Safety and Efficacy of Photocatalytic Micro-Mist Desktop Humidifier for Dry Eye Caused by Digital Environment: A Randomized Controlled Trial
Abstract
1. Introduction
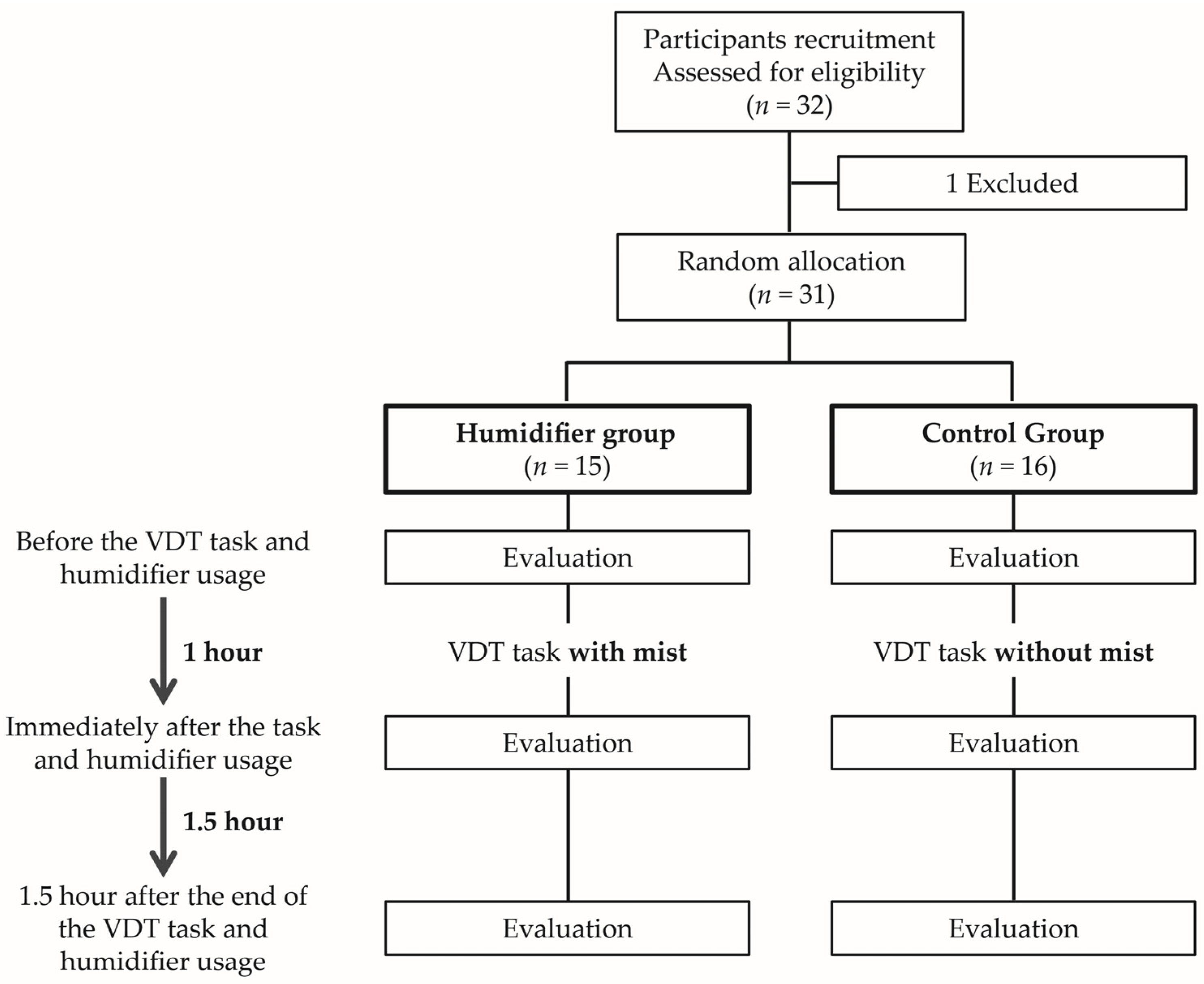
2. Methods
2.1. Participants
2.2. Features of Humidifier
2.3. Examinations
2.4. Ocular Symptoms
2.5. Ocular Surface Parameters
2.6. Adverse Events
2.7. Measurement of Temperature and Relative Humidity around Humidifiers
2.8. Statistical Analysis
3. Results
3.1. Demographic Data of Participants
3.2. Ocular Symptoms
3.3. Ocular Surface Parameters
3.4. Temperature and Relative Humidity around Humidifiers
3.5. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolffsohn, J.S.; Lingham, G.; Downie, L.E.; Huntjens, B.; Inomata, T.; Jivraj, S.; Kobia-Acquah, E.; Muntz, A.; Mohamed-Noriega, K.; Plainis, S.; et al. TFOS Lifestyle: Impact of the digital environment on the ocular surface. Ocul. Surf. 2023, 28, 213–252. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Alves, M.; Wolffsohn, J.S.; Downie, L.E.; Efron, N.; Galor, A.; Gomes, J.A.P.; Jones, L.; Markoulli, M.; Stapleton, F.; et al. TFOS Lifestyle Report Executive Summary: A Lifestyle Epidemic—Ocular Surface Disease. Ocul. Surf. 2023, 30, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Information and Communications in Japan 2021. Available online: https://www.soumu.go.jp/johotsusintokei/whitepaper/eng/WP2021/2021-index.html (accessed on 26 April 2024).
- Fjaervoll, K.; Fjaervoll, H.; Magno, M.; Noland, S.T.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Review on the possible pathophysiological mechanisms underlying visual display terminal-associated dry eye disease. Acta Ophthalmol. 2022, 100, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, E.; Aronowicz, J.D.; Butovich, I.A.; McCulley, J.P. Increased evaporative rates in laboratory testing conditions simulating airplane cabin relative humidity: An important factor for dry eye syndrome. Eye Contact Lens 2007, 33, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Greiner, J.V.; Glonek, T.; Esbah, R.; Finnemore, V.M.; Whalen, A.C. Effect of periocular humidity on the tear film lipid layer. Cornea 1996, 15, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Chan, E.; Ea, L.; Kam, C.; Lu, Y.; Misra, S.L.; Craig, J.P. Randomized Trial of Desktop Humidifier for Dry Eye Relief in Computer Users. Optom. Vis.Sci. Off. Publ. Am. Acad. Optom. 