Establishment and Validation of Patient-Derived Non-Small Cell Lung Cancer Organoids as In Vitro Lung Cancer Models
Abstract
1. Introduction
2. Materials and Methods
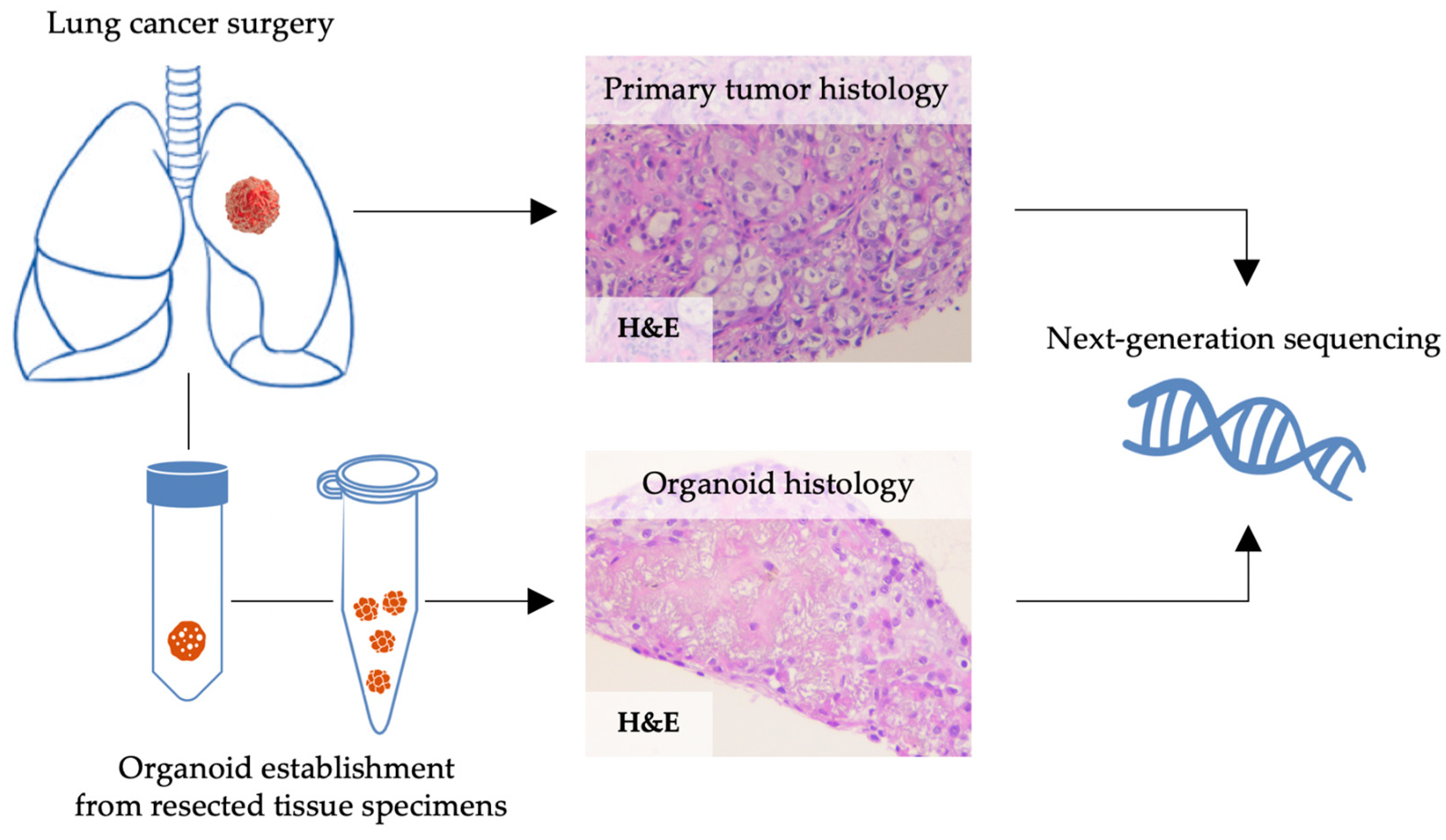
2.1. Human NSCLC Tissue Specimens for Organoid Culture
2.2. Tissue Processing and Culture of Patient-Derived NSCLC Organoids
2.3. Histology and Immunohistochemistry (IHC) for Validation of Patient-Derived Organoids
2.4. Next Generation Sequencing (NGS)
3. Results
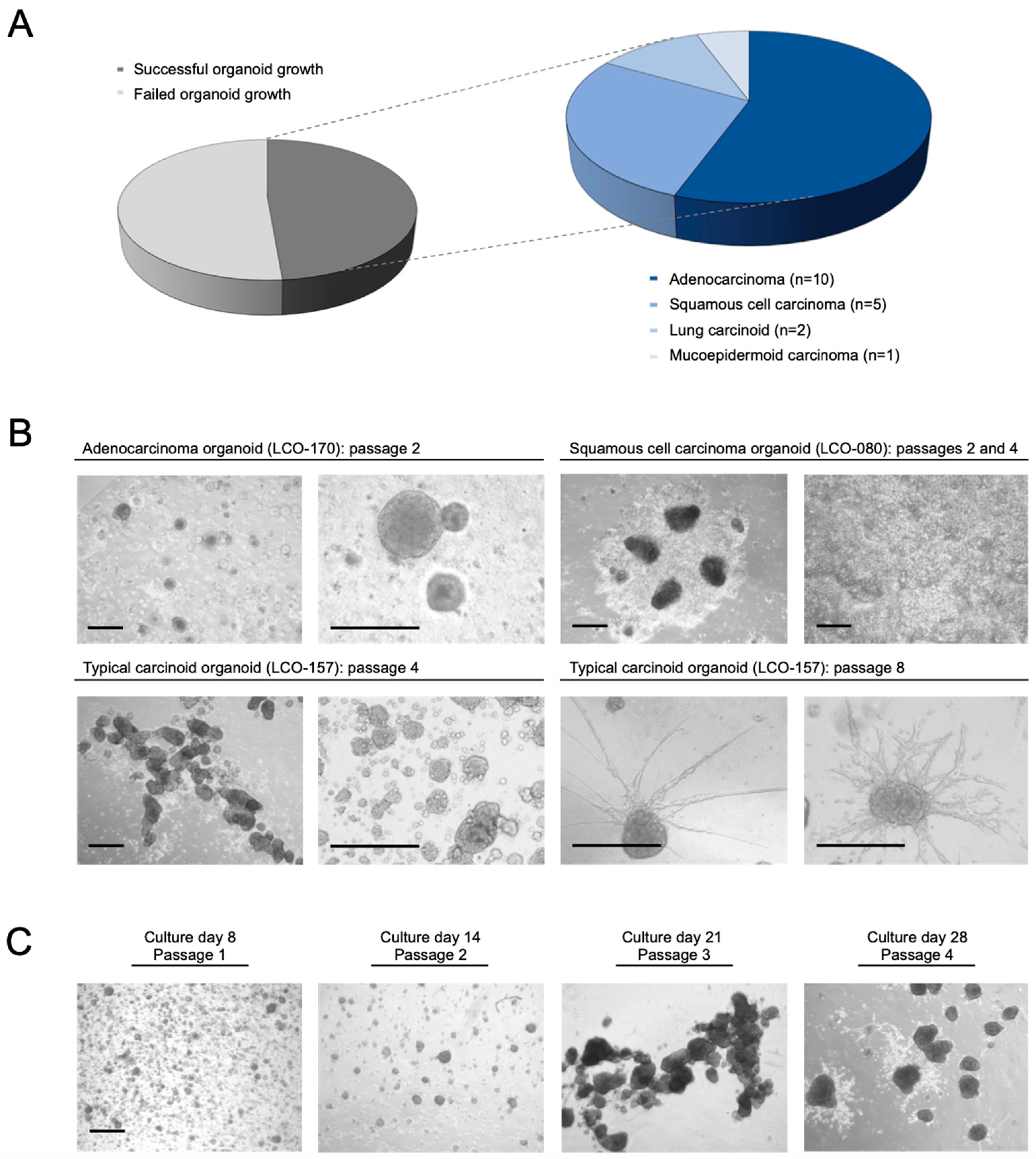
3.1. Establishment of Patient-Derived NSCLC Organoids
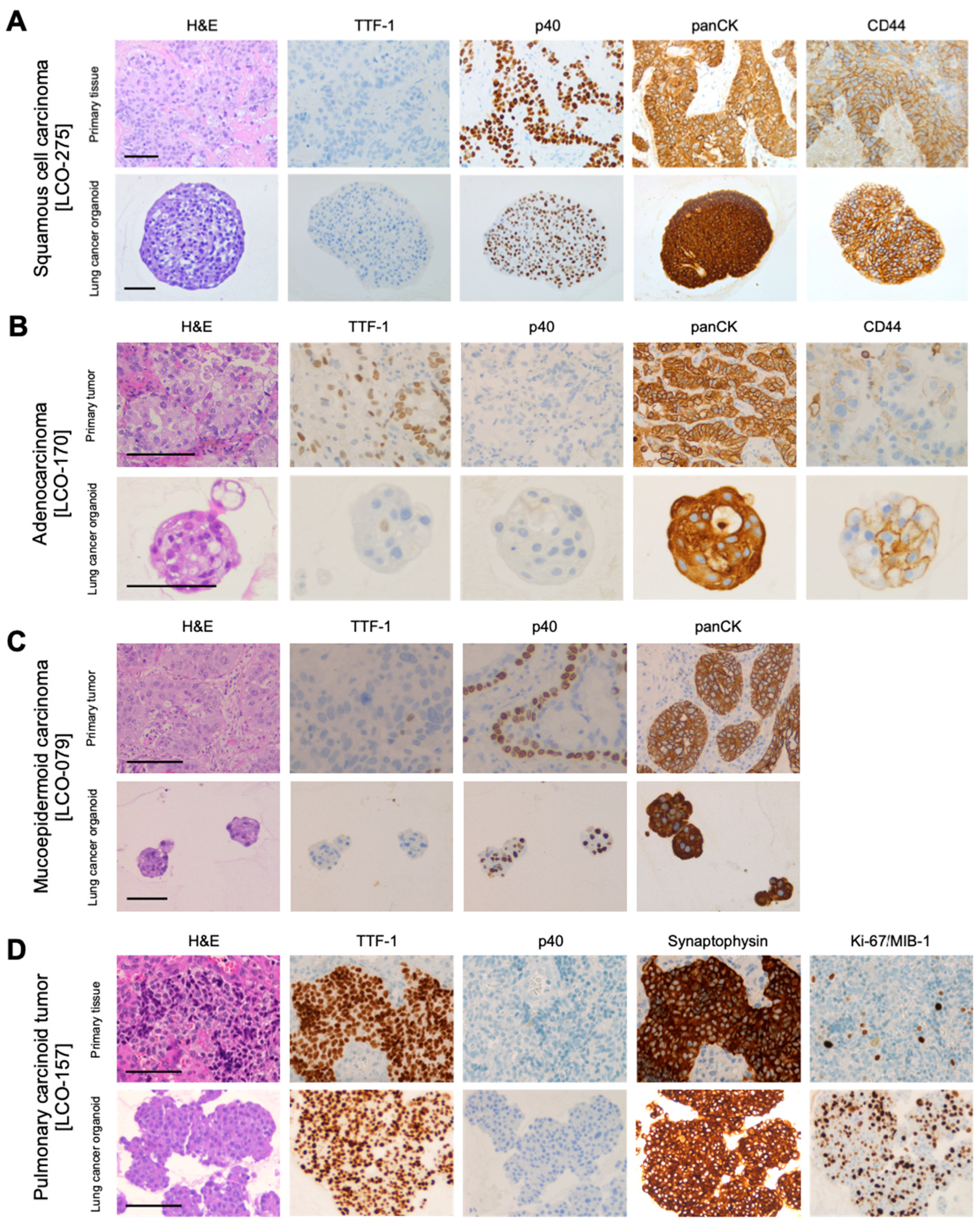
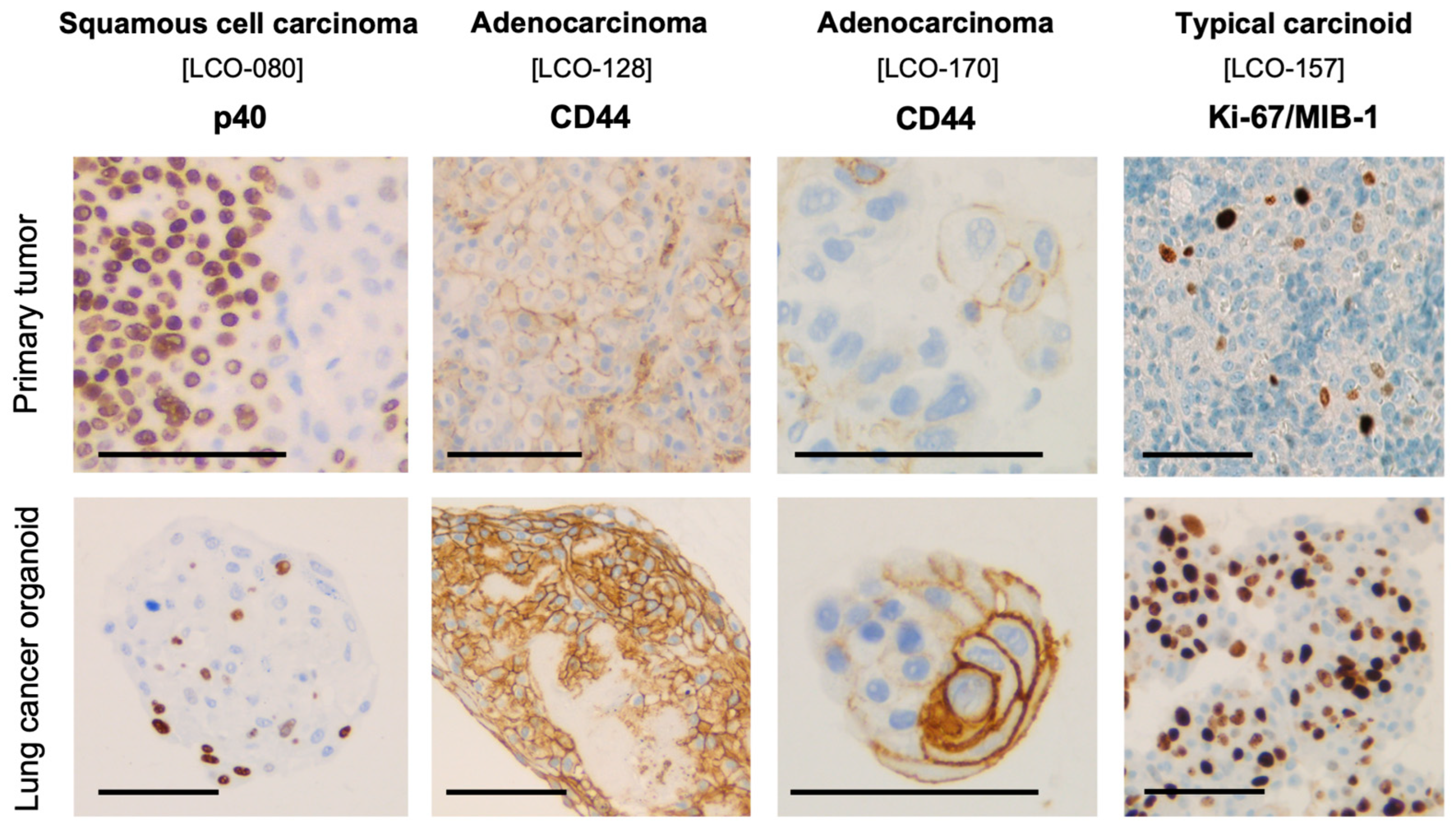
3.2. Histological and Immunohistochemical Characterization of NSCLC Organoids
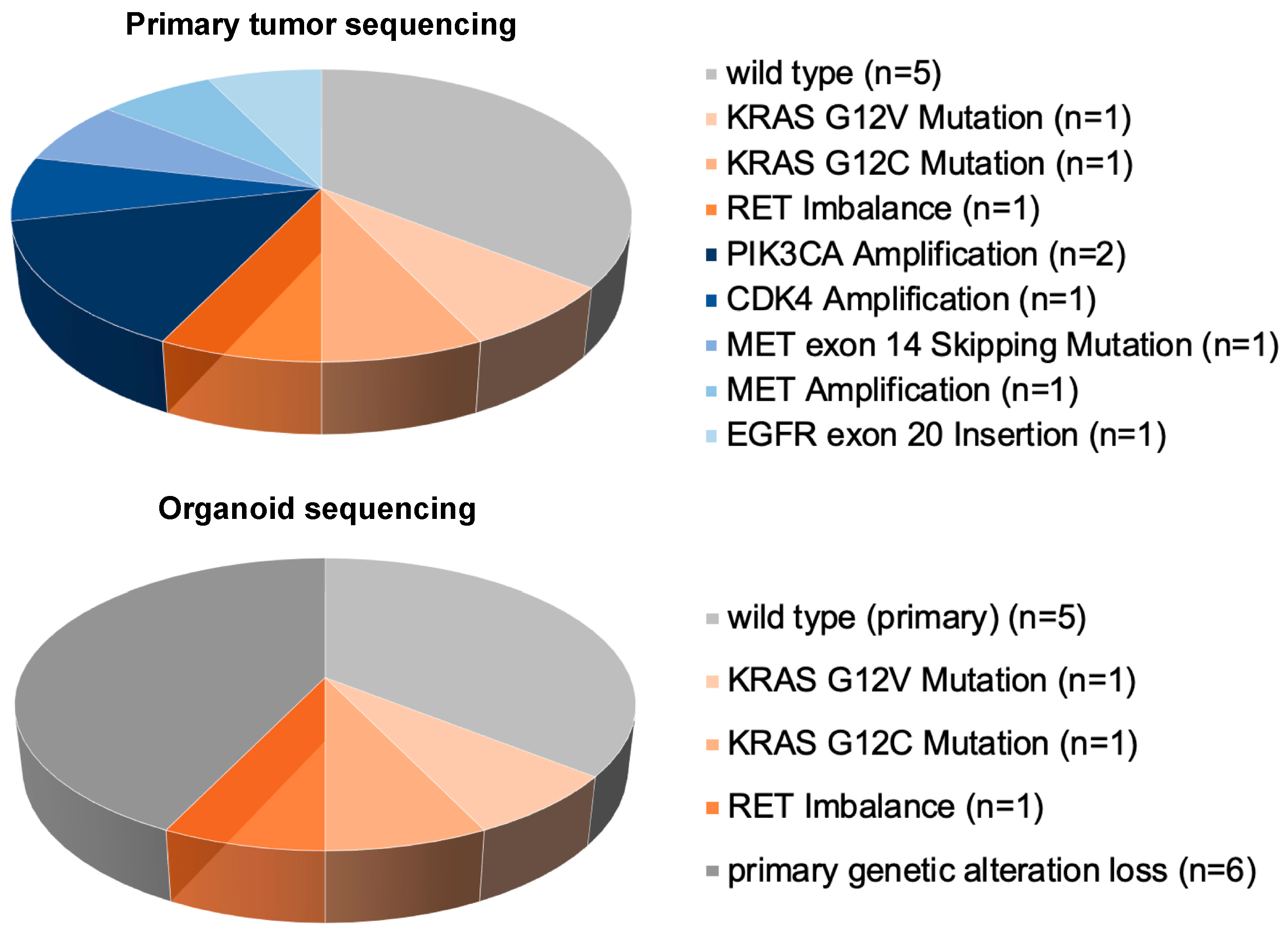
3.3. Genetic Characterization by NGS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adenocarcinoma |
| bFGF | Basic fibroblast growth factor |
| CNV | Copy number variation |
| DPBS | Dulbecco’s phosphate-buffered saline |
| EGFR | Epidermal growth factor receptor |
| hEGF | Human epidermal growth factor |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemistry |
| KRAS | Kirsten rat sarcoma virus |
| NGS | Next-generation sequencing |
| NSCLC | Non-small cell lung cancer |
| panCK | Pan-cytokeratin |
| PDX | Patient-derived xenograft |
| SCC | Squamous cell carcinoma |
| TTF-1 | Thyroid transcription factor-1 |
