Utilisation of Liquefied Biomass in Water Co-Electrolysis for the Production of Synthesis Gas †
Abstract
1. Introduction
2. Materials and Methods
- (i)
- Cathode reactions:
- (ii)
- Anode reactions:
- (iii)
- Global reaction:
- (i)
- Have an oxygen content as low as possible, to minimise the risk of catalyst deactivation during methanation;
- (ii)
- A CO2:H2 ratio ideally close to the one associated with the conversion of CO2 into methane (1:4) via the following reaction:
- (iii)
- A good relation between the flow of gas produced and the energy consumed to produce it.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- bp Statistical Review. Statistical Review of World Energy Globally Consistent Data. 2022. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf (accessed on 6 February 2025).
- Ma, X.; Fu, Q. The influence of financial development on energy consumption: Worldwide evidence. Int. J. Environ. Res. Public Health 2020, 17, 1428. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Oyeakale, J.; Petrollese, M.; Tola, V.; Cau, G. Impacts of renewable energy resources on effectiveness of grid-integrated systems: Succint review of current challenges and potential solution strategies. Energies 2020, 13, 4856. [Google Scholar] [CrossRef]
- Kelly, N. Hydrogen production by water electrolysis. In Advances in Hydrogen Production, Storage and Distribution; Basile, A., Iulianelli, A., Eds.; Woodhead Publishing Series in Energy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–185. [Google Scholar]
- Shiva-Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Mazzeo, D.; Sacit-Herdem, M.; Matera, N.; Wen, J. Green hydrogen production: Analysis for different single or combined large-scale photovoltaic and wind renewable systems. Renew. Energy 2022, 200, 360–378. [Google Scholar] [CrossRef]
- Chen, W.; Lin, T.; Dai, Y.; An, Y.; Yu, F.; Zhong, L.; Li, S.; Sun, Y. Recent advances in the investigation of nanoeffects of Fischer-Tropsch catalysts. Catal. Today 2018, 311, 8–22. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Makkee, M.; Van Diepen, A.E. Chemical Process Technology, 2nd ed; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Dittrich, L.; Nohl, M.; Jaekel, E.E.; Foit, S.; Haart, B.; Eicchel, R. High-Temperature Co-Electrolysis: A Versatile Method to Sustainably Produce Tailored Syngas Compositions. J. Electrochem. Soc. 2019, 13, F971. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Shi, Y.; Meng, N.; Lu, S.; Yu, Y.; Zhang, B. Electrosynthesis of Syngas via the Co-Reduction of CO2 and H2O. Cell Rep. Phys. Sci. 2020, 1, 100237. [Google Scholar] [CrossRef]
