Relationship Between the Severity of Subjective Cognitive Decline and Health-Related Quality of Life in Community-Dwelling Older Adults: A Cross-Sectional Study Focusing on Sex Differences
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
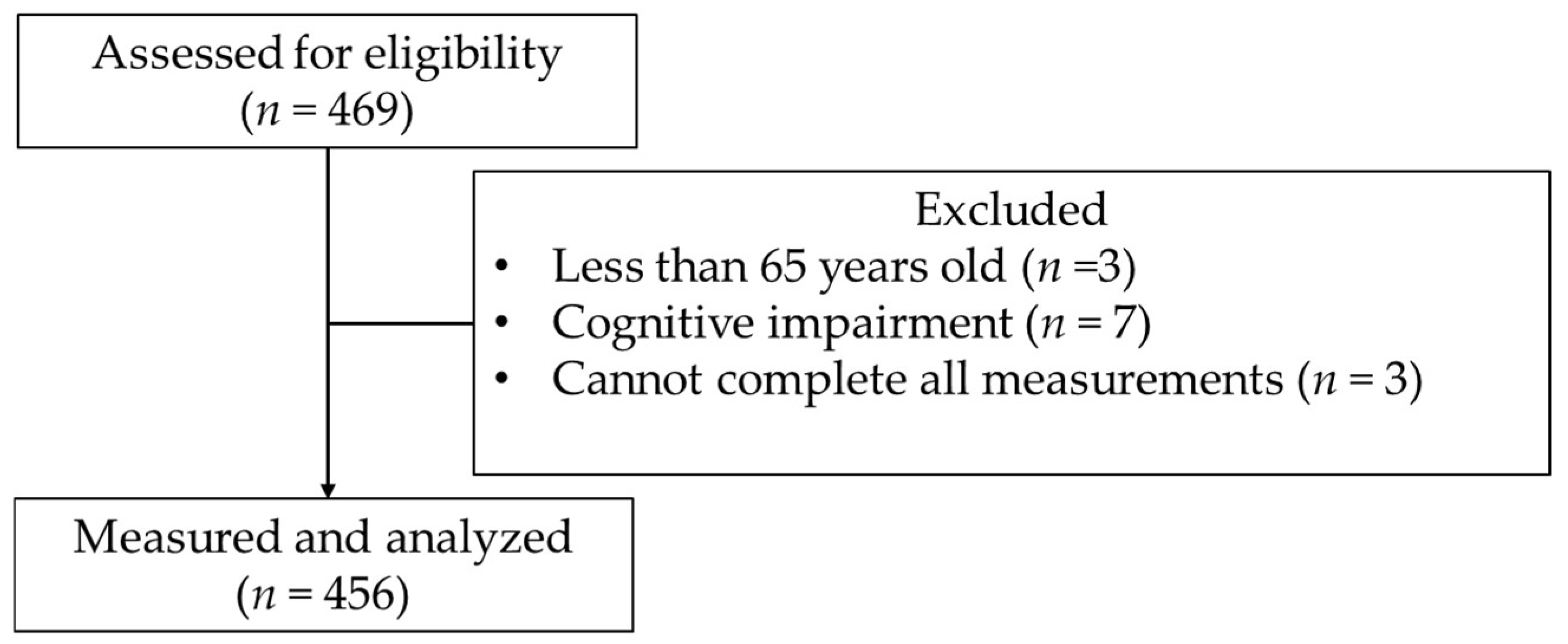
2.2. Participants
2.3. Measurement Items
- (1)
- Basic attributes
- (2)
- HRQOL
- (3)
- Severity of SCD
- (4)
- MMSE
- (5)
- GDS-5
- (6)
- AIS-J
- (7)
- Number of items matching the J-CHS criteria
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutai, H.; Obuchi, K.; Yokoi, K.; Furukawa, T. Factors associated with health-related quality of life among community-dwelling older adults without social participation in Japan: A cross-sectional study. J. Appl. Gerontol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Nakano, H.; Goda, A.; Mori, K.; Abiko, T.; Mitsumaru, N.; Murata, S. The influence of physical, mental, and cognitive factors on health-related quality of life among community-dwelling older adults: A focus on central sensitization-related symptoms. Geriatrics 2024, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.; Park, E.; Jang, S. The association between the changes in general, family, and financial aspects of quality of life and their effects on cognitive function in an elderly population: The Korean longitudinal study of aging, 2008–2016. Int. J. Environ. Res. Public Health 2020, 17, 1106. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ho Chung, J. The association between subjective cognitive decline and quality of life: A population-based study. J. Clin. Neurosci. 2022, 98, 60–65. [Google Scholar] [CrossRef]
- Roehr, S.; Luck, T.; Pabst, A.; Bickel, H.; König, H.H.; Lühmann, D.; Fuchs, A.; Wolfsgruber, S.; Wiese, B.; Weyerer, S.; et al. Subjective cognitive decline is longitudinally associated with lower health-related quality of life. Int. Psychogeriatr. 2017, 29, 1939–1950. [Google Scholar] [CrossRef]
- Königsberg, A.; Belau, M.; Ascone, L.; Gallinat, J.; Kühn, S.; Jensen, M.; Gerloff, C.; Cheng, B.; Thomalla, G. Subjective cognitive decline is associated with health-related quality of life in the middle-aged to elderly population. J. Alzheimers Dis. 2023, 91, 427–436. [Google Scholar] [CrossRef]
- Cutler, R.A.; Mirjalili, S.; Pham, P.; Devulapalli, H.; Zafar, S.; Duarte, A. Semantic memory space becomes denser with age. Neuropsychologia 2025, 208, 109083. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, L.; Wei, Y.; Mai, W.; Duan, G.; Su, J.; Nong, X.; Yu, B.; Li, C.; Mo, X.; et al. Structural and functional hippocampal changes in subjective cognitive decline from the community. Front. Aging Neurosci. 2020, 12, 64. [Google Scholar] [CrossRef]