2017, 94, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.L.C.; Wada, S.; Somei, J.; Ochiai, H.; Murakami, T.; Saito, N.; Ogawa, T.; Shinjo, A.; Benno, Y.; Nakagawa, Y.; et al. SARS-CoV-2 Disinfection of Air and Surface Contamination by TiO2 Photocatalyst-Mediated Damage to Viral Morphology, RNA, and Protein. Virus 2021, 13, 942. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.K.A.; Matsumoto, Y.; Fukushima, T.; Fujimoto, K.; Ochiai, H.; Somei, J.; Aida, Y. Rutile-TiO2/PtO2 Glass Coatings Disinfects Aquatic Legionella pneumophila via Morphology Change and Endotoxin Degradation under LED Irradiation. Catalysts 2022, 12, 856. [Google Scholar] [CrossRef]
- Ngo, W.; Situ, P.; Keir, N.; Korb, D.; Blackie, C.; Simpson, T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013, 32, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Vigo, L.; Pellegrini, M.; Bernabei, F.; Carones, F.; Scorcia, V.; Giannaccare, G. Diagnostic Performance of a Novel Noninvasive Workup in the Setting of Dry Eye Disease. J. Ophthalmol. 2020, 2020, 5804123. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Srivastav, S.; Modiwala, Z.; Ali, M.H.; Basu, S. Repeatability, reproducibility and agreement between three different diagnostic imaging platforms for tear film evaluation of normal and dry eye disease. Eye 2023, 37, 2042–2047. [Google Scholar] [CrossRef]
- McMonnies, C.W. Incomplete blinking: Exposure keratopathy, lid wiper epitheliopathy, dry eye, refractive surgery, and dry contact lenses. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2007, 30, 37–51. [Google Scholar] [CrossRef]
- van Bijsterveld, O.P. Diagnostic tests in the Sicca syndrome. Arch. Ophthalmol. 1969, 82, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Chlasta-Twardzik, E.; Gorecka-Niton, A.; Nowinska, A.; Wylegala, E. The Influence of Work Environment Factors on the OcularSurface in a One-Year Follow-Up Prospective Clinical Study. Diagnostics 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Arita, R. Tear film lipid layer increase after diquafosol instillation in dry eye patients with meibomian gland dysfunction: A randomized clinical study. Sci Rep. 2019, 9, 9091. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, M.; Kort, H.; Murphy, P.; Evans, K. The influence of dry eye and office environment on visual functioning. Stud. Health Technol. Inform. 2015, 217, 427–431. [Google Scholar] [PubMed]