- Andika, R.; Nandiyant, A.; Putra, Z.; Bilad, M.; Kim, Y.; Yuan, C.; Lee, M. Co-electrolysis for power-to-methane applications. Renew. Sustain. Energy Rev. 2018, 95, 227–241. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, S.; Zhu, X.; Yang, W. Electrochemical reduction of CO2 in solid oxide electrolysis cells. J. Energy Chem. 2017, 26, 593–601. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Wang, G.; Bao, X. Co-electrolysis of CO2 and H2O in high temperature solid oxide electrolysis cells: Recent advances in cathodes. J. Energy Chem. 2017, 26, 839–853. [Google Scholar] [CrossRef]
- Patente Portuguesa 106779T; Btenção de Gás de Síntese por Eletrólise Alcalina da Água. Politécnico de Lisboa: Lisbon, Portugal, 2013. (In Portuguese)
- Guerra, L.; Gomes, J.; Puna, J.; Rodrigues, J. Production of renewable synthetic fuels from electricity using the ELECTROFUEL® concept. Energy 2015, 89, 1050–1056. [Google Scholar] [CrossRef]
- Guerra, L.; Moura, K.; Rodrigues, J.; Gomes, J.; Puna, J.; Santos, M. Synthesis gas production from water electrolysis, using the Electrocracking concept. J. Environ. Chem. Eng. 2018, 6, 604–609. [Google Scholar] [CrossRef]