- Levine, D.A.; Gross, A.L.; Briceño, E.M.; Tilton, N.; Giordani, B.J.; Sussman, J.B.; Hayward, R.A.; Burke, J.F.; Hingtgen, S.; Elkind, M.S.V.; et al. Sex differences in cognitive decline among US adults. JAMA Netw. Open. 2021, 4, e210169. [Google Scholar] [CrossRef]
- Overton, M.; Skoog, J.; Laukka, E.J.; Bodin, T.H.; Mattsson, A.D.; Sjöberg, L.; Hofer, S.M.; Johansson, L.; Kulmala, J.; Kivipelto, M.; et al. Sleep disturbances and change in multiple cognitive domains among older adults: A multicenter study of five Nordic cohorts. Sleep 2023, 47, zsad244. [Google Scholar] [CrossRef] [PubMed]
- Exalto, L.G.; Hendriksen, H.M.A.; Barkhof, F.; van den Bosch, K.; Ebenau, J.L.; van Leeuwenstijn-Koopman, M.; Prins, N.D.; Teunissen, C.E.; Visser, L.N.C.; Scheltens, P.; et al. Subjective cognitive decline and self-reported sleep problems: The science project. Alzheimer’s Dement. 2022, 14, e12287. [Google Scholar] [CrossRef] [PubMed]
- Sanprakhon, P.; Suriyawong, W.; Chusri, O.; Rattanaselanon, P. Exploring the association between loneliness, subjective cognitive decline, and quality of life among older Thai adults: A convergent parallel mixed-method study. J. Appl. Gerontol. 2024, 43, 1795–1807. [Google Scholar] [CrossRef]
- Benson, G.; Schwarz, C.; Horn, N.; Marchant, N.; Flöel, A.; Wirth, M. Beyond anxiety and depression: Rumination, stress coping, and quality of life in subjective cognitive decline. Alzheimer’s Dement. 2018, 14, 416. [Google Scholar] [CrossRef]
- Bouldin, E.; Taylor, C.; Knapp, K.; Miyawaki, C.; Mercado, N.; Wooten, K.; McGuire, L. Unmet needs for assistance related to subjective cognitive decline among community-dwelling middle-aged and older adults in the US: Prevalence and impact on health-related quality of life. Int. Psychogeriatr. 2020, 33, 689–702. [Google Scholar] [CrossRef]
- Pusswald, G.; Tropper, E.; Kryspin-Exner, I.; Moser, D.; Klug, S.; Auff, E.; Dal-Bianco, P.; Lehrner, J. Health-related quality of life in patients with subjective cognitive decline and mild cognitive impairment and its relation to activities of daily living. J. Alzheimers Dis. 2015, 47, 479–486. [Google Scholar] [CrossRef]
- Pan, C.W.; Wang, X.; Ma, Q.; Sun, H.P.; Xu, Y.; Wang, P. Cognitive dysfunction and health-related quality of life among older Chinese. Sci. Rep. 2015, 5, 17301. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, R.; Zhang, C.; Chen, H.; Wang, R.; Zhao, Q.; Zhu, T.; Chen, C. Evidence for cognitive decline in chronic pain: A systematic review and meta-analysis. Front. Neurosci. 2021, 15, 737874. [Google Scholar] [CrossRef]
