Abstract
An integrated visual energy system consisting of conjugated polymer electrodes is promising for combining electrochromism with energy storage. In this work, we obtained copolymer bifunctional electrodes poly(3,6-dimethoxythieno[3,2-b]thiophene-co-2,3-dihydrothieno[3,4-b][1,4]dioxin-3-ylmethanol)(P(TT-OMe-co-EDTM)) by one-step electrochemical copolymerization, which exhibits favorable electrochromic and capacitive energy storage properties. Because of the synergistic effect of PTT-OMe and PEDTM, the prepared copolymers show better flexibility. Moreover, the morphology and electrochemical properties of the copolymers could be adjusted by depositing different molar ratios of 3,6-dimethoxythieno[3,2-b]thiophene (TT-OMe) and 2,3-dihydrothieno[3,4-b][1,4] dioxin-3-ylmethanol (EDTM). The P(TT-OMe-co-EDTM) electrodes realized a high specific capacitance (190 F/g at 5 mV/s) and recognizable color conversion. This work provides a novel and simple way to synergistically improve electrochromic and energy storage properties and develop thiophene-based conducting polymers for electrochromic energy storage devices.
1. Introduction
As the demand for renewable energy rises, the development of sustainable energy storage devices has become a popular research direction [1]. Supercapacitors (SCs), characterized by a long cycle life, a high power density, and fast charge/discharge rates [2,3,4,5,6], stand out as the most promising candidate among the energy storage devices for renewable energy systems. Among others, electrochromic supercapacitors (ESCs) have the capability of reversible color transformation in specific electrode materials during the electrochemical oxidation/reduction processes induced by an applied voltage [7]. The capacity of ESCs to both change colors and store energy enables them to fulfill the essential requirements for electronic devices, smart wearables, and military gear, which has emerged as a hot area of research [8,9,10,11]. However, the exploitation of bifunctional electrode materials for electrochromic supercapacitors is particularly critical for next-generation energy storage devices.
Over the years, the bifunctional electrode materials for ESCs have consisted of two main categories: transition metal oxides [12,13,14] and conducting polymers [15,16,17]. Transition metal oxides, such as MnO2 and RuO2, have emerged as the most utilized substances. Their prevalent use is attributed to their commendable performance, such as environmental friendliness and high energy density. However, transition metal oxide materials suffer from drawbacks, such as low conductivity, extended response times, limited flexibility, and single color variability [18,19,20]. In contrast to transition metal oxides, conductive polymers, such as PANI, PPy, PTh, and PEDOT, which offer advantages like diverse color options, rapid response times, and exceptional flexibility, herald a promising trend for future large-scale device manufacturing in industry and application as electrode materials for flexible functional devices [21,22,23]. Furthermore, the properties of these conductive polymers could be easily adjusted through various physical and chemical strategies, enhancing their suitability for multiple applications [24,25,26,27,28].
Among the different conducting polymers, Polythiophene (PTh) and its analogue Poly2,3-dihydrothieno[3,4-b][1,4]dioxin-3-ylmethanol (PEDTM) are extensively researched due to their ease of synthesis, flexibility, and excellent electrochemical properties [29,30]. Moreover, the derivatives of thiophene-based polymers, exemplified by fused thiophenes (e.g., thieno[3,2-b]thiophene, TT), have attracted extensive attention for their extended π-conjugated structures, which can significantly increase the charge carrier mobility [31]. Furthermore, the modification of the side chains can further refine the morphology of conductive polymer films, which is beneficial for enhancing their electrochemical performance [32]. For instance, TT modified with methoxy side chains demonstrates exceptional electropolymerization capability, and its polymer present remarkable electrochromic characteristics, positioning it as a promising material in the field of advanced organic electronics. Nonetheless, poly(3,6-dimethoxythieno[3,2-b]thiophene) (TT-OMe)P(TT-OMe) polymers directly electropolymerized from their monomers exhibit poor electrochemical activity due to an unsatisfactory microstructure, constraining their use in electrochemical energy storage devices [33,34,35]. Electrochemical copolymerization offers the advantage of precisely tailoring the microstructure of conducting polymer films through a controlled synthesis process directly on the electrode surfaces, which provides a promising platform for developing high-performance electrochemical devices.
In this study, P(TT-OMe-co-EDTM) copolymer thin films were synthesized by one-step electrochemical polymerization. By adjusting the molar ratio of TT-OMe and EDTM, bifunctional electrodes with a customizable morphology and flexibility were achieved. These electrodes exhibit an enhanced specific capacitance (from 104 F/g to 190 F/g) and controllable color conversion (from purple to yellow). A supercapacitor is a novel energy storage device with the features of a high power density, fast charging and discharging speeds, high reliability, environmental friendliness, and so on. Furthermore, when integrated into an electrochromic supercapacitor, the charge status can be visually monitored due to the reversible and stable chromatic changes. This suggests the potential for developing P(TT-OMe-co-EDTM) electrodes into smart electrochromic supercapacitors.
2. Experiment
2.1. Materials
3,6-dimethoxythieno[3,2-b]thiophene(TT-OMe) was purchased from Bide Pharmatech Ltd., Shanghai, China, and 2,3-dihydrothieno[3,4-b][1,4]dioxin-3-ylmethanol (EDTM) was purchased from Innokai Technology Ltd., Beijing, China. Tetrabutylammonium hexafluorophosphate (Bu4NPF6, 99%; Energy Chemical, Shanghai, China) was from Bide Pharmatech Ltd. and dried under a vacuum at 80 °C for 24 h before use. Aceto-nitrile (ACN, AR, Beijing, China) was purchased from Beijing InnoChem Science & Technology Co., Ltd., Beijing, China.
2.2. Measurement and Characterization
The CHI 660E electrochemical workstation (Shanghai Chenhua Ins., Shanghai, China) was used in all electrochemical measurements. The electrochemical polymerization of the monomers was performed in a precisely technical standard-setting three-electrode system using Pt wire as the counter electrode, Ag/AgCl wire as the reference electrode, and ITO-coated glass as the working electrode to supply a superior polymerization stability and deposition on the surface area (effective area = 1.5 cm2). The 3600i Plus UV-Vis-NIR spectrophotometer (Tokyo, Japan) was used to evaluate the absorption spectra. We used the ZEISS-Sigma-300 scanning electron microscope (SEM) (Oberkochen, Germany) to measure the morphology of samples. Infrared spectra were obtained by using the Nicolet-iS10 FTIR spectrometer with KBr pellets (Thermo Fisher Scientific, Waltham, MA, USA). The thermal property was analyzed by thermogravimetric analysis (TGA) on an NETZSCH STA 449F3 (NETZSCH-Gerätebau GmbH, Selb, Germany) in the temperature range of 200–1000 °C with a heating rate of 10 °C/min under N2 flow. The bending stability of the polymer film was assessed at a certain bending angle and under the same mechanical stress.