- Gonçalves, A.; Puna, J.; Guerra, L.; Rodrigues, J.; Gomes, J.; Santos, M.; Alves, D. Towards the Development of Syngas/Biomethane Electrolytic Production, Using Liquefied Biomass and Heterogeneous Catalyst. Energies 2019, 12, 3787. [Google Scholar] [CrossRef]
- Gomes, J.; Puna, J.; Marques, A.; Gominho, J.; Lourenço, A.; Galhano, R.; Ozkan, S. Clean Forest–Project concept and early results. Energies 2022, 15, 9294. [Google Scholar] [CrossRef]
- Chen, X.; Guan, C.; Xiao, G.; Du, X.; Wang, J. Syngas production by high temperature steam/CO2 co-electrolysis using solid oxide electrolysis cells. Faraday Discuss. 2015, 182, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Rossi, S.; Rodrigues, J.; Gomes, J.; Puna, J.; Santos, M. Methane production by a combined Sabatier reaction/water electrolysis process. J. Environ. Chem. Eng. 2018, 6, 671–676. [Google Scholar] [CrossRef]
- Mateus, M.; Acero, N.; Bordado, J.; Santos, R. Sonication as a foremost tool to improve cork liquefaction. Ind. Crops Prod. 2015, 74, 9–13. [Google Scholar] [CrossRef]
- Ozkan, S.; Sousa, H.; Gonçalves, D.; Puna, J.; Carvalho, A.; Bordado, J.; Santos, R.; Gomes, J. Unlocking Nature’s Potential: Modelling Acacia Melanoxylon as a Renewable Resource for Bio-Oil Production through Thermochemical Liquefaction. Energies 2024, 17, 4899. [Google Scholar] [CrossRef]