- Wolkoff, P. External eye symptoms in indoor environments. Indoor Air 2017, 27, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Uchino, Y.; Dogru, M.; Kawashima, M.; Yokoi, N.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Schaumberg, D.A.; et al. Dry eye disease and work productivity loss in visual display users: The Osaka study. Am. J. Ophthalmol. 2014, 157, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Murat, D.; Liu, Y.; Kojima, T.; Kawakita, T.; Tsubota, K. Efficacy of a novel moist cool air device in office workers with dry eye disease. Acta Ophthalmol. 2013, 91, 756–762. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All (n = 31) | Humidifier Group (n = 15) | Control Group (n = 16) | p Value |
|---|---|---|---|---|
| Age, mean ± SD (range) (years) | 41.1 ± 8.8 (21–62) | 41.7 ± 9.2 (25–62) | 40.5 ± 8.7 (21–60) | 0.86 |
| Sex (male/female) | 5/26 | 2/13 | 3/13 | 1.0 |
| History of dry eye (n (%)) | 14 (45%) | 6 (40%) | 8 (50%) | 0.72 |
| Contact lens wear (n (%)) | 4 (13%) | 3 (20%) | 1 (6%) | 0.33 |
| Allergic conjunctivitis (n (%)) | 2 (6%) | 2 (13%) | 0 (0%) | 0.23 |
| Seasonal allergy (n (%)) | 12 (39%) | 8 (53%) | 4 (25%) | 0.15 |
| Symptom | Humidifier Group (n = 15) | p Value vs. Before | Control Group (n =16) | p Value vs. Before | p Value for Humidifier vs. Control | |
|---|---|---|---|---|---|---|
| SPEED (0–28) | Before | 8.3 ± 4.6 | 9.3 ± 5.0 | 0.61 | ||
| Immediately after | 2.0 ± 2.6 | <0.001 ** | 7.1 ± 4.3 | 0.059 | <0.001 †† | |
| 1.5 h after | 6.0 ± 4.5 | 0.006 * | 8.3 ± 5.2 | 0.71 | 0.22 | |
| VAS score (0–100) | ||||||
| Dryness | Before | 41.5 ± 27.6 | 55.0 ± 29.0 | 0.17 | ||
| Immediately after | 9.9 ± 14.2 | 0.002 * | 49.4 ± 34.2 | 1.0 | 0.001 † | |
| 1.5 h after | 32.3 ± 24.6 | 0.47 | 58.4 ± 26.6 | 0.51 | 0.012 † | |
| Eye strain | Before | 49.9 ± 23.9 | 58.9 ± 29.2 | 0.28 | ||
| Immediately after | 14.7 ± 16.6 | <0.001 ** | 51.4 ± 34.6 | 1.0 | 0.003 † | |
| 1.5 h after | 28.4 ± 24.4 | 0.004 * | 58.6 ± 28.2 | 1.0 | 0.007 † | |
| Discomfort | Before | 32.0 ± 23.3 | 44.4 ± 29.5 | 0.25 | ||
| Immediately after | 7.7 ± 15.9 | 0.002 * | 39.1 ± 35.2 | 0.88 | 0.002 † | |