2.3. Fabricating the Polymer Electrodes
The polymer film was electrodeposited on the surface of ITO-coated glass by a constant potential method in a standard-setting three-electrode system, as shown in Figure 1. The effective area (1.5 cm2) of the working electrode was immersed in 0.1 M Bu4NPF6/ACN solution, including 0.05 M monomer or a mixture of TT-OMe and EDTM monomers in molar ratios of 10:1, 8:1, and 4:1. The polymer films were deposited with 1.4 V constant electrical potential (amount of deposition = 5 mC/cm2) on the ITO for UV-Vis spectroscopy measurement. The films were electrodeposited using the same method for 3500 s, and then immersed the films adhered to the ITO-coated glass substrates in deionized water, peeled off, and dried to yield free-standing and soft films. For TGA and FTIR testing, the polymers were electrodeposited on ITO (2 cm × 1 cm), and then scraped off the ITO surface, and then cleaned.
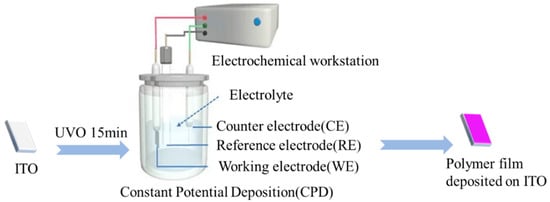
Figure 1.
Preparation process of polymer film (P(TT-OMe-co-EDTM), PTT-OMe, PEDTM).
The monomers were electrochemically polymerized potentiostatically at 1.4 V. Assuming a current efficiency (η) of 100%, the mass of polymers was computed following Equation (1) below [36].
where M is the molecular weight of the monomer. F is the Faraday constant (96,485 C/mol). Z is the quantity of electrons transferred per monomer attached to the polymer, and Z = 2 + f. The part of the charge f is known as the doping level, with f being approximately 0.33 [37].
All electrochemical characterizations of the polymer electrodes were carried out using the standard-setting three-electrode system with 0.1 M Bu4NPF6/ACN electrolyte solution. For electrochemical characterization, polymer-coated ITO was used as the working electrode, Pt wire was used as the counter electrode, and Ag/AgCl was used as the reference electrode. Cyclic voltammetry (CV) curves were used to calculate the specific capacitance of the polymers electrode using Equation (2) [38]:
where is the total integrated charge through the anode and cathode scans in the CV curve, is the mass of the active polymer deposited on the electrode, is the scan rate, is the potential window for CV, and is the specific capacitance.
In addition, the specific capacitance can be calculated from the CD curves using Equation (3) [39,40]:
where I and are the discharge current and the discharge time, respectively, m is the mass of the polymer film electrode, and is the potential window in the discharging process.
3. Results and Discussion
3.1. Electrochemical Polymerization of Monomers
The electrochemical polymerization of the monomer and the mixture occurred in 0.1 M ACN-Bu4NPF6, as depicted in Figure 2 and Figure S1. The appearance of a redox peak in the second cycle indicates the formation of a conducting polymer in the initial cycle [41]. The redox peak current density gradually increases as the cycle grows, indicating that the amount of polymer on the working electrode is increasing. At the same time, the potential of the redox peaks has shifted because of the higher resistance of the conducting polymers grown on the working electrode [42]. In addition, with the increase in EDTM content in the electrochemical polymerization process, the reduction peak gradually moved to a negative potential, which suggested that the copolymer of TT-OMe and EDTM was formed. It is generally believed that there are two mechanisms for the electro-polymerization of heterocyclic compounds, either radical–radical or radical–monomer coupling [43,44]. The monomer undergoes oxidation to form a cationic radical, which then reacts with the neutral molecules to produce a cationic dimer. This dimer loses electrons to become a neutral dimer, and the process continues through oxidation, recombination, and deionization, ultimately yielding the final polymer product [45]. In addition, the CV behavior of the copolymers is quite different from the CV of the monomers, which results in the enhanced electrochemical properties of the copolymers [45].
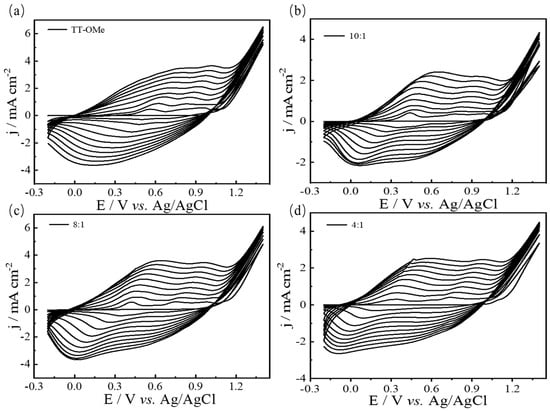
Figure 2.
CVs of 0.05 M TT−OMe (a), and EDTM monomer mixtures with different molar ratios of 10:1 (b), 8:1 (c), and 4:1 (d) in ACN-Bu4NPF6 at 50 mV/s.