- Liu, H.; Li, W. Recent advances in paired electrolysis of biomass-derived compounds toward cogeneration of value-added chemicals and fuels. Curr. Opin. Electrochem. 2021, 30, 100795. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, X.; Deng, W.; Xiang, Z.; Xu, J.; Jiang, L.; Su, S.; Hu, S.; Hu, X.; Gao, X.; et al. Synchronous bio-oil upgrading and CO2 fixation by co-electrolysis. Energy Convers. Manag. 2023, 288, 117135. [Google Scholar] [CrossRef]

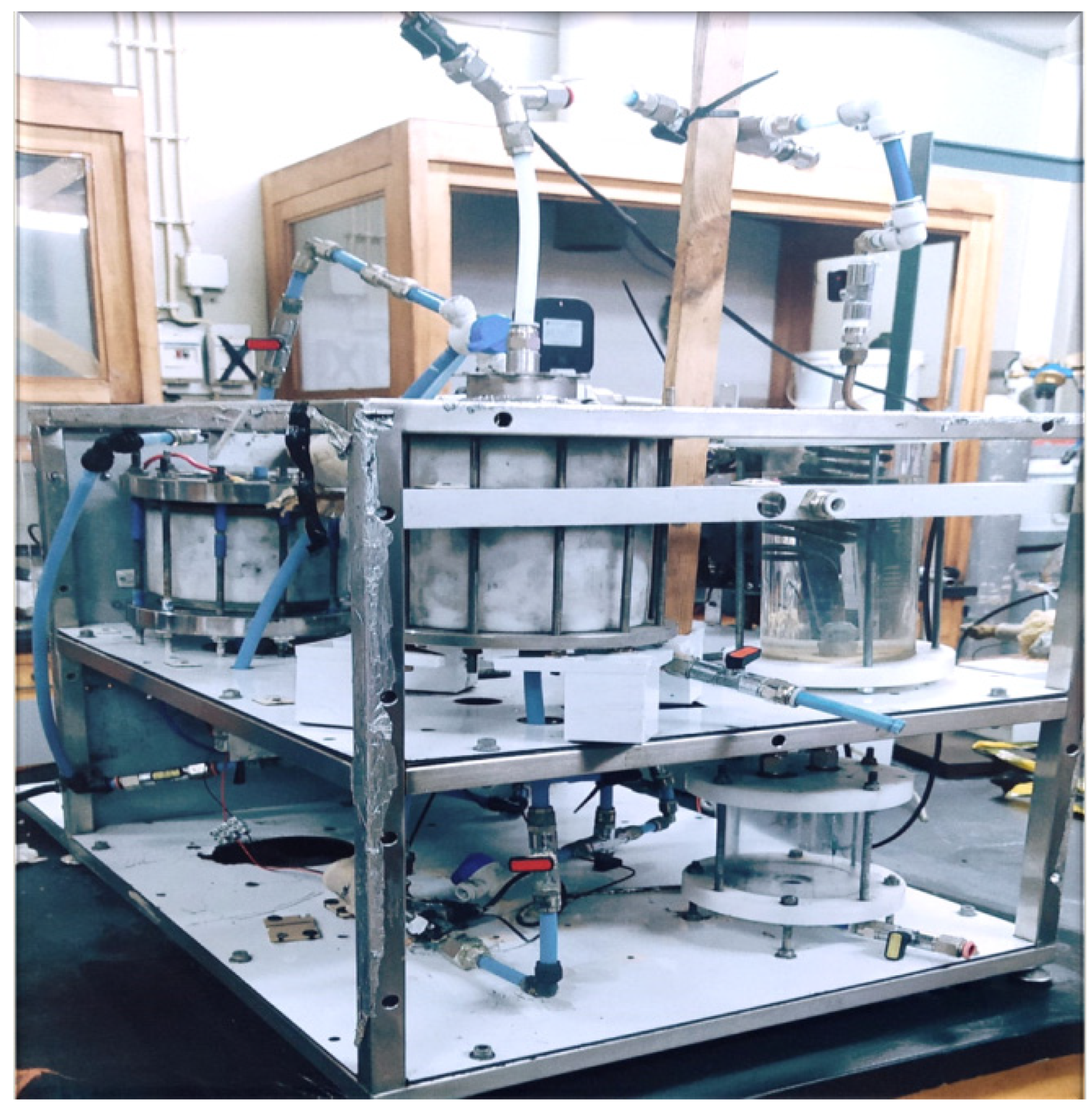
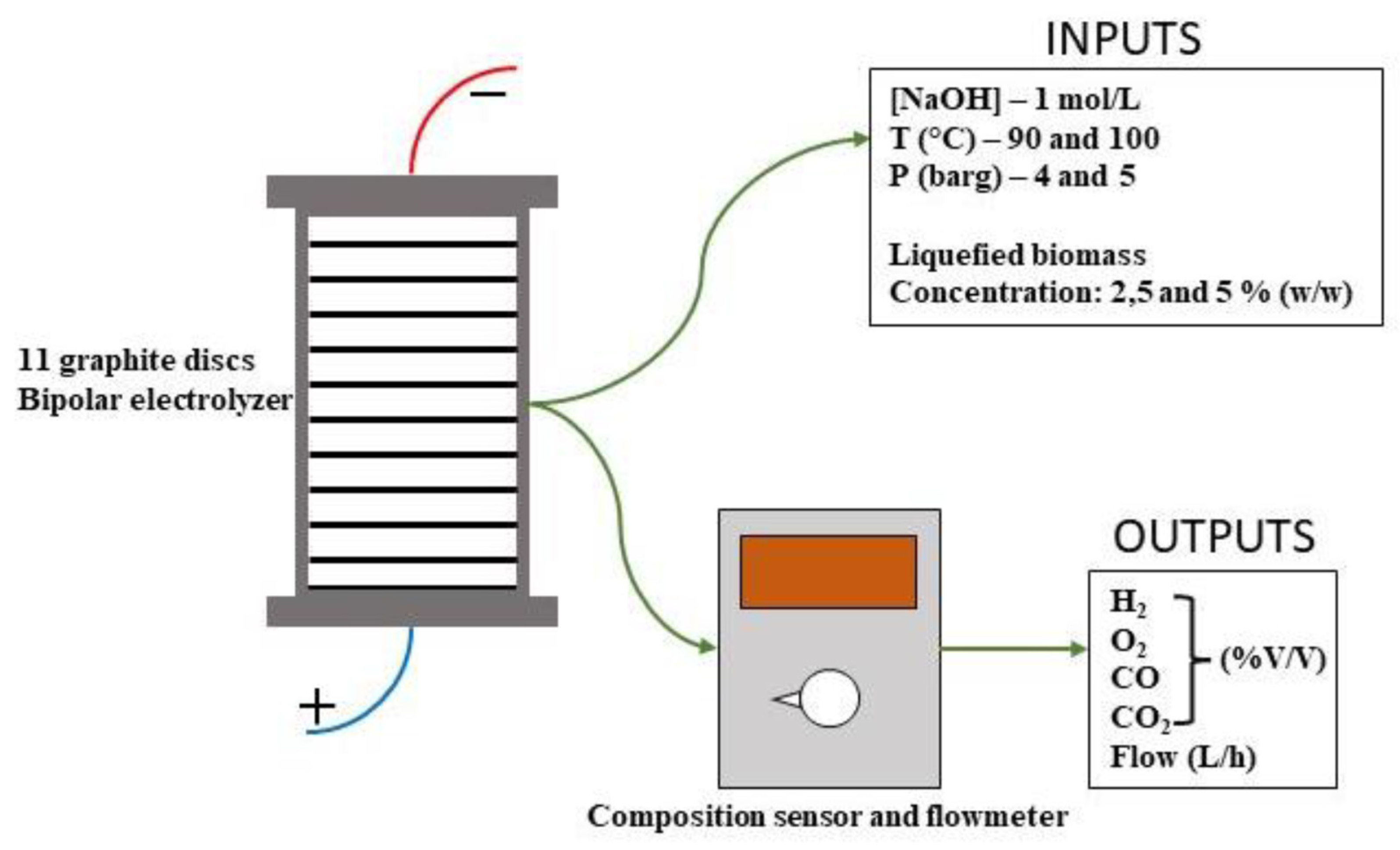
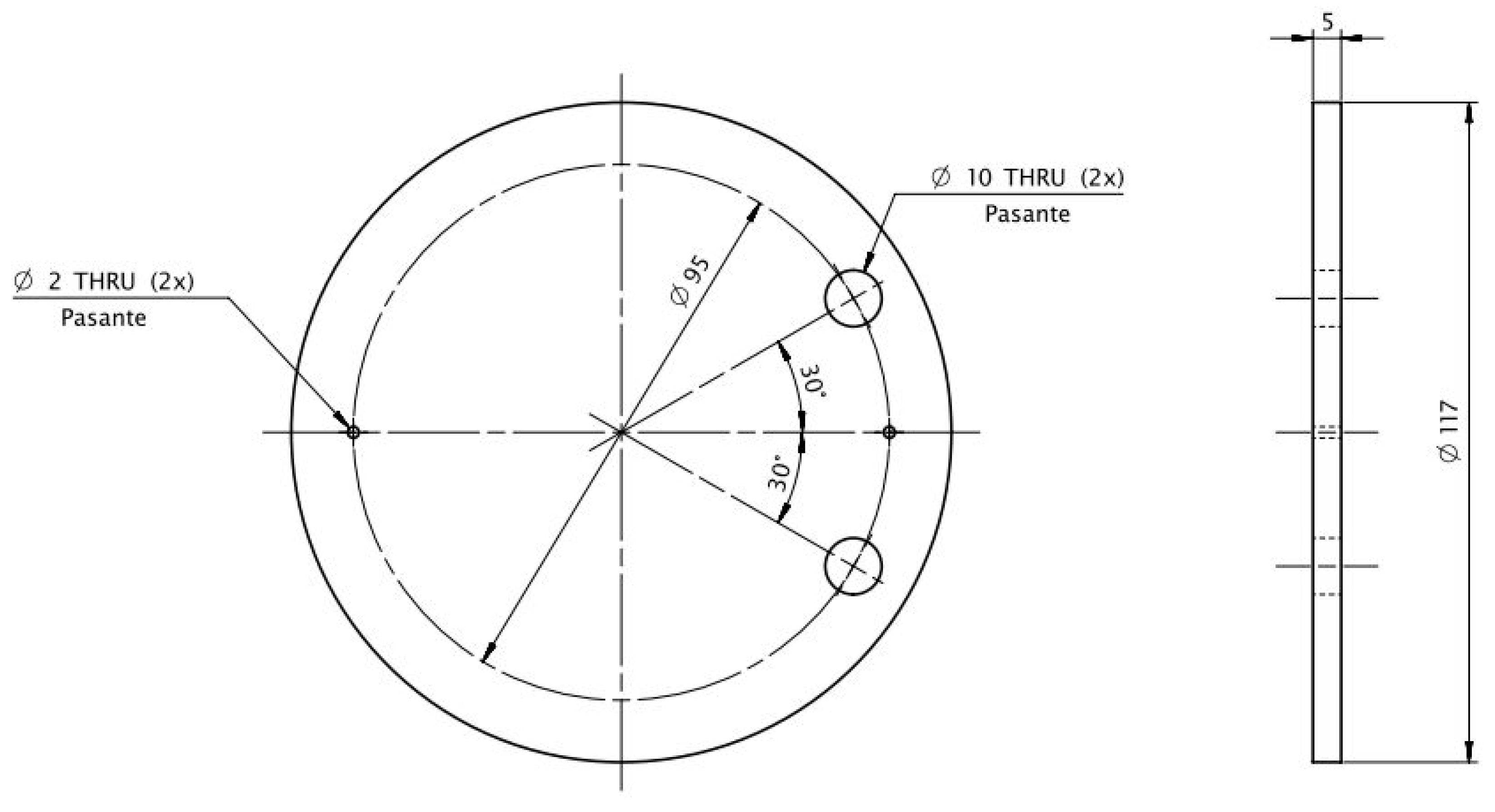
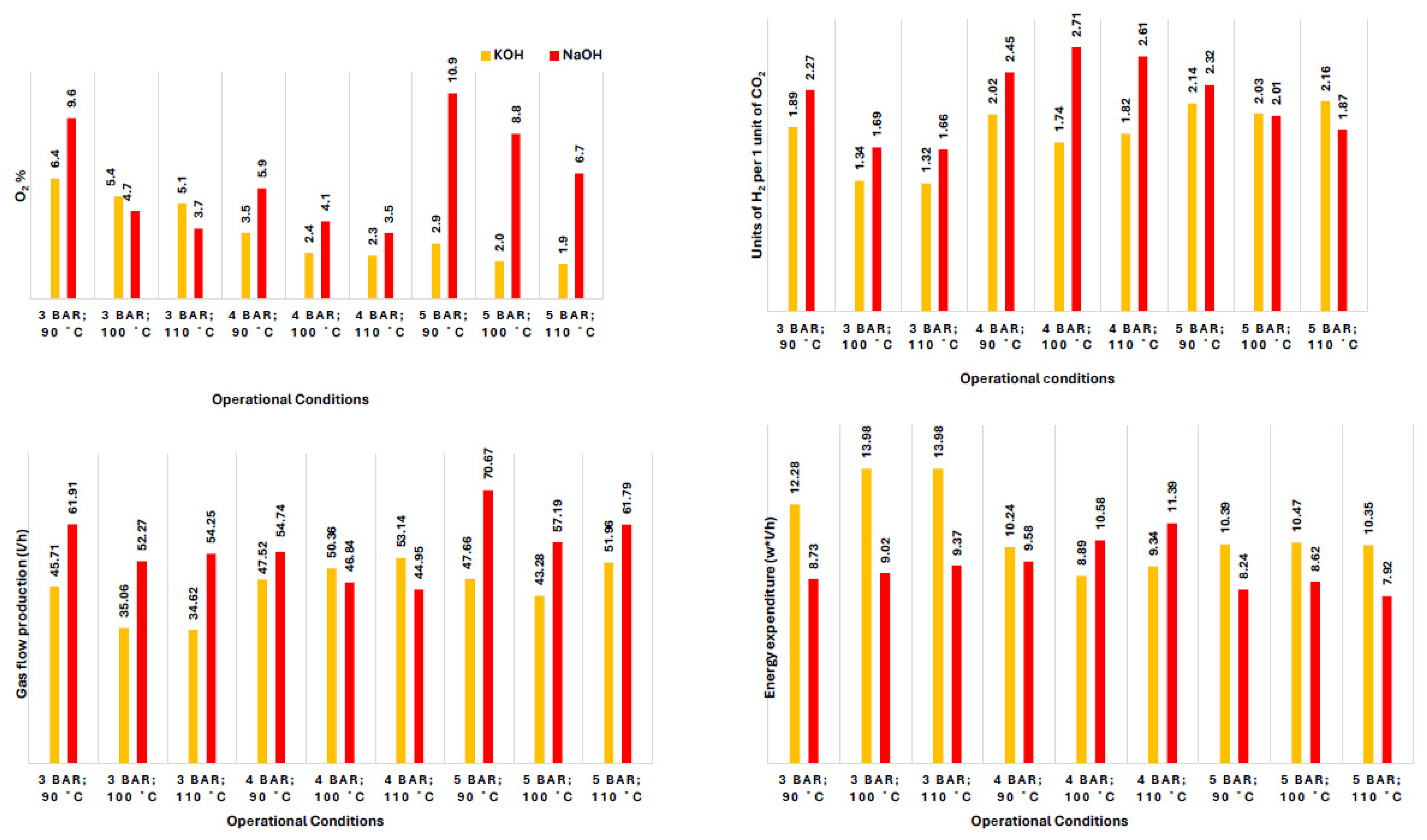
| Biomass Types | C (%) | H (%) | N (%) | S (%) | O (%) | |
|---|---|---|---|---|---|---|
| Energreen | Fresh | 46.0 | 5.3 | 1.2 | 0.1 | 47.2 |
| Bio-oil | 65.3 | 9.7 | 1.2 | 0.3 | 23.5 | |
| Acacia | Fresh | 47.4 | 5.8 | <0.5 | <0.1 | 46.8 |
| Bio-oil | 68.2 | 10.7 | <0.2 | <2.0 | 21.1 | |
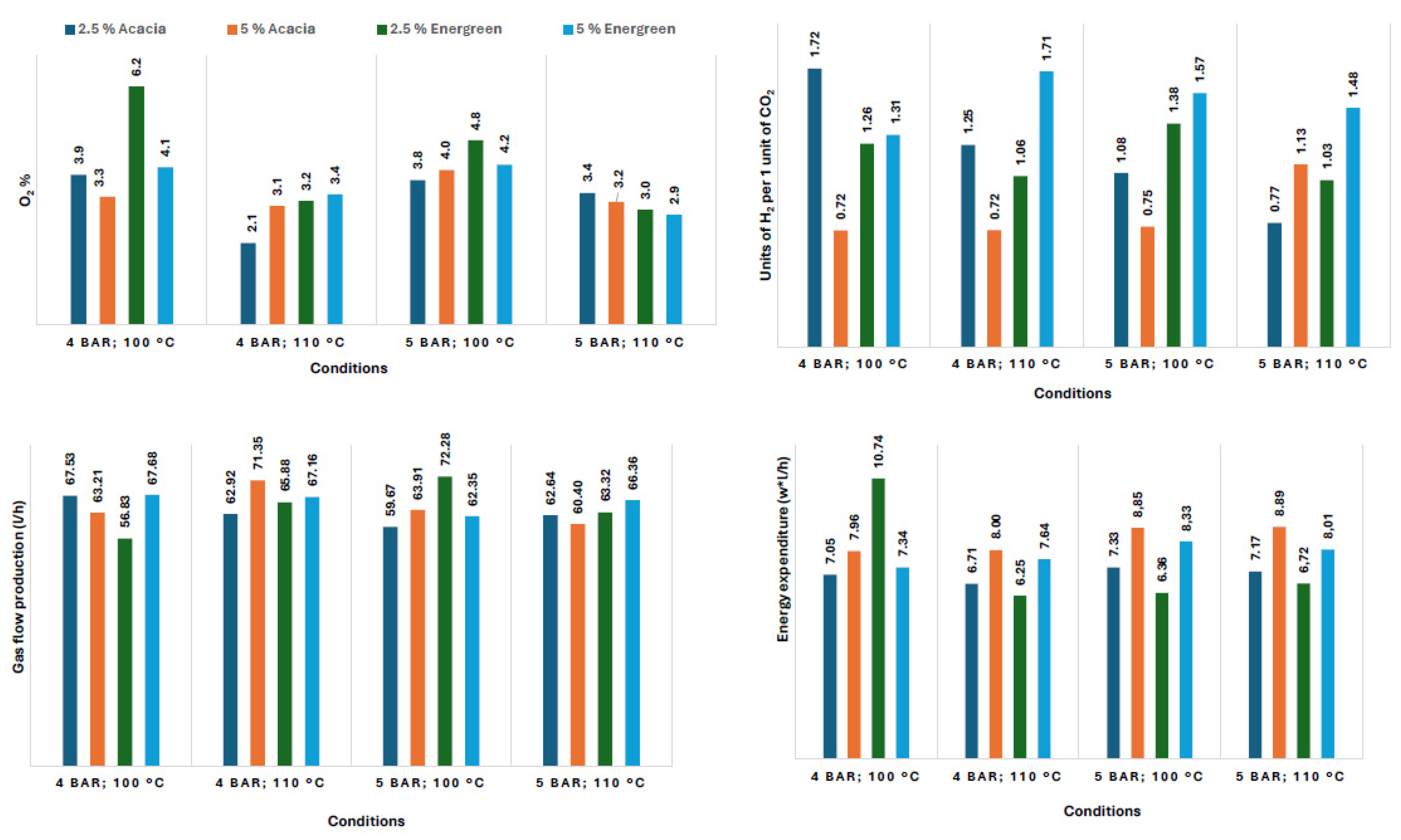
| Carbon Source | Test Conditions | O2 (%) | CO (%) | CO2 (%) | H2 (%) | F (l/h) | W/f (Wh/L) | CO2:H2 Ratio |
|---|---|---|---|---|---|---|---|---|
| No biomass added | 4 bar gauge; 100 °C | 4.1 | 1.8 | 25.4 | 68.7 | 46.84 | 10.58 | 2.71 |