- Khera, T.; Rangasamy, V. Cognition and pain: A review. Front. Psychol. 2021, 12, 673962. [Google Scholar] [CrossRef]
- Sugishita, M.; Hemmi, I.; Takeuchi, T. Reexamination of the validity and reliability of the Japanese version of the Mini-Mental State Examination (MMSE-J). Jpn. J. Cogn. Neurosci. 2016, 18, 168–183. [Google Scholar]
- Ikeda, S.; Shiroiwa, T.; Igarashi, A.; Noto, S.; Fukuda, T.; Saito, S.; Shimozuma, K. Developing a Japanese version of the EQ-5D-5L value set. J. Natl. Inst. Public Health 2015, 64, 47–55. [Google Scholar]
- Okada, N.; Komorisono, M. Efficacy of women’s health drugs for menopausal complaints. Shinryo to Shinyaku (Med. Cons. New-Remed.) 2024, 61, 537–547. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Ideno, Y.; Takayama, M.; Hayashi, K.; Takagi, H.; Sugai, Y. Evaluation of a Japanese version of the Mini-Mental State Examination in elderly persons. Geriatr. Gerontol. Int. 2012, 12, 310–316. [Google Scholar] [CrossRef]
- Hoyl, M.T.; Alessi, C.A.; Harker, J.O.; Josephson, K.R.; Pietruszka, F.M.; Koelfgen, M.; Mervis, J.R.; Fitten, L.J.; Rubenstein, L.Z. Development and testing of a five-item version of the Geriatric Depression Scale. J. Am. Geriatr. Soc. 1999, 47, 873–878. [Google Scholar] [CrossRef]
- Wada, Y.; Murata, C.; Hirai, H.; Kondo, N.; Kondo, K.; Ueda, K.; Ichida, N. Predictive validity of GDS5 using AGES project data. Kousei no Shihyou 2014, 61, 7–12. (In Japanese) [Google Scholar]
- Okajima, I.; Nakajima, S.; Kobayashi, M.; Inoue, Y. Development and validation of the Japanese version of the Athens Insomnia Scale. Psychiatry Clin. Neurosci. 2013, 67, 420–425. [Google Scholar] [CrossRef]
- Enomoto, K.; Adachi, T.; Yamada, K.; Inoue, D.; Nakanishi, M.; Nishigami, T.; Shibata, M. Reliability and validity of the Athens Insomnia Scale in chronic pain patients. J. Pain Res. 2018, 11, 793–801. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef]
- Yamada, M.; Arai, H. Predictive value of frailty scores for healthy life expectancy in community-dwelling older Japanese adults. J. Am. Med. Dir. Assoc. 2015, 16, 1002.e7–1002.e11. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Suzuki, T. Impact of physical frailty on disability in community-dwelling older adults: A prospective cohort study. BMJ Open 2015, 5, e008462. [Google Scholar] [CrossRef] [PubMed]
- Statistics Bureau, Ministry of Internal Affairs and Communications, Population of the Elderly (Population Estimates, World Population Prospects). Statistics Bureau of Japan. Available online: https://www.stat.go.jp/data/topics/pdf/topi142_01.pdf (accessed on 18 February 2025).