3.2. Morphology of Polymers
In order to compare the morphologies of the polymers PTT-OMe, PEDTM, P10:1(TT-OMe-co-EDTM), P8:1(TT-OMe-co-EDTM), and P4:1(TT-OMe-co-EDTM), the surface morphology was recorded by using SEM techniques, and the material elements were analyzed by Energy-Dispersive X-ray Spectroscopy (EDS). And the combination of the two monomers is beneficial in the morphology modulation of polymers. Figure 3a–d shows the low- and high-resolution morphologies of PTT-OMe, PEDTM, P10:1(TT-OMe-co-EDTM), P8:1(TT-OMe-co-EDTM), and P4:1(TT-OMe-co-EDTM), respectively. The SEM images reveal that three copolymer images are porous and evenly distributed over the entire surface to form a film, a critical feature that promotes charge storage and ion diffusion as well as reduces the dead volume as it allows for the maximum penetration of electrolyte ions [46]. As shown in Figure S2 and Figure 3a, the PEDTM film has a relatively large number of round holes on its surface, whereas the PTT-OMe film has a dense surface. This explains why the film of PEDTM is a flexible free-standing film. As the proportion of EDTM in the precursor mixture increases from one eleventh to one fifth, the polymer surface gradually forms more pores, with reduced pore depth and increased surface roughness. It is worth noting that at a mixing ratio of 8:1, the surface becomes compact, and the microporous layers are graded. This copolymer-modified nanostructure creates an effective diffusion path that will facilitate ion transport, greatly accelerating ion penetration on the surface of an electrode and increasing the effective use of the electrode material [46]. The morphological changes in the P(TT-OMe-co-EDTM) films are attributed to the synergistic effect of TT-OMe and EDTM. As can be seen from the CV of monomer polymerization, the EDTM (Figure S1) monomer polymerizes more slowly than TT-OMe, resulting in a porous and smooth film after polymerization at the same driving voltage. The different ratios of TT-OMe to EDTM monomers during the copolymerization process can affect the polymerization behavior, leading to morphological changes of polymers, and resulting in copolymer modification. The elemental mapping images of P8:1(TT-OMe-co-EDTM) (Figure 3e) confirm the presence of carbon (C), oxygen (O), and sulfur (S) as the major components present with atomic percentages of 49.96%, 47.57%, and 2.48%, respectively (Table S1).
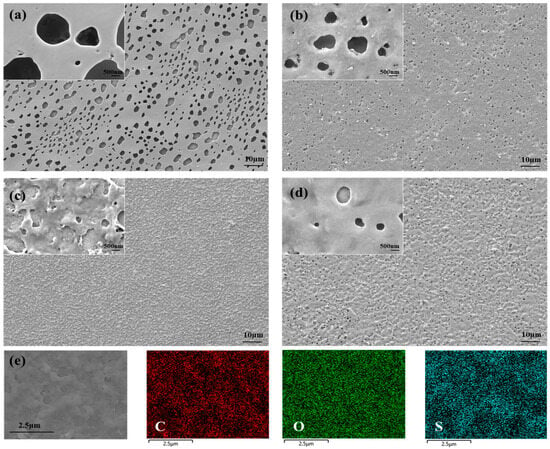
Figure 3.
SEM images of (a) PEDTM, (b) P10:1(TT-OMe-co-EDTM), (c) P8:1(TT-OMe-co-EDTM), and (d) P4:1(TT-OMe-co-EDTM)-coated ITO glass, and (e) EDS elemental color mapping images of P8:1(TT-OMe-co-EDTM).
3.3. Structure, Thermal Analysis, and Mechanical Bending of Polymers
Figure 4a shows the FT-IR spectra of the monomers and polymers. It can be seen from the figure that the intensity of the peaks at around 1204 cm−1 and 1087 cm−1 (represented of C-O-C bonding) [47] gradually increases as the copolymerization of the two monomers is completed. For the FTIR spectrum of P8:1(TT-OMe-co-EDTM), the characteristic peak of it at 3400 cm−1 corresponds to the stretching of the –OH bond. In addition, EDTM and TT-OMe show a peak of aromatic C=C-H (3110 cm−1), but P8:1(TT-OMe-co-EDTM) did not show this. Moreover, EDTM, PEDTM, PTT-Ome, and P8:1(TT-OMe-co-EDTM) show a peak of methylene C-C-H (1480 cm−1), except for TT-OMe [14]. For the FTIR spectra of P8:1(TT-OMe-co-EDTM), the characteristic peaks of PEDTM and PTT-OMe all appear in the composite films of P8:1(TT-OMe-co-EDTM). Through the above analysis, we can conclude that the monomer blends of EDTM and TT-OMe were successfully prepared as copolymers by electrochemical polymerization. In the field of supercapacitors, the stability of conducting polymers is very significant. The thermal stability analysis of the polymer films was carried out in a nitrogen atmosphere at a temperature increase rate of 10 °C/min up to 1000 °C, as shown in Figure 4b. At 140 °C and below, the weight loss of PTT-OMe, PEDTM, and P8:1(TT-OMe-co-EDTM) was about 2.07%, 4.63%, and 2.69%, respectively, which may be related to the evaporation of water or the removal of other impurities. From 140 °C to 450 °C, the weight loss of PTT-OMe, PEDTM, and P8:1(TT-OMe-co-EDTM) mainly originated from the release flow of oligomers and the decomposition of dopant ions. With further increase in temperature, the weight loss was mainly related to the degradation of the polymer backbone and the skeleton. The synergistic effect of the mixing of TT-OMe and EDTM monomers at the molecular level during copolymerization resulted in the copolymer mass retention reaching 50% at 712 °C. In addition, the temperatures at the maximum decomposition rates of PTT-OMe, PEDTM, and P8:1(TT-OMe-co-EDTM) were 575 °C, 330 °C, and 194 °C, respectively. The test results confirm that the thermal stability of the P8:1(TT-OMe-co-EDTM) composite copolymer has been substantially improved compared with that of the PTT-OMe single polymer.
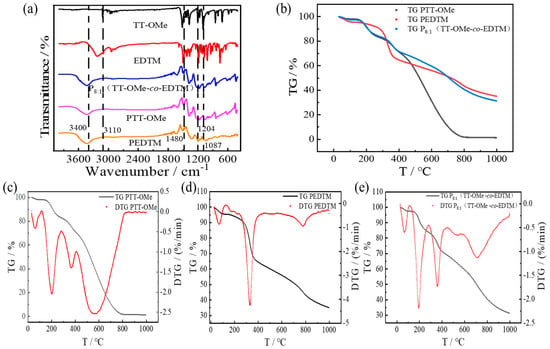
Figure 4.
(a) FTIR spectra of TT−OMe, EDTM, P8:1(TT−OMe-co-EDTM), PTT−OMe, and PEDTM; (b) TG curves of PTT−OMe, PEDTM, and P8:1(TT−OMe-co-EDTM); (c) TG and DTG curves of PTT−OMe; (d) TG and DTG curves of PEDTM; and (e) TG and DTG curves of P8:1(TT−OMe-co-EDTM).