| 4 bar gauge; 110 °C | 3.5 | 1.8 | 26.2 | 68.5 | 44.95 | 11.39 | 2.61 | |
| 5 bar gauge; 100 °C | 8.8 | 2.1 | 29.6 | 59.5 | 57.19 | 8.62 | 2.01 | |
| 5 bar gauge; 110 °C | 6.7 | 2.1 | 31.8 | 59.4 | 61.79 | 7.92 | 1.87 | |
| 2.5% Acacia biomass | 4 bar gauge 100 °C | 3.9 | 2.0 | 34.5 | 59.6 | 67.53 | 7.05 | 1.72 |
| 4 bar gauge 110 °C | 2.1 | 2.0 | 42.6 | 53.3 | 62.92 | 6.71 | 1.25 | |
| 5 bar gauge 100 °C | 3.8 | 2.0 | 45.4 | 48.9 | 59.67 | 7.33 | 1.08 | |
| 5 bar gauge 110 °C | 3.4 | 2.0 | 53.4 | 41.1 | 62.64 | 7.17 | 0.77 | |
| 5% Acacia biomass | 4 bar gauge 100 °C | 3.3 | 2.0 | 54.9 | 39.7 | 63.21 | 7.96 | 0.72 |
| 4 bar gauge 110 °C | 3.1 | 2.0 | 55.0 | 39.9 | 71.35 | 8.00 | 0.72 | |
| 5 bar gauge 100 °C | 4.0 | 2.0 | 53.8 | 40.1 | 63.91 | 8.85 | 0.75 | |
| 5 bar gauge 110 °C | 3.2 | 2.0 | 44.5 | 50.3 | 60.40 | 8.89 | 1.13 | |
| 2.5% Energreen biomass | 4 bar gauge 100 °C | 6.2 | 2.0 | 40.6 | 51.2 | 56.83 | 10.74 | 1.26 |
| 4 bar gauge 110 °C | 3.2 | 2.0 | 46.0 | 48.7 | 65.88 | 6.25 | 1.06 | |
| 5 bar gauge 100 °C | 4.8 | 2.0 | 39.1 | 54.1 | 72.28 | 6.36 | 1.38 | |
| 5 bar gauge 110 °C | 3.0 | 2.0 | 46.7 | 48.3 | 63.32 | 6.72 | 1.03 | |
| 5% Energreen biomass | 4 bar gauge 100 °C | 4.1 | 2.0 | 40.6 | 53.3 | 67.68 | 7.34 | 1.31 |