| UICC | Union for International Cancer Control |
References
- Wild, C.; Weiderpass, E.; Steward, B. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.; Veeriah, S.; Swanton, C. Tracking the Evolution of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Girard, L.; Lockwood, W.W.; Lam, W.L.; Minna, J.D. Lung cancer cell lines as tools for biomedical discovery and research. J. Natl. Cancer Inst. 2010, 102, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; El-Naggar, A.K.; Kim, E.S.; Kurie, J.M.; Lozano, G. A genetic mouse model for metastatic lung cancer with gender differences in survival. Oncogene 2007, 26, 6896–6904. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pham, N.A.; Tong, J.; Sakashita, S.; Allo, G.; Kim, L.; Tsao, M.S. Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors. Int. J. Cancer 2017, 140, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.P.; Varma, S.; Gottesman, M.M. The clinical relevance of cancer cell lines. J. Natl. Cancer Inst. 2013, 105, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-H.; Karlsson, K.; Kuo, C.J. Applications of organoids for cancer biology and precision medicine. Nat. Cancer 2020, 1, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Clevers, H. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.J.; Chun, S.M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, K.K.; Monkhorst, K.; Schipper, L.J.; Hartemink, K.J.; Smit, E.F.; Kaing, S.; de Groot, R.; Wolkers, M.C.; Clevers, H.; Cuppen, E.; et al. Challenges in Establishing Pure Lung Cancer Organoids Limit Their Utility for Personalized Medicine. Cell Rep. 2020, 31, 107588. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qian, Y.; Li, W.; Liu, L.; Yu, L.; Liu, X.; Wu, G.; Wang, Y.; Luo, W.; Fang, F.; et al. Human Lung Adenocarcinoma-Derived Organoid Models for Drug Screening. iScience 2020, 23, 101411. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.S.; Kirschner, M.B.; Opitz, I. Primary Lung Cancer Organoids for Personalized Medicine-Are They Ready for Clinical Use? Cancers 2021, 13, 4832. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Radulovich, N.; Ng, C.; Liu, N.; Notsuda, H.; Cabanero, M.; Martins-Filho, S.N.; Raghavan, V.; Li, Q.; Mer, A.S.; et al. Organoid Cultures as Preclinical Models of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, Y.; Dacic, S.; Borczuk, A.C.; Warth, A.; Russell, P.A.; Lantuejoul, S.; Beasley, M.B.; Thunnissen, E.; Pelosi, G.; Rekhtman, N.; et al. Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J. Thorac. Oncol. 2019, 14, 377–407. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.; Iversen, P.W.; Schumacher, D.; Lallena, M.J.; Haro, R.; Amat, J.; Haybaeck, J.; Liebs, S.; Lange, M.; Schäfer, R.; et al. Assay Establishment and Validation of a High-Throughput Screening Platform for Three-Dimensional Patient-Derived Colon Cancer Organoid Cultures. J. Biomol. Screen. 