Additionally, the mechanical bending cycle test of the as-prepared conducting polymer films was conducted, as depicted in Figure 5. The PEDTM film exhibited exceptional robustness, remaining intact without any significant degradation even after 500 bending cycles. In contrast, the PTT-OMe film demonstrated fragility, being prone to fracturing and lacking bendability. This observation corroborates the notion that the flexibility of copolymer films is predominantly imparted by the incorporation of the PEDTM moiety, resulting in copolymer films that retain their structural integrity after 500 mechanical bending cycles, thereby showcasing superior mechanical flexibility. The enhanced flexibility of the copolymer films can be ascribed to alterations in the conjugated backbone structure of the EDTM and TT-OMe monomers subsequent to the electrochemical deposition process. Furthermore, improved mechanical bending stability will facilitate the integration of these films into flexible electronic devices, enhancing their reliability and longevity for practical applications in flexible electronics.

Figure 5.
Photographs of the free-standing films, and the bending test of PTT-OMe, PEDTM, and P8:1(TT-OMe-co-EDTM).
3.4. Electrochemical Performance of Polymer Electrodes
The CV curves for P10:1(TT-OMe-co-EDTM), P8:1(TT-OMe-co-EDTM), and P4:1(TT-OMe-co-EDTM), PTT-OMe, and PEDTM are presented in Figure 6 and Figure S3, respectively. The CV measurements of the polymer thin films were conducted in 0.1 M ACN-Bu4NPF6 electrolyte within a potential window from −0.4 to 1.4 V at various scan rates (5, 20, 50, 100, 150, and 200 mV/s), enabling a detailed investigation of their capacitive properties. As depicted in Figure 6, the conducting polymer films maintained relatively distinct redox peaks even as the scan rate increased, indicating that the electrode materials underwent stable and efficient electrochemical redox processes, which are crucial for their application in high-performance energy storage devices. Furthermore, the CV curve shape remained largely unchanged at the higher scan rates, signifying the material has a superior pseudocapacitive performance. The three copolymer films exhibited reversible redox reaction behaviors across a broad potential window (1.8 V), indicative of their excellent electrochemical activity [48]. Figure S3 shows that the conducting polymer films derived from the EDTM monomer exhibited higher current densities compared to those formed from the TT-OMe monomer via electropolymerization, suggesting a greater number of electroactive sites and a higher degree of electronic conductivity within the PEDTM-based films. The current densities of the copolymer films were found to be intermediate between those of the two homopolymer films (PTT-OMe and PEDTM), which indirectly confirms the successful combination of the two monomers.
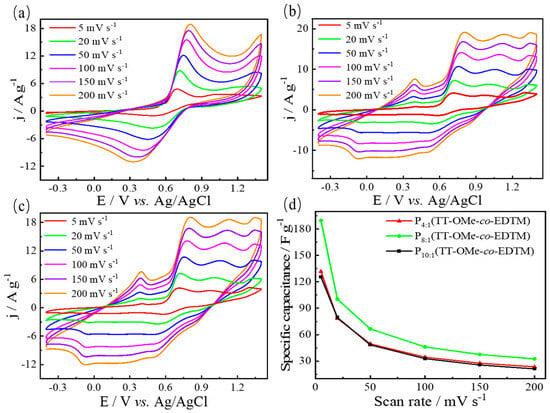
Figure 6.
CV curves of 0.05 M (a) P10:1(TT−OMe-co-EDTM), (b) P8:1(TT−OMe-co-EDTM), (c) and P4:1(TT−OMe-co-EDTM) in ACN−Bu4NPF6 at different scan rates. (d) Plots of specific capacitances versus scan rates of polymers.
Figure 6d reveals that the specific capacitances derived from the CV curves are 125 F/g, 190 F/g, and 132 F/g at a scan rate of 5 mV/s for P10:1(TT-OMe-co-EDTM), P8:1(TT-OMe-co-EDTM), and P4:1(TT-OMe-co-EDTM), respectively, outperforming PTT-OMe (104 F/g) and PEDTM (119 F/g), as indicated in Figure S3c. The suboptimal capacitive performance of the PTT-OMe thin film electrodes can be ascribed to their overly compact surface microstructure, which leads to a reduced electrochemically active surface area and a diminished rate of electrochemical reactions. The introduction of the EDTM monomer into the TT-OMe monomer, leveraging the porous microstructure of PEDTM (Figure 3a), fosters effective synergy between EDTM and TT-OMe, resulting in an increased electrochemically active surface area and an enhanced specific capacitance. Through a series of experiments exploring various monomer copolymerization ratios, the polymer films exhibited the most favorable electrochemical energy storage properties when the molar ratio of TT-OMe to EDTM monomers was 8:1. Moreover, the capacitive performance of the copolymer films prepared via an electrochemical copolymerization strategy significantly surpasses that of most previously reported bifunctional electrode materials (Table S2 [14,15,16,42,49,50,51,52,53,54,55]), highlighting the prominent advantages of these materials in the field of supercapacitor technology. Electrochemical stability can be measured by calculating the capacitance retention from a voltammogram of the first versus the last cycle. The electrochemical stability of PTT-OMe, PEDTM, and P(TT-OMe-co-EDTM) was calculated using repetitive CV data for 3000 cycles at 200 mV/s (Figure S4). The stability of PTT-OMe, PEDTM, and P(TT-OMe-co-EDTM) was 7.78%, 91.25%, and 39.15% after 500 cycles, respectively. In the future, we will choose specific dopants, improve the polymer preparation process, or design specific molecular structures to enhance the stability of conducting polymers.
The galvanostatic charge–discharge (GCD) curves of the copolymers with different monomer ratios measured at different current densities are shown in Figure 7a–c. A nonlinear voltage–time curve shows the typical pseudocapacitance contribution of a polymer [56]. It can be found that the GCD curve changes drastically near 0.6–0.7 V, which indicates that the specific capacitance performance is mainly pseudocapacitive and is consistent with the CV curves [57]. Capacitance variation versus the current density is shown in Figure 7d. Increasing the current density will decrease the specific capacitance due to an insufficient redox reaction. According to the calculation formula of specific capacitance, the calculated specific capacitances are 65 F/g, 86 F/g, 102 F/g, and 91 F/g at 4 A/g for PTT-OMe, P10:1(TT-OMe-co-EDTM), P8:1(TT-OMe-co-EDTM), and P4:1(TT-OMe-co-EDTM), respectively. Specifically, as the current density increases, there is little interaction between the active sites on the electrode and the electrolyte ions [18]. Therefore, at high current densities, this ion diffusion limitation leads to a decrease in specific capacitance [18]. For the PTT-OMe electrode (Figure S5a), the charging and discharging times are very short when the current density is high, which indicates its poor capacitive performance. When the current density was increased from 4 to 15 A/g, the copolymer film electrode exhibited a better rate capability compared to that of the PTT-OMe electrode, with a capacitance retention of 27%. An increase in specific capacitance and an enhanced rate capability can be attributed to the introduction of porous PEDTM units in the PTT-OMe backbone, and the effective synergy of the two monomers ensures a higher specific surface area and efficient charge transportation.
