, V.A.; Mahmood, T.; Wasim, R.; Akhtar, J.; Verma, A. Navigating the dementia landscape: Biomarkers and emerging therapies. Ageing Res. Rev. 2024, 94, 102193. [Google Scholar] [CrossRef] [PubMed]
- Winchester, L.M.; Harshfield, E.L.; Shi, L.; Badhwar, A.; Khleifat, A.A.; Clarke, N.; Dehsarvi, A.; Lengyel, I.; Lourida, I.; Madan, C.R.; et al. Artificial intelligence for biomarker discovery in Alzheimer’s disease and dementia. Alzheimer’s Dement. 2023, 19, 5860–5871. [Google Scholar] [CrossRef] [PubMed]
- Oluwagbemigun, K.; Anesi, A.; Vrhovsek, U.; Mattivi, F.; Martino Adami, P.; Pentzek, M.; Scherer, M.; Riedel-Heller, S.G.; Weyerer, S.; Bickel, H.; et al. An investigation into the relationship of circulating gut microbiome molecules and inflammatory markers with the risk of incident dementia in later life. Mol. Neurobiol. 2024, 61, 9776–9793. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.A.; Kleinman, D.V.; Pronk, N.; Wrenn Gordon, G.L.; Ochiai, E.; Blakey, C.; Johnson, A.; Brewer, K.H. Addressing health equity and social determinants of health through healthy people 2030. J. Public Health Manag. Pract. 2021, 27, S249–S257. [Google Scholar] [CrossRef] [PubMed]
| Gut Microbiota Genera | AD Outcome | References |
|---|---|---|
| Collinsella | The APOE C rs429358 SNP genetic variant is positively correlated with Collinsella and AD diagnosis, independent of sex and age. | Cammann, et al. [36] |
| Veillonella, Bacteroides and Lachnospira | Identified as a risk factor for AD. Bacteroides release pro-inflammatory liposaccharides capable of bypassing the mucosal barrier of the gastrointestinal tract endothelium leading to induced systemic inflammation. Liposaccharide-induced systemic inflammation is associated with synaptic loss and cognitive decline, with its role in AD pathophysiology well documented. | Cammann, et al. [36], Lukiw W.J [71], Zhan, et al. [72] |
| Adlercreutzia, Eubacterium nodatum group, Eisenbergiella, Eubacterium fissicatena group, Gordonibacter, and Prevotella9 | Showed a negative correlation to AD diagnosis and were identified as having protective properties. | Cammann, et al. [36] |
| Medication | Outcome | References |
|---|---|---|
| Donepezil (Cholinesterase Inhibitor) and Gastrointestinal Nutrient Absorption | A clinical trial with 188 people with mild-to-moderate AD, donepezil (5–10 mg daily) was associated with nausea, vomiting, and diarrhoea in 15–20% of participants, persisting after initial dose adjustment. | Rogers, et al. [103] |
| Memantine and Vitamin D Metabolism | A randomised controlled trial investigated memantine (20 mg daily), an NMDA receptor antagonist, in 561 people with moderate-to-severe AD over 24 weeks. Memantine’s metabolism via cytochrome P450 enzymes in the liver was found to increase vitamin D metabolism, reducing its bioavailability. This led to lower serum 25-hydroxyvitamin D levels in 30% of treated participants compared to placebo, exacerbating bone health issues and potentially worsening cognitive outcomes. | Sano, et al. [104] |
| Rivastigmine (Cholinesterase Inhibitor) and Protein Metabolism Interference | A 26-week open-label study of rivastigmine (3–12 mg daily) in 114 people with mild AD found that its cholinergic effects increased gastric acid secretion, altering protein digestion and amino acid absorption. The plasma levels of essential amino acids (e.g., tryptophan, tyrosine) dropped by 10–15% in 25% of participants, linked to nausea and appetite loss in 18% of cases. | Corey-Bloom J [105] |
| Galantamine (Cholinesterase Inhibitor) and Iron Absorption Inhibition | A 26-week RCT of galantamine (8–24 mg daily) in 636 people with mild-to-moderate AD showed that its cholinergic stimulation of gastric motility reduced iron absorption by 15–20% in 35% of participants, as evidenced by lower ferritin levels. This was linked to nausea and altered gastric pH, affecting dietary iron bioavailability over time. | Raskind, et al. [106] |
| Author | Perceived Barriers |
|---|---|
| Mathew et al., 2023 [131] | Nutrigenomic subjects should be a requirement in master’s level dietetic education |
| Further research into precision nutrition is required for clinical implementation | |
| Precision nutrition is a valuable tool for weight loss management | |
| Genetic testing to inform precision nutrition strategies will improve medical nutrition therapy for diseases that require weight management | |
| Nacis et al., 2022 [132] | Cost concerns |
| Ethical considerations | |
| It is a cellular approach to nutrition | |
| Genes affect nutrient metabolism and nutrients affect genes | |
| Greyvensteyn et al., 2022 [133] | Limited experts to convey professional advice |
| Limited access to nutrigenomics for clients or patients | |
| Do Rosario et al., 2025 [134] | Cost concerns (p < 0.001) |
| Limited experts to convey professional advice (p < 0.001) | |
| Limited ongoing education for healthcare professionals (p < 0.005) | |
| Confidentiality concerns (p < 0.005) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jewell, T.J.; Minehan, M.; Williams, J.; D’Cunha, N.M. Precision Nutrition for Dementia: Exploring the Potential in Mitigating Dementia Progression. J. Dement. Alzheimer's Dis. 2025, 2, 28. https://doi.org/10.3390/jdad2030028
Jewell TJ, Minehan M, Williams J, D’Cunha NM. Precision Nutrition for Dementia: Exploring the Potential in Mitigating Dementia Progression. Journal of Dementia and Alzheimer's Disease. 2025; 2(3):28. https://doi.org/10.3390/jdad2030028
Chicago/Turabian StyleJewell, Tara J., Michelle Minehan, Jackson Williams, and Nathan M. D’Cunha. 2025. "Precision Nutrition for Dementia: Exploring the Potential in Mitigating Dementia Progression" Journal of Dementia and Alzheimer's Disease 2, no. 3: 28. https://doi.org/10.3390/jdad2030028
APA StyleJewell, T. J., Minehan, M., Williams, J., & D’Cunha, N. M. (2025). Precision Nutrition for Dementia: Exploring the Potential in Mitigating Dementia Progression. Journal of Dementia and Alzheimer's Disease, 2(3), 28. https://doi.org/10.3390/jdad2030028