2016, 21, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Pomares, A.; de-Maya-Girones, J.D.; Calabuig-Fariñas, S.; Lucas, R.; Martínez, A.; Pardo-Sánchez, J.M.; Camps, C. Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer. Cell Death Dis. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Dijkstra, K.K.; Rawlins, E.L.; Hynds, R.E. Open questions in human lung organoid research. Front. Pharmacol. 2022, 13, 1083017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zuo, X.; Xie, K.; Wei, D. The Role of CD44 and Cancer Stem Cells. Methods Mol. Biol. 2018, 1692, 31–42. [Google Scholar] [CrossRef] [PubMed]
| Additive | Final Concentration |
|---|---|
| Advanced DMEM/F12 (ThermoFisher) | |
| GlutaMax (ThermoFisher) | 4 mM |
| HEPES Buffer (ThermoFisher) | 25 mM |
| Penicillin/streptomycin (1%) | 10 μL/mL |
| Human epidermal growth factor (hEGF, PeproTech) | 50 ng/mL |
| Basic fibroblast growth factor (bFGF, PeproTech) | 50 ng/mL |
| B-27 serum-free supplement (ThermoFisher) | 25 μL/mL |
| N-2 supplement (ThermoFisher) | 10 μL/mL |
| rho-kinase (ROCK) inhibitor Y-27632 (Enzo Life Sciences) | 10 μM |
| A83-01 (Stemcell) | 500 nM |