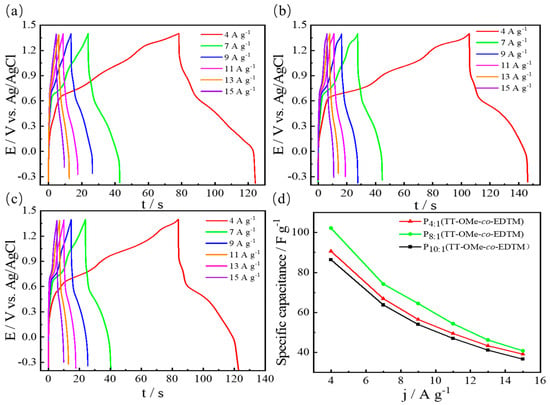
Figure 7.
CD curves of 0.05 M (a) P10:1(TT−OMe-co-EDTM), (b) P8:1(TT−OMe-co-EDTM), and (c) P4:1(TT−OMe-co-EDTM) in ACN−Bu4NPF6 at different current densities. (d) Plots of specific capacitances versus current densities of polymers.
The absorption spectra of the five kinds of conducting polymer film are shown in Figure 8 and Figure S6. The PTT-OMe film displays light yellow coloration (indicative of a reduced state) at low potentials on the indium tin oxide (ITO) substrate. With the elevation of the potential, the film undergoes a transition to a purple hue (corresponding to an oxidized state). The maximum absorption peaks shift towards the blue region with an increasing EDTM content, with the peaks situated at 420 nm for PTT-OMe, 409 nm for P10:1(TT-OMe-co-EDTM), 404 nm for P8:1(TT-OMe-co-EDTM), 402 nm for P4:1(TT-OMe-co-EDTM), and 360 nm for PEDTM. The observed shift in the absorption peaks also indirectly confirms the transformation of the intrinsic π-conjugated structure of the conducting polymer during the electrochemical copolymerization process. As the applied voltage progresses (from −0.4 V to 0.3 V to 1.4 V), the ultraviolet absorption peaks attenuate, while new absorption bands emerge in the near-infrared spectrum, indicative of the formation of bipolar electrons and polarizers [49,58]. These copolymer films display pronounced color variations and rapid, reversible color transitions across a broad voltage spectrum. The observed color-shifting behavior in these polymer films promises to accelerate the development of electrochromic supercapacitors, which are useful in diverse applications, such as wearable electronics, energy-efficient windows, and smart sensors for building integration.
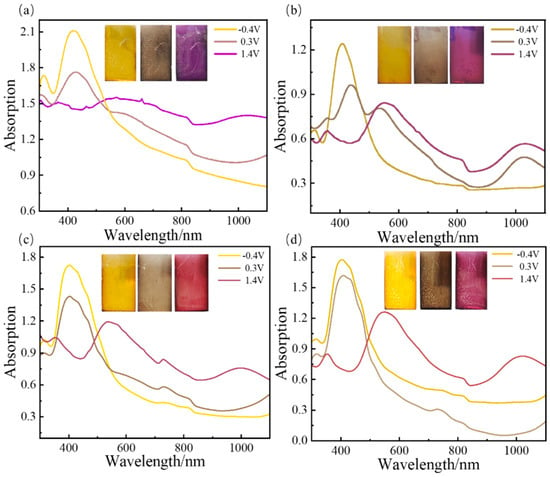
Figure 8.
Absorption spectra of (a)TT−OMe, (b) P10:1(TT−OMe-co-EDTM), (c) P8:1(TT−OMe-co-EDTM), and (d) P4:1(TT−OMe-co-EDTM) films under different potentials in ACN−Bu4NPF6 electrolyte.
The energy storage capacity of polymer-based electrochromic supercapacitor devices was assessed, as shown in Figure 9 and Figure S7. The GCD curves of PTT-OMe exhibit its poor electrochemical performance, but there is a clear color change in the process. It can be seen that the working electrode coated on the ITO surface is light yellow in the charging state and purple in the discharging state. With the addition of EDTM, the charging and discharging performances of the copolymer film are improved, which is attributed to increased electronic exchange [59]. This visually recognizable and reversible color change reflects the states of charge and discharge. Moreover, the gradual return to the initial state after charged/discharged indicates good cycle reversibility. The above results indicate that P(TT-OMe-co-EDTM) is a promising bifunctional electrode material for electrochromic energy storage.
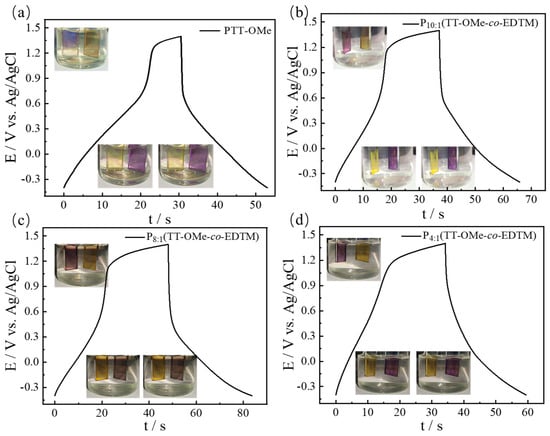
Figure 9.
GCD curves and corresponding color change in (a) PTT−OMe, (b) P10:1(TT−OMe-co-EDTM), (c) P8:1(TT−OMe-co-EDTM), and (d) P4:1(TT−OMe-co-EDTM) electrodes based on dual function electrochromic-supercapacitor device.