| 1.5 h after | 13.1 ± 20.2 | 0.035 * | 43.5 ± 30.6 | 1.0 | 0.005 † | |
| Blurred vision | Before | 38.5 ± 24.1 | 38.6 ± 33.8 | 0.87 | ||
| Immediately after | 9.7 ± 17.0 | <0.001 ** | 29.3 ± 28.1 | 0.25 | 0.029 † | |
| 1.5 h after | 15.9 ± 18.4 | <0.001 ** | 32.6 ± 31.7 | 0.070 | 0.23 | |
| Foreign body sensation | Before | 28.9 ± 26.6 | 37.3 ± 28.4 | 0.47 | ||
| Immediately after | 3.3 ± 6.2 | 0.002 * | 34.5 ± 29.6 | 1.0 | <0.001 †† | |
| 1.5 h after | 4.5 ± 9.4 | 0.002 * | 34.5 ± 34.0 | 1.0 | 0.004 † | |
| Eye pain | Before | 17.3 ± 19.5 | 21.4 ± 17.8 | 0.51 | ||
| Immediately after | 2.3 ± 5.0 | 0.004 * | 18.8 ± 17.7 | 1.0 | 0.005 † | |
| 1.5 h after | 6.1 ± 12.9 | 0.098 | 23.8 ± 25.3 | 1.0 | 0.032 † | |
| Difficulty opening eyelids | Before | 21.4 ± 30.9 | 21.6 ± 20.8 | 0.60 | ||
| Immediately after | 3.7 ± 5.5 | 0.016 * | 24.4 ± 27.6 | 1.0 | 0.031 † | |
| 1.5 h after | 12.8 ± 19.4 | 0.59 | 32.3 ± 32.2 | 0.082 | 0.074 |
| Characteristic | Humidifier Group (n = 15) | p Value vs. Before | Control Group (n = 16) | p Value vs. Before | p Value for Humidifier vs. Control | |
|---|---|---|---|---|---|---|
| LLT (nm) | Before | 68.5 ± 10.9 | 75.5 ± 21.2 | 0.50 | ||
| Immediately after | 79.2 ± 13.0 | 0.012 * | 66.6 ± 16.6 | 0.029 * | 0.05 | |
| 1.5 h after | 67.9 ± 8.5 | 1.0 | 59.4 ± 15.2 | 0.013 * | 0.034 † | |
| Blink frequency (/min) | Before | 32.2 ± 18.9 | 39.0 ± 26.7 | 0.74 | ||
| Immediately after | 24.9 ± 9.9 | 0.17 | 28.9 ± 22.3 | 0.15 | 0.97 | |
| 1.5 h after | 27.9 ± 14.6 | 1.0 | 24.1 ± 10.8 | 0.17 | 0.43 | |
| IBR | Before | 0.3 ± 0.3 | 0.4 ± 0.3 | 0.19 | ||
| Immediately after | 0.4 ± 0.3 | 0.83 | 0.2 ± 0.2 | 0.31 | 0.078 | |
| 1.5 h after | 0.4 ± 0.4 | 0.59 | 0.4 ± 0.4 | 1.0 | 0.89 | |
| TMH (mm) | Before | 0.24 ± 0.09 | 0.26 ± 0.08 | 0.42 | ||
| Immediately after | 0.32 ± 0.11 | 0.001 * | 0.20 ± 0.06 | 0.007 * | <0.001 †† | |
| 1.5 h after | 0.24 ± 0.06 | 1.0 | 0.21 ± 0.05 | 0.13 | 0.43 | |
| NIBUT first (s) | Before | 6.28 ± 2.24 | 7.36 ± 2.67 | 0.24 | ||
| Immediately after | 9.65 ± 2.55 | 0.005 * | 6.20 ± 2.45 | 0.15 | 0.001 † | |
| 1.5 h after | 8.13 ± 3.10 | 0.27 | 5.41 ± 1.91 | 0.042 * | 0.025 † | |
| NIBUT average (s) | Before | 8.72 ± 1.60 | 9.55 ± 2.57 | 0.21 | ||
| Immediately after | 11.02 ± 2.82 | 0.017 * | 8.78 ± 2.19 | 0.49 | 0.040 † | |
| 1.5 h after | 9.61 ± 2.47 | 0.65 | 8.16 ± 2.47 | 0.32 | 0.23 | |