| ID | Specimen Diameter (mm) | Organoid Culture Established | Targeted Sequencing | Sex | Age | Smoking History (py) | Histology | TNM (8th Edition) | UICC Stage | Hemangiosis | Lymphangiosis | Differentiation | Neoadjuvant Treatment | Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LCO-63 | 6 | Y (1) | - | f | 58 | 35 | AC | pT1b pN0 cM0 | IA2 | V0 | L0 | n/a | N | RATS lobectomy |
| LCO-79 | 15 | Y (2) | wt | m | 84 | 19 | MUC | pT2b pN0 cM0 | IIA | V0 | L1 | G3 | N | RATS lobectomy |
| LCO-80 | 6 | Y (3) | PIK3CA amplification | m | 68 | 95 | SCC | pT2a pN1 cM0 | IIB | V1 | L1 | n/a | N | Open double-sleeve lobectomy |
| LCO-82 | 8 | Y (4) | wt | f | 82 | 0 | AtC | pT3 pN1 pM1b | IV | V1 | L1 | n/a | N | RATS lobectomy |
| LCO-83 | 5 | N | - | m | 71 | 100 | SCC | pT4 pN1 pM1a | IV | V0 | L1 | n/a | Y: Chemotherapy | VATS wedge resection (metastasis) |
| LCO-89 | 16 | Y (5) | CDK4 amplification | f | 69 | 30 | AC | pT2a pN1 cM0 | IIB | V1 | L0 | G3 | N | VATS lobectomy |
| LCO-92 | 18 | N | - | f | 60 | 45 | AC | pT3 pN0 cM0 | IIB | V1 | L0 | G3 | N | RATS lobectomy |
| LCO-97 | 10 | Y (6) | wt | m | 70 | 60 | AC | pT1b pN0 cM0 | IA2 | V1 | L0 | G2 | N | RATS lobectomy |
| LCO-94 | 4 | N | - | m | 62 | 120 | AC | ypT4 ypN1 cM0 | IIIA | V1 | L0 | n/a | Y: Radio-chemotherapy | Open lobectomy, chest wall resection |
| LCO-99 | 9 | Y (7) | PIK3CA amplification | m | 62 | 80 | SCC | pT2a pN0 cM0 | IB | V1 | L1 | G3 | N | RATS lobectomy |