- Kamyab, F.; Hoseinzadeh, A. The psychological impact of social expectations on women’s personal choices. Psywoman 2023, 4, 169–176. [Google Scholar] [CrossRef]
- Haussmann, R.; Sauer, C.; Birnbaum, A.; Donix, M. Gender differences in the perception of cognitive decline. Asian J. Psychiatr. 2019, 46, 6. [Google Scholar] [CrossRef] [PubMed]
- Mazzonna, F.; Peracchi, F. Self-Assessed Cognitive Ability and Financial Wealth: Are People Aware of Their Cognitive Decline? Einaudi Institute for Economics and Finance (EIEF): Rome, Italy, 2018. [Google Scholar]
- Wood, R.H.; Gardner, R.E.; Ferachi, K.A.; King, C.; Ermolao, A.; Cherry, K.E.; Cress, M.E.; Jazwinski, S.M. Physical function and quality of life in older adults: Sex differences. South. Med. J. 2005, 98, 504–512. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Nakano, H.; Abiko, T.; Goda, A.; Murata, S. Central sensitization-related symptoms and influencing factors on health-related quality of life among frail older adults in senior day care centers: A cross-sectional study. Healthcare 2024, 12, 1201. [Google Scholar] [CrossRef]
- Zis, P.; Daskalaki, A.; Bountouni, I.; Sykioti, P.; Varrassi, G.; Paladini, A. Depression and chronic pain in the elderly: Links and management challenges. Clin. Interv. Aging 2017, 12, 709–720. [Google Scholar] [CrossRef]
- Adriany, K.; Cendoroglo, M.; Santos, F. Anxiety disorder in elderly persons with chronic pain: Frequency and associations. Rev. Bras. Geriatr. Gerontol. 2017, 20, 91–98. [Google Scholar]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Xing, W.; Zhang, S.; Wang, Z.; Jiang, D.; Han, S.; Luo, Y. Self-awareness protects working memory in people under chronic stress: An ERP study. Front. Psychol. 2022, 13, 1003719. [Google Scholar] [CrossRef]
- Moreno, G.L.; Ammann, E.; Kaseda, E.T.; Espeland, M.A.; Wallace, R.; Robinson, J.; Denburg, N.L. The influence of social support on cognitive health in older women: A Women’s Health Initiative study. J. Women Aging 2021, 34, 394–410. [Google Scholar] [CrossRef]
- Hong, Y.J.; Lee, J.H.; Choi, E.J.; Han, N.; Kim, J.E.; Park, S.H.; Kim, H.J.; Kang, D.W. Efficacies of cognitive interventions in the elderly with subjective cognitive decline: A prospective, three-arm, controlled trial. J. Clin. Neurol. 2020, 16, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.; Kumar, P. Multi-component interventions in older adults having subjective cognitive decline (SCD): A review article. Geriatrics 2022, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tse, M.; Tang, A. The effectiveness of a dyadic pain management program for community-dwelling older adults with chronic pain: A pilot randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 4966. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.J.; Baron, K.G.; Lu, B.; Naylor, E.; Wolfe, L.; Zee, P.C. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010, 11, 934–940. [Google Scholar] [CrossRef]
- Tanaka, H.; Shirakawa, S. Sleep health, lifestyle and mental health in the Japanese elderly: Ensuring sleep to promote a healthy brain and mind. J. Psychosom. Res. 2004, 56, 465–477. [Google Scholar] [CrossRef]
| Variable | Total (n = 456) | Male (n = 109) | Female (n = 347) | p-Value (Before Weighting) | p-Value (After Weighting) |
|---|---|---|---|---|---|
| Age (year) | 75.81 ± 5.96 | 77.61 ± 6.26 | 75.24 ± 5.76 | <0.01 | <0.01 |
| Number of health conditions (n) | 1.21 ± 0.93 | 1.10 ± 0.94 | 1.25 ± 0.93 | 0.11 | 0.04 |
| Presence of physical pain [Yes/No] (n [%]) * | 280 (61.4)/176 (38.6) | 60 (55.0)/49 (45.0) | 220 (69.6)/127 (40.2) | 0.14 | 0.07 |