| 4 bar gauge 110 °C | 3.4 | 2.0 | 35.0 | 59.6 | 67.16 | 7.64 | 1.71 | |
| 5 bar gauge 100 °C | 4.2 | 2.0 | 36.5 | 57.4 | 62.35 | 8.33 | 1.57 | |
| 5 bar gauge 110 °C | 2.9 | 2.0 | 38.3 | 56.8 | 66.36 | 8.01 | 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, D.; Cabrita, T.; Rodrigues, J.; Puna, J.; Gomes, J. Utilisation of Liquefied Biomass in Water Co-Electrolysis for the Production of Synthesis Gas. Energy Storage Appl. 2025, 2, 2. https://doi.org/10.3390/esa2010002
Martins D, Cabrita T, Rodrigues J, Puna J, Gomes J. Utilisation of Liquefied Biomass in Water Co-Electrolysis for the Production of Synthesis Gas. Energy Storage and Applications. 2025; 2(1):2. https://doi.org/10.3390/esa2010002
Chicago/Turabian StyleMartins, Diogo, Tiago Cabrita, João Rodrigues, Jaime Puna, and João Gomes. 2025. "Utilisation of Liquefied Biomass in Water Co-Electrolysis for the Production of Synthesis Gas" Energy Storage and Applications 2, no. 1: 2. https://doi.org/10.3390/esa2010002
APA StyleMartins, D., Cabrita, T., Rodrigues, J., Puna, J., & Gomes, J. (2025). Utilisation of Liquefied Biomass in Water Co-Electrolysis for the Production of Synthesis Gas. Energy Storage and Applications, 2(1), 2. https://doi.org/10.3390/esa2010002