| Number of obstructed meibomian gland orifices (0–8) | Before | 3.0 ± 2.7 | 4.6 ± 2.1 | 0.057 | ||
| Immediately after | 1.2 ± 1.5 | 0.004 * | 4.6 ± 2.1 | 1.0 | <0.001 †† | |
| 1.5 h after | 1.7 ± 1.9 | 0.016 * | 4.6 ± 2.1 | 1.0 | 0.001 † |
| Characteristic | Humidifier Group (n = 15) | Control Group (n = 16) | p Value |
|---|---|---|---|
| FBUT (s) | 6.8 ± 2.4 | 3.2 ± 1.2 | <0.001 ** |
| Corneal fluo score (0–3) | 0.0 ± 0.0 | 0.3 ± 0.7 | 0.18 |
| Temporal conjunctival fluo score (0–3) | 0.4 ± 0.8 | 0.4 ± 0.9 | 1.0 |
| Nasal conjunctival fluo score (0–3) | 0.6 ± 1.0 | 0.4 ± 0.9 | 0.62 |
| Corneal and conjunctival fluo score (0–9) | 1.0 ± 1.7 | 1.1 ± 2.4 | 0.73 |
| Characteristic | Humidifier Group | p Value vs. Before | Control Group | p Value vs. Before | p Value for Humidifier vs. Control | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Before | 24.5 ± 0.6 | 24.4 ± 0.3 | 0.55 | ||
| Immediately after | 22.2 ± 1.1 | 0.002 * | 24.4 ± 0.5 | 1.0 | 0.003 † | |
| 1.5 h after | 25.1 ± 0.3 | 0.080 | 25.0 ± 0.2 | 0.063 | 0.21 | |
| Relative humidity (%) | Before | 47.6 ± 1.9 | 47.0 ± 0.9 | 0.11 | ||
| Immediately after | 70.3 ± 2.6 | 0.002 * | 45.5 ± 2.4 | 0.19 | <0.001 †† | |
| 1.5 h after | 44.5 ± 2.5 | 0.051 | 45.4 ± 1.7 | 0.070 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arita, R.; Fukuoka, S. Safety and Efficacy of Photocatalytic Micro-Mist Desktop Humidifier for Dry Eye Caused by Digital Environment: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3720. https://doi.org/10.3390/jcm13133720
Arita R, Fukuoka S. Safety and Efficacy of Photocatalytic Micro-Mist Desktop Humidifier for Dry Eye Caused by Digital Environment: A Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(13):3720. https://doi.org/10.3390/jcm13133720
Chicago/Turabian StyleArita, Reiko, and Shima Fukuoka. 2024. "Safety and Efficacy of Photocatalytic Micro-Mist Desktop Humidifier for Dry Eye Caused by Digital Environment: A Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 13: 3720. https://doi.org/10.3390/jcm13133720
APA StyleArita, R., & Fukuoka, S. (2024). Safety and Efficacy of Photocatalytic Micro-Mist Desktop Humidifier for Dry Eye Caused by Digital Environment: A Randomized Controlled Trial. Journal of Clinical Medicine, 13(13), 3720. https://doi.org/10.3390/jcm13133720