| HRQOL score (score) | 0.83 ± 0.13 | 0.84 ± 0.15 | 0.83 ± 0.13 | 0.67 | 0.59 |
| VAS for SCD severity (score) | 5.98 ± 2.16 | 5.91 ± 2.14 | 6.00 ± 2.18 | 0.73 | 0.67 |
| MMSE (score) | 28.27 ± 1.82 | 28.10 ± 1.90 | 28.33 ± 1.79 | 0.18 | 0.09 |
| GDS-5 (score) | 0.64 ± 1.00 | 0.63 ± 0.93 | 0.65 ± 1.02 | 0.79 | 0.74 |
| AIS-J (score) | 3.98 ± 3.02 | 3.83 ± 2.86 | 4.03 ± 3.07 | 0.51 | 0.08 |
| Number of items that match the J-CHS judgment criteria (n) | 0.63 ± 0.81 | 0.72 ± 0.92 | 0.60 ± 0.77 | 0.30 | 0.19 |
| HRQOL Score (Score) | VAS for SCD Severity (Score) | |||||||
|---|---|---|---|---|---|---|---|---|
| Male (n = 109) | Female (n = 347) | Male (n = 109) | Female (n = 347) | |||||
| ρ | p-Value | ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Age (year) | −0.023 | 0.813 | 0.011 | 0.833 | 0.115 | 0.232 | 0.075 | 0.166 |
| Number of health conditions (n) | −0.345 | p < 0.001 | −0.111 | 0.040 | 0.060 | 0.534 | −0.050 | 0.354 |
| HRQOL score (score) | −0.087 | 0.371 | 0.292 | <0.001 | ||||
| VAS for SCD severity (score) | −0.087 | 0.371 | 0.292 | <0.001 | ||||
| MMSE (score) | 0.090 | 0.351 | 0.002 | 0.974 | 0.002 | 0.987 | 0.049 | 0.362 |
| GDS-5 (score) | −0.298 | 0.002 | −0.328 | <0.001 | −0.107 | 0.268 | −0.245 | <0.001 |
| AIS-J (score) | −0.330 | p < 0.001 | −0.395 | <0.001 | −0.175 | 0.068 | −0.159 | 0.003 |
| Number of items that match the J-CHS judgment criteria (n) | −0.337 | p < 0.001 | −0.356 | <0.001 | 0.119 | 0.218 | −0.188 | <0.001 |
| HRQOL Score (Score) | VAS for SCD Severity (Score) | |||||||
|---|---|---|---|---|---|---|---|---|
| Presence of Body Pain | Absence of Body Pain | p-Value (Before Weighting) | p-Value (After Weighting) | Presence of Body Pain | Absence of Body Pain | p-Value (Before Weighting) | p-Value (After Weighting) | |
| Male (n = 109) | 0.77 ± 0.12 | 0.92 ± 0.12 | <0.01 | <0.01 | 5.85 ± 1.67 | 5.99 ± 2.61 | 0.71 | 0.60 |
| Female (n = 347) | 0.78 ± 0.11 | 0.93 ± 0.11 | <0.01 | <0.01 | 5.75 ± 2.14 | 6.43 ± 2.19 | <0.01 | <0.01 |
| Before Weighting | After Weighting | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI for β | 95% CI for β | ||||||||||||
| Group | Independent Variable | β | std-β | Lower | Upper | p-Value | VIF | β | std-β | Lower | Upper | p-Value | VIF |
| Male (n = 109) | Age (year) | −0.001 | −0.032 | −0.004 | 0.003 | 0.668 | 1.077 | −0.001 | −0.032 | −0.003 | 0.002 | 0.556 | 1.077 |
| Number of medical histories (n) | −0.015 | −0.100 | −0.040 | 0.009 | 0.221 | 1.305 | −0.015 | −0.100 | −0.033 | 0.003 | 0.092 | 1.305 | |
| Presence of physical pain [1: Yes/0: No] | −0.136 | −0.471 | −0.180 | −0.092 | <0.001 | 1.189 | −0.136 | −0.471 | −0.168 | −0.104 | <0.001 | 1.189 | |
| VAS for SCD severity (score) | −0.007 | −0.102 | −0.017 | 0.003 | 0.180 | 1.136 | −0.007 | −0.102 | −0.014 | 0.000 | 0.065 | 1.136 | |
| MMSE (score) | 0.007 | 0.093 | −0.004 | 0.018 | 0.201 | 1.043 | 0.007 | 0.093 | −0.001 | 0.015 | 0.078 | 1.043 | |
| GDS-5 (score) | −0.018 | −0.115 | −0.043 | 0.007 | 0.158 | 1.299 | −0.018 | −0.115 | −0.036 | 0.000 | 0.052 | 1.299 | |
| AIS-J (score) | −0.011 | −0.229 | −0.019 | −0.004 | 0.004 | 1.163 | −0.011 | −0.229 | −0.017 | −0.006 | <0.001 | 1.163 | |
| Number of items that match the J-CHS judgment criteria (n) | −0.038 | −0.241 | −0.063 | −0.013 | 0.004 | 1.301 | −0.038 | −0.241 | −0.056 | −0.020 | <0.001 | 1.301 | |
| Adjusted R2 (p-value) | 0.462 (p < 0.001) | 0.481 (p < 0.001) | |||||||||||