4. Conclusions
In this work, a novel conducting polymer of P(TT-OMe-co-EDTM) was synthesized via the one-step electrochemical copolymerization of TT-OMe and EDTM monomers. By optimizing the ratio of TT-OMe to EDTM, copolymer film with a uniform microporous morphology can be fabricated, and the resulting free-standing film exhibits robust mechanical flexibility. Such a P(TT-OMe-co-EDTM) film combines the advantageous properties of the two types of conductive polymer materials, endowing it with both an excellent electrochromic performance and electrochemical energy storage capabilities. These copolymer film electrodes displayed enhanced specific capacitance (190 F/g at 5 mV/s) compared to those of the individual PTT-OMe (104 F/g) and PEDTM (119 F/g) electrodes. Furthermore, they demonstrated an excellent electrochromic performance, undergoing significant reversible color changes within a voltage range of −0.4 V to 1.4 V. The P(TT-OMe-co-EDTM) polymer films displayed three distinct color changes at different voltages. These reversible color transitions provide visual feedback on energy storage level changes during the charging and discharging processes. This study presents a straightforward and effective approach for fabricating bifunctional conducting polymer electrodes, with promising prospects for future applications in electrochromic energy storage devices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym16162313/s1, Figure S1. CVs of 0.05 M EDTM in ACN-Bu4NPF6 at 50 mV s−1. Figure S2. SEM images of PTT-OMe coated ITO glass. Figure S3. CV curves of 0.05 M (a) PEDTM, (b) PTT-OMe in ACN-Bu4NPF6 at different scan rates, and (c) Plots of specific capacitances versus scan rates of polymers. Figure S4. Cycle stability of PTT-OMe, PEDTM and P8:1(TT-OMe-co-EDTM) electrodes. Figure S5. Charging-discharging of 0.05 M (a) PTT-OMe (b) PEDTM in ACN-Bu4NPF6 at different current densities. (c) Plots of specific capacitances versus current densities of polymers. Figure S6. UV-Vis-NIR spectroscopy, absorption spectra of PEDTM. Figure S7. Charging-discharging curves and corresponding color change of PEDTM based electrochromic-capacitance device. Table S1. EDS analyse of P8:1(TT-OMe-co-EDTM) copolymer film. Table S2. Comparison of the specific capacitance and color change of the materials in this study with other reported similar materials of conducting polymers.
Author Contributions
Software, H.T.; Resources, J.Y.; Data curation, Z.Y.; Writing—original draft, Z.Y.; Writing—review & editing, R.W. and D.Z.; Visualization, Z.Y.; Supervision, D.Z. and J.Y.; Project administration, D.Z.; Funding acquisition, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Science Foundation of China (U21A20492 and 62275041) and the Sichuan Science and Technology Program (2023ZYZYTS0016, 2022YFH0081, 2022YFG0012, 2022YFG0013 and 2023ZYD0162). This work was also sponsored by the Sichuan Province Key Laboratory of Display Science and Technology and the Qiantang Science & Technology Innovation Center.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Yi, M.; Lu, B.; Zhang, X.; Tan, Y.; Zhu, Z.; Pan, Z.; Zhang, J. Ionic liquid-assisted synthesis of nickel cobalt phosphide embedded in N, P codoped-carbon with hollow and folded structures for efficient hydrogen evolution reaction and supercapacitor. Appl. Catal. B Environ. 2021, 283, 119635. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, S.; Xu, K.; Zhang, Y.; Zhang, L.; Lou, G.; Wu, Y.; Zhu, E.; Chen, H.; Shen, Z.; et al. Sustainable activated carbons from dead ginkgo leaves for supercapacitor electrode active materials. Chem. Eng. Sci. 2018, 181, 36–45. [Google Scholar] [CrossRef]
- Mao, T.; Chen, H.; Li, J.; Liu, F.; Wang, X.; Wang, S. Hydroxypolybenzimidazole Electrolyte with Excellent Stability for High Power Density All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 5163–5172. [Google Scholar] [CrossRef]
- Dong, W.; Xie, M.; Zhao, S.; Qin, Q.; Huang, F. Materials design and preparation for high energy density and high power density electrochemical supercapacitors. Mater. Sci. Eng. R Rep. 2023, 152, 100713. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zhou, W.; Jiang, F.; Zhang, H.; Jiang, Q.; Jia, Y.; Wang, R.; Liang, A.; Xu, J.; et al. Fused Heterocyclic Molecule-Functionalized N-Doped Reduced Graphene Oxide by Non-Covalent Bonds for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 45202–45213. [Google Scholar] [CrossRef]
- Shao, M.; Lv, X.; Zhou, C.; Ouyang, M.; Zhu, X.; Xu, H.; Feng, Z.; Wright, D.S.; Zhang, C. A colorless to multicolored triphenylamine-based polymer for the visualization of high-performance electrochromic supercapacitor. Sol. Energy Mater. Sol. Cells 2023, 251, 112134. [Google Scholar] [CrossRef]
- Yun, T.G.; Kim, D.; Kim, Y.H.; Park, M.; Hyun, S.; Han, S.M. Photoresponsive Smart Coloration Electrochromic Supercapacitor. Adv. Mater. 2017, 29, 1606728. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, J.; Zhang, L.; Ouyang, M.; Tameev, A.; Nekrasov, A.; Kim, G.; Zhang, C. High-performance electrochromic supercapacitor based on quinacridone dye with good specific capacitance, fast switching time and robust stability. Chem. Eng. J. 2022, 431, 133733. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Chen, Z.; Cong, S.; Zhao, Z. Fabry–Perot Cavity-Type Electrochromic Supercapacitors with Exceptionally Versatile Color Tunability. Nano Lett. 2020, 20, 1915–1922. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Wang, J.; Chen, J.; Magdassi, S.; Lee, P.S. Highly Stable Transparent Conductive Silver Grid/PEDOT:PSS Electrodes for Integrated Bifunctional Flexible Electrochromic Supercapacitors. Adv. Energy Mater. 2016, 6, 1501882. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, L.; Yang, X.; Luo, X.; Bi, P.; Fu, Z.; Pang, A.; Li, W.; Yi, Y. Revealing the Charge Storage Mechanism of Nickel Oxide Electrochromic Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 39098–39107. [Google Scholar] [CrossRef]