| LCO-102 | 4 | N | - | f | 75 | 35 | AC | pT1b cN0 cM0 | IA2 | n/a | n/a | n/a | N | VATS wedge resection |
| LCO-103 | 8 | Y (8) | - | f | 77 | 50 | AC | pT3 pN1 cM0 | IIIA | V1 | L1 | G3 | N | Open bilobectomy |
| LCO-106 | 10 | Y (9) | - | m | 71 | 50 | SCC | pT1b pN0 cM0 | IA2 | V0 | L0 | G2 | N | VATS lobectomy |
| LCO-093 | 12 | N | - | m | 73 | 120 | SCC | pT1c pN0 cM0 | IA3 | V0 | L0 | G2 | N | RATS lobectomy |
| LCO-110 | 4 | N | - | m | 66 | 30 | SCC | pT2a pN1 cM0 | IIA | V1 | L1 | G2 | N | Open double-sleeve lobectomy |
| LCO-111 | 8 | N | - | m | 58 | 40 | SCC | ypT3 ypN0 cM0 | IIIA | V0 | L1 | n/a | Y: Radio-chemotherapy | Pneumonectomy |
| LCO-114 | 6 | Y (10) | MET fusion | f | 62 | 10 | AC | pT3 pN0 cM0 | IIB | V0 | L0 | G1 | N | VATS lobectomy |
| LCO-113 | 15 | Y (11) | - | m | 71 | 50 | SCC | pT3 pN1 cM0 | IIIA | V1 | L1 | G3 | N | VATS lobectomy |
| LCO-127 | 10 | N | - | f | 65 | 40 | AC | pT4 pN0 cM0 | IIIA | V1 | L0 | G2 | N | VATS lobectomy |
| LCO-128 | 7 | Y (12) | KRAS G12V mutation | m | 62 | 40 | AC | ypT4 ypN2 pM1b | IVA | n/a | n/a | n/a | Y: Chemotherapy | Open mediastinal lymphadenectomy |
| LCO-135 | 7 | N | - | f | 61 | 20 | AC | pT2b pN2 cM0 | IIIA | V1 | L1 | G2 | Y: Chemotherapy | VATS lobectomy |
| LCO-140 | 6 | N | - | m | 71 | 80 | PC | pT3 pN0 cM1b | IVA | V0 | L0 | G3 | N | VATS lobectomy |