| Female (n = 316) | Age (year) | 0.000 | −0.008 | −0.002 | 0.002 | 0.860 | 1.119 | 0.000 | −0.008 | −0.002 | 0.002 | 0.879 | 1.119 |
| Number of medical histories (n) | −0.009 | −0.063 | −0.020 | 0.002 | 0.124 | 1.028 | −0.009 | −0.063 | −0.022 | 0.004 | 0.187 | 1.028 | |
| Presence of physical pain [1: Yes/0: No] | −0.125 | −0.461 | −0.147 | −0.103 | <0.001 | 1.081 | −0.125 | −0.461 | −0.151 | −0.099 | 0.000 | 1.081 | |
| VAS for SCD severity (score) | 0.007 | 0.123 | 0.002 | 0.012 | 0.004 | 1.113 | 0.007 | 0.123 | 0.002 | 0.013 | 0.014 | 1.113 | |
| MMSE (score) | 0.002 | 0.028 | −0.004 | 0.008 | 0.493 | 1.050 | 0.002 | 0.028 | −0.005 | 0.009 | 0.556 | 1.050 | |
| GDS-5 (score) | −0.019 | −0.151 | −0.031 | −0.007 | 0.001 | 1.376 | −0.019 | −0.151 | −0.033 | −0.006 | 0.006 | 1.376 | |
| AIS-J (score) | −0.007 | −0.169 | −0.011 | −0.004 | <0.001 | 1.200 | −0.007 | −0.169 | −0.012 | −0.003 | 0.001 | 1.200 | |
| Number of items that match the J-CHS judgment criteria (n) | −0.025 | −0.149 | −0.041 | −0.010 | 0.001 | 1.304 | −0.025 | −0.149 | −0.043 | −0.007 | 0.006 | 1.304 | |
| Adjusted R2 (p-value) | 0.447 (p < 0.001) | 0.442 (p < 0.001) | |||||||||||
| 95% CI for β | |||||||
|---|---|---|---|---|---|---|---|
| Independent Variable | β | std-β | Lower | Upper | p-Value | VIF | |
| Before weighting | VAS for SCD severity (score) | −0.005 | −0.076 | −0.016 | 0.007 | 0.420 | 4.309 |
| Sex (0: Male, 1: Female) | −0.003 | −0.009 | −0.031 | 0.025 | 0.841 | 1.000 | |
| VAS for SCD severity × Sex | 0.022 | 0.310 | 0.009 | 0.035 | 0.001 | 4.308 | |
| Adjusted R2 (p-value) | 0.054 (p < 0.001) | ||||||
| After weighting | VAS for SCD severity (score) | −0.005 | −0.075 | −0.014 | 0.004 | 0.291 | 2.357 |
| Sex (0: Male, 1: Female) | −0.003 | −0.010 | −0.028 | 0.022 | 0.821 | 1.000 | |
| VAS for SCD severity × Sex | 0.022 | 0.262 | 0.010 | 0.034 | <0.001 | 2.356 | |
| Adjusted R2 (p-value) | 0.038 (p < 0.001) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, A.; Nakano, H.; Kikuchi, Y.; Horie, J.; Shiraiwa, K.; Abiko, T.; Katsurasako, T.; Mori, K.; Murata, S. Relationship Between the Severity of Subjective Cognitive Decline and Health-Related Quality of Life in Community-Dwelling Older Adults: A Cross-Sectional Study Focusing on Sex Differences. J. Dement. Alzheimer's Dis. 2025, 2, 11. https://doi.org/10.3390/jdad2020011
Goda A, Nakano H, Kikuchi Y, Horie J, Shiraiwa K, Abiko T, Katsurasako T, Mori K, Murata S. Relationship Between the Severity of Subjective Cognitive Decline and Health-Related Quality of Life in Community-Dwelling Older Adults: A Cross-Sectional Study Focusing on Sex Differences. Journal of Dementia and Alzheimer's Disease. 2025; 2(2):11. https://doi.org/10.3390/jdad2020011
Chicago/Turabian StyleGoda, Akio, Hideki Nakano, Yuki Kikuchi, Jun Horie, Kayoko Shiraiwa, Teppei Abiko, Tsuyoshi Katsurasako, Kohei Mori, and Shin Murata. 2025. "Relationship Between the Severity of Subjective Cognitive Decline and Health-Related Quality of Life in Community-Dwelling Older Adults: A Cross-Sectional Study Focusing on Sex Differences" Journal of Dementia and Alzheimer's Disease 2, no. 2: 11. https://doi.org/10.3390/jdad2020011
APA StyleGoda, A., Nakano, H., Kikuchi, Y., Horie, J., Shiraiwa, K., Abiko, T., Katsurasako, T., Mori, K., & Murata, S. (2025). Relationship Between the Severity of Subjective Cognitive Decline and Health-Related Quality of Life in Community-Dwelling Older Adults: A Cross-Sectional Study Focusing on Sex Differences. Journal of Dementia and Alzheimer's Disease, 2(2), 11. https://doi.org/10.3390/jdad2020011