- Xue, J.; Xu, H.; Wang, S.; Hao, T.; Yang, Y.; Zhang, X.; Song, Y.; Li, Y.; Zhao, J. Design and synthesis of 2D rGO/NiO heterostructure composites for high-performance electrochromic energy storage. Appl. Surf. Sci. 2021, 565, 150512. [Google Scholar] [CrossRef]
- Mohanadas, D.; Sulaiman, Y. Recent advances in development of electroactive composite materials for electrochromic and supercapacitor applications. J. Power Sources 2022, 523, 231029. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Q.; Hao, Z.; Tang, Y.; Wang, H.; Liu, J.; Yan, H. Integrated electrochromic supercapacitors with visual energy levels boosted by coating onto carbon nanotube conductive networks. Sol. Energy Mater. Sol. Cells 2020, 206, 110330. [Google Scholar] [CrossRef]
- Li, H.; Cao, J.; Liu, F.; Zhou, W.; Chen, X.; Deng, Y.; Wu, Z.; Lu, B.; Mo, D.; Xu, J.; et al. Stable Three-Dimensional PEDOT Network Construction for Electrochromic-Supercapacitor Dual Functional Application. ACS Appl. Energy Mater. 2022, 5, 12315–12323. [Google Scholar] [CrossRef]
- Feng, T.; Liu, L.; Mao, S.; Xue, H.; Zhao, J.; Bai, Y.; Zhao, W. Polyoxometalate/poly(3,4-ethylenedioxythiophene) nanocomposites enabling visualization of energy storage status in multicolor electrochromic supercapacitors. Appl. Surf. Sci. 2023, 641, 158450. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yun, T.Y.; Yu, K.S.; Moon, H.C. Reliable, High-Performance Electrochromic Supercapacitors Based on Metal-Doped Nickel Oxide. ACS Appl. Mater. Interfaces 2020, 12, 51978–51986. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, X.; Li, Z.; Wang, D.; Nie, G. A novel solid-state electrochromic supercapacitor with high energy storage capacity and cycle stability based on poly(5-formylindole)/WO3 honeycombed porous nanocomposites. Chem. Eng. J. 2020, 384, 123370. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Le, Q.V.; Peng, S.; Dai, Z.; Ahn, S.H.; Kim, S.Y. Exploring Conducting Polymers as a Promising Alternative for Electrochromic Devices. Adv. Mater. Technol. 2023, 8, 2300474. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 12, 1978–1987. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; You, J.; Park, M.-S.; Hossain, M.S.A.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: Recent progress and new functions. Mater. Horiz. 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Camurlu, P.; Gültekin, C.; Gürbulak, V. Optoelectronic Properties and Electrochromic Device Application of Novel Pyrazole Based Conducting Polymers. J. Macromol. Sci. Part A 2013, 50, 588–595. [Google Scholar] [CrossRef]
- Li, M.; Jiang, F.; Yang, J.; Wang, Y.; Zhao, F.; Xu, X.; Liu, M.; Yan, J.; Xu, J. Electrochemical Preparation and Regulation of Flexible Polypyrrole Film toward Enhanced Thermoelectric Performance. ACS Appl. Energy Mater. 2021, 4, 12982–12988. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Yen, S.-C.; Hsu, C.-S.; Wang, W.-T.; Yougbaré, S.; Lin, L.-Y.; Wu, Y.-F. Novel synthesis of polyaniline, manganese oxide and nickel sulfide lavandula-like composites as efficient active material of supercapacitor. J. Energy Storage 2023, 66, 107390. [Google Scholar] [CrossRef]
- Yang, X.; Liu, A.; Zhao, Y.; Lu, H.; Zhang, Y.; Wei, W.; Li, Y.; Liu, S. Three-Dimensional Macroporous Polypyrrole-Derived Graphene Electrode Prepared by the Hydrogen Bubble Dynamic Template for Supercapacitors and Metal-Free Catalysts. ACS Appl. Mater. Interfaces 2015, 7, 23731–23740. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xu, J. Progress in Conjugated Polyindoles: Synthesis, Polymerization Mechanisms, Properties, and Applications. Polym. Rev. 2017, 57, 248–275. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, D.; Ma, X.; Xu, J.; Zhou, W.; Zhao, F. High-performance capacitive behavior of layered reduced graphene oxide and polyindole nanocomposite materials. RSC Adv. 2016, 6, 29840–29847. [Google Scholar] [CrossRef]
- Hsiao, A.-E.; Tuan, C.-S.; Lu, L.-H.; Liao, W.-S.; Teng, W.-J. Preparation of poly (hydroxymethyl EDOT)/nano-silver composite films by oxidative polymerization with low-baking temperature, low resistance and good adhesion on PET substrate. Synth. Met. 2010, 160, 2319–2322. [Google Scholar] [CrossRef]
- da Silva, A.C.; Augusto, T.; Andrade, L.H.; Córdoba de Torresi, S.I. One pot biocatalytic synthesis of a biodegradable electroactive macromonomer based on 3,4-ethylenedioxytiophene and poly(l-lactic acid). Mater. Sci. Eng. C 2018, 83, 35–43. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef]
- Liu, J.; Tang, D.; Hou, W.; Ding, D.; Yao, S.; Liu, Y.; Chen, Y.; Chi, W.; Zhang, Z.; Ouyang, M.; et al. Conductive polymer electrode materials with excellent mechanical and electrochemical properties for flexible supercapacitor. J. Energy Storage 2023, 74, 109329. [Google Scholar] [CrossRef]
- Turbiez, M.; Hergué, N.; Leriche, P.; Frère, P. Rigid oligomers based on the combination of 3,6-dimethoxythieno[3,2-b]thiophene and 3,4-ethylenedioxythiophene. Tetrahedron Lett. 2009, 50, 7148–7151. [Google Scholar] [CrossRef]
- Alunni, S.; Linda, P.; Marino, G.; Santini, S.; Savelli, G. The mechanism of the Vilsmeier–Haack reaction. Part II. A kinetic study of the formylation of thiophen derivatives with dimethylformamide and phosphorus oxychloride or carbonyl chloride in 1,2-dichloroethane. J. Chem. Soc. Perkin Trans. 1972, 2, 2070–2073. [Google Scholar] [CrossRef]
- Matharu, A.; Huddleston, P.; Jeeva, S.; Wood, M.; Chambers-Asman, D. An efficient direct method for the azo-coupling of methoxythiophenes. Dye. Pigment. 2008, 78, 89–92. [Google Scholar] [CrossRef]