| LCO-141 | 7 | N | - | f | 66 | 40 | AC | pT2a pN0 cM0 | IB | V1 | L0 | G3 | N | RATS lobectomy |
| LCO-145 | 5 | N | - | f | 60 | 40 | AC | pT1c pN0 cM0 | IA3 | V0 | L0 | G2 | N | RATS lobectomy |
| LCO-148 | 6 | N | - | m | 63 | 80 | SCC | pT2 pN0 cM0 | IB | V0 | L0 | G2 | N | Open sleeve-lobectomy |
| LCO-149 | 5 | N | - | m | 62 | 135 | SCC | pT1c pN0 cM0 | IA3 | V0 | L0 | n/a | N | Open lobectomy |
| LCO-153 | 9 | N | - | f | 69 | 0 | PC | pT3 pN1 cM0 | IIIA | V1 | L1 | G3 | N | Pneumonectomy |
| LCO-154 | 8 | N | - | m | 74 | 90 | SCC | pT2a pN0 cM0 | IB | V1 | L0 | G3 | N | VATS lobectomy |
| LCO-155 | 6 | N | - | m | 65 | 13 | AC | pT1c pN0 cM0 | IA3 | V1 | L0 | G2 | N | Pneumonectomy |
| LCO-157 | 4 | Y (14) | RET imbalance | m | 54 | 40 | AtC | pT1b pN0 cM0 | IA2 | V0 | L0 | n/a | N | RATS lobectomy |
| LCO-158 | 18 | N | - | f | 52 | 30 | AC | cTX pN2 cM0 | IIIA | n/a | n/a | n/a | Y: Chemotherapy | Open mediastinal lymphadenectomy |
| LCO-166 | 5 | Y (13) | - | m | 83 | 1 | AC | pT1c pN0 cM0 | IA3 | V0 | L0 | G2 | N | RATS lobectomy |
| LCO-167 | 9 | N | - | m | 76 | 0 | AC | pT1b pN2 cM0 | IIIA | V0 | L0 | G2 | N | RATS wedge resection |
| LCO-170 | 8 | Y (15) | MET amplification | m | 57 | 40 | AC | pT1b pN1 cM0 | IIB | V1 | L1 | G2 | N | VATS lobectomy |
| LCO-275 | 9 | Y (16) | wt | f | 77 | 50 | SCC | pT2b pN0 cM0 | IIA | V1 | L0 | G2 | N | VATS lobectomy |
| LCO-279 | 6 | Y (17) | EGFR Ex20Ins | f | 56 | 15 | AC | pT2a pN0 cM0 | IB | V0 | L0 | G2 | N | VATS lobectomy |
| LCO-283 | 18 | Y (18) | KRAS G12C mutation | f | 55 | 25 | AC | pT4 pN1 cM0 | IIIB | V1 | L0 | G3 | Y: Chemotherapy | Open lobectomy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, R.S.; Jang, J.-H.; Rechsteiner, M.; Kirschner, M.B.; Opitz, I. Establishment and Validation of Patient-Derived Non-Small Cell Lung Cancer Organoids as In Vitro Lung Cancer Models. Organoids 2024, 3, 281-294. https://doi.org/10.3390/organoids3040017
Werner RS, Jang J-H, Rechsteiner M, Kirschner MB, Opitz I. Establishment and Validation of Patient-Derived Non-Small Cell Lung Cancer Organoids as In Vitro Lung Cancer Models. Organoids. 2024; 3(4):281-294. https://doi.org/10.3390/organoids3040017
Chicago/Turabian StyleWerner, Raphael S., Jae-Hwi Jang, Markus Rechsteiner, Michaela B. Kirschner, and Isabelle Opitz. 2024. "Establishment and Validation of Patient-Derived Non-Small Cell Lung Cancer Organoids as In Vitro Lung Cancer Models" Organoids 3, no. 4: 281-294. https://doi.org/10.3390/organoids3040017
APA StyleWerner, R. S., Jang, J.-H., Rechsteiner, M., Kirschner, M. B., & Opitz, I. (2024). Establishment and Validation of Patient-Derived Non-Small Cell Lung Cancer Organoids as In Vitro Lung Cancer Models. Organoids, 3(4), 281-294. https://doi.org/10.3390/organoids3040017