- Wang, R.; Lin, K.; Jiang, F.; Zhou, W.; Wang, Z.; Wu, Y.; Ding, Y.; Hou, J.; Nie, G.; Xu, J.; et al. Fluoro-substituted conjugated polyindole for desirable electrochemical charge storage materials. Electrochim. Acta 2019, 320, 134641. [Google Scholar] [CrossRef]
- Guo, Y.; Li, W.; Yu, H.; Perepichka, D.F.; Meng, H. Flexible Asymmetric Supercapacitors via Spray Coating of a New Electrochromic Donor–Acceptor Polymer. Adv. Energy Mater. 2017, 7, 1601623. [Google Scholar] [CrossRef]
- Song, R.Y.; Park, J.H.; Sivakkumar, S.R.; Kim, S.H.; Ko, J.M.; Park, D.-Y.; Jo, S.M.; Kim, D.Y. Supercapacitive properties of polyaniline/Nafion/hydrous RuO2 composite electrodes. J. Power Sources 2007, 166, 297–301. [Google Scholar] [CrossRef]
- Antiohos, D.; Folkes, G.; Sherrell, P.; Ashraf, S.; Wallace, G.G.; Aitchison, P.; Harris, A.T.; Chen, J.; Minett, A.I. Compositional effects of PEDOT-PSS/single walled carbon nanotube films on supercapacitor device performance. J. Mater. Chem. 2011, 21, 15987–15994. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, J. High-operating-voltage all-solid-state symmetrical supercapacitors based on poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) films treated by organic solvents. Electrochim. Acta 2016, 222, 1895–1902. [Google Scholar] [CrossRef]
- Nie, G.; Bai, Z.; Yu, W.; Chen, J. Electrochemiluminescence Biosensor Based on Conducting Poly(5-formylindole) for Sensitive Detection of Ramos Cells. Biomacromolecules 2013, 14, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, J.; Gao, L.; Yu, J. One-step electropolymerized thieno[3,2-b]thiophene-based bifunctional electrode with controlled color conversion for electrochromic energy storage application. Chem. Eng. J. 2022, 445, 136731. [Google Scholar] [CrossRef]
- Visy, C.; Lukkari, J.; Kankare, J. Study of the role of the deprotonation step in the electrochemical polymerization of thiophene-type monomers. Synth. Met. 1994, 66, 61–65. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Liu, T.-A.; Prabhakar, C.; Yu, J.-Y.; Chen, C.-H.; Huang, H.-H.; Yang, J.-S. Star-Shaped Oligothiophenes Containing an Isotruxene Core: Synthesis, Electronic Properties, Electropolymerization, and Film Morphology. Macromolecules 2012, 45, 4529–4539. [Google Scholar] [CrossRef]
- Khumujam, D.D.; Kshetri, T.; Singh, T.I.; Kim, N.H.; Lee, J.H. Fibrous asymmetric supercapacitor based on wet spun MXene/PAN Fiber-derived multichannel porous MXene/CF negatrode and NiCo2S4 electrodeposited MXene/CF positrode. Chem. Eng. J. 2022, 449, 137732. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Zhao, Y.; Kang, J.; Chen, J.; Yan, B.; Gu, Y.; Yang, F.; Cao, Y. All-Solid-State Electrochromic Device Based on Nanocellulose/PANI/PEDOT Ternary Hybrid System for High Optical Contrast and Excellent Cycling Stability. J. Electrochem. Soc. 2019, 166, H77. [Google Scholar] [CrossRef]
- Lee, H.; Kumbhar, V.S.; Lee, J.; Choi, Y.; Lee, K. Highly reversible crystal transformation of anodized porous V2O5 nanostructures for wide potential window high-performance supercapacitors. Electrochim. Acta 2020, 334, 135618. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color Control in π-Conjugated Organic Polymers for Use in Electrochromic Devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, S.; Bansal, L.; Ghanghass, A.; Ghosh, T.; Rani, C.; Sahu, B.; Rath, D.K.; Bhatia, R.; Sameera, I.; Kumar, R. Bifunctional solid state electrochromic device using WO3/WS2 nanoflakes for charge storage and dual-band color modulation. J. Mater. Chem. C 2023, 11, 12590–12598. [Google Scholar] [CrossRef]
- Yao, W.; Liu, P.; Liu, C.; Xu, J.; Lin, K.; Kang, H.; Li, M.; Lan, X.; Jiang, F. Flexible conjugated polyfurans for bifunctional electrochromic energy storage application. Chem. Eng. J. 2022, 428, 131125. [Google Scholar] [CrossRef]
- Du, C.; Li, H.; Zhang, G.; Wan, R.; Zhang, W.; Xu, X.; Zheng, L.; Deng, X.; Xu, J.; Lu, B.; et al. Design of robust fluorinated interpenetrating poly(thieno[3,2-b]thiophene) network for highly stable flexible electrochromic-supercapacitor devices. Chem. Eng. J. 2024, 495, 153692. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, X.; Yang, M.; Qi, Y. Investigation of a Branchlike MoO3/Polypyrrole Hybrid with Enhanced Electrochemical Performance Used as an Electrode in Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 1125–1130. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Chen, X.; Du, X. Electrochemical supercapacitor electrode material based on poly(3,4-ethylenedioxythiophene)/polypyrrole composite. J. Power Sources 2007, 163, 1120–1125. [Google Scholar] [CrossRef]
- Song, H.-K.; Lee, E.J.; Oh, S.M. Electrochromism of 2,2‘-Azinobis(3-ethylbenzothiazoline-6-sulfonate) Incorporated into Conducting Polymer as a Dopant. Chem. Mater. 2005, 17, 2232–2233. [Google Scholar] [CrossRef]
- Helseth, L.E. The nonlinearities in the galvanostatic charging curves of supercapacitors provide insights into charging mechanisms. J. Energy Storage 2022, 55, 105440. [Google Scholar] [CrossRef]
- Li, T.; Zhu, W.; Shen, R.; Wang, H.-Y.; Chen, W.; Hao, S.-J.; Li, Y.; Gu, Z.-G.; Li, Z. Three-dimensional conductive porous organic polymers based on tetrahedral polythiophene for high-performance supercapacitors. New J. Chem. 2018, 42, 6247–6255. [Google Scholar] [CrossRef]
- Collier, G.S.; Pelse, I.; Österholm, A.M.; Reynolds, J.R. Electrochromic Polymers Processed from Environmentally Benign Solvents. Chem. Mater. 2018, 30, 5161–5168. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Wang, C.; Wang, Y.; Shen, Y. Electropolymerization and properties of 3,4-ethylenedioxythiophene backbone polymer with tetrathiafulvalene as pendant. J. Appl. Polym. Sci. 2013, 127, 3356–3364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).