A Study of the Impact of Additives on the Physicochemical Properties of Eptifibatide-Loaded Microspheres for Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Microspheres
2.1.1. The Inclusion of Additives in the Drug Phase
2.1.2. The Inclusion of Additives in the Polymer Phase
2.2. Evaluation of Microspheres
2.2.1. Percent Drug Entrapment
Preparation of Sample Solution (Total Drug Content)
Preparation of Test Solution (Free Drug Content)
2.2.2. Particle Size Analysis
2.2.3. In Vitro Drug Release (Accelerated In Vitro Dissolution)
2.2.4. Surface Morphology
2.2.5. Drug Stability
3. Results and Discussion
3.1. Percentage Entrapment Efficiency
3.2. Particle Size Analysis
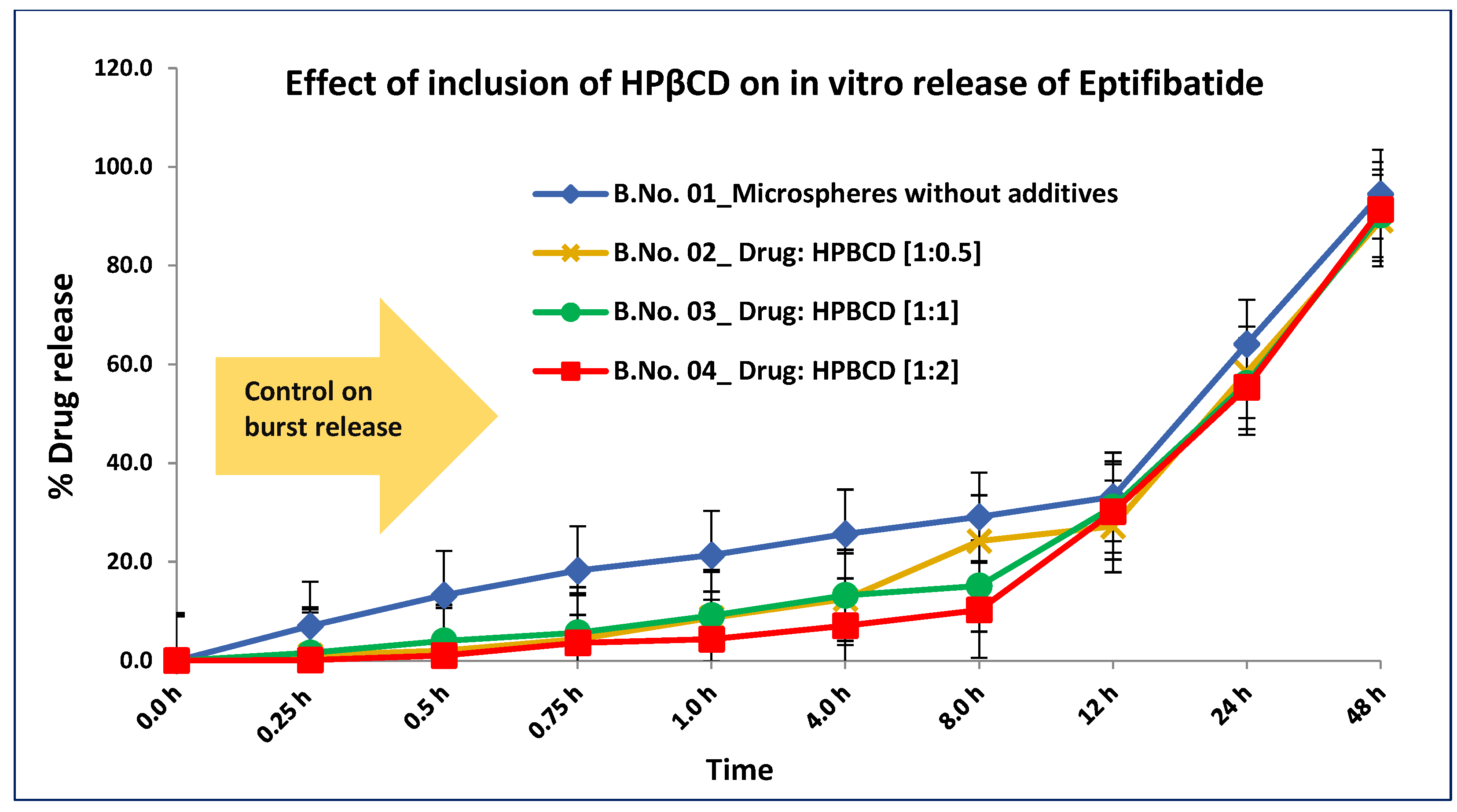
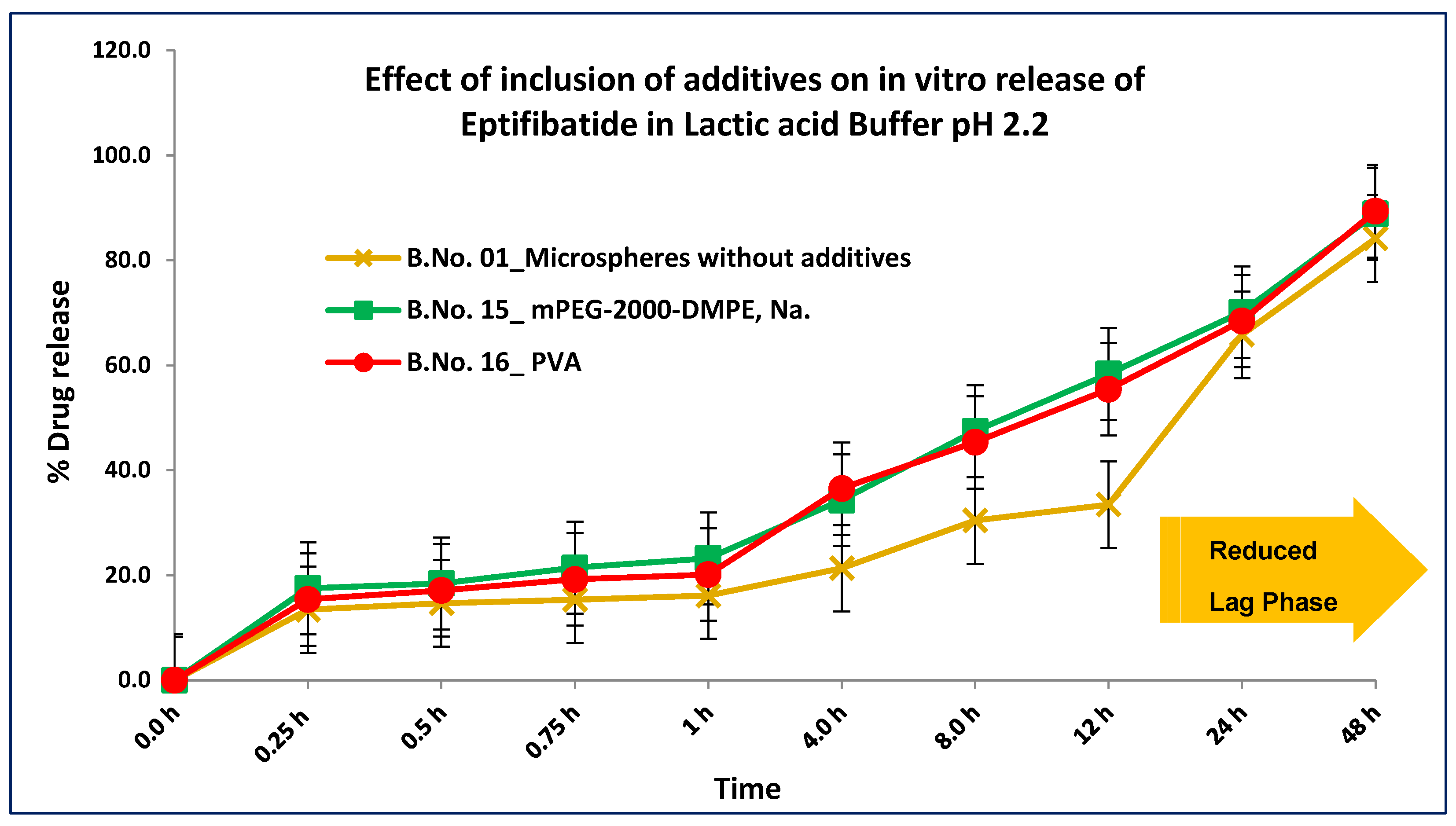
3.3. In Vitro Accelerated Drug Release
3.4. Surface Morphology
3.5. Drug Stability
| Details of Additives | MSP | MSP, Trehalose | Con. Inj, Trehalose | Con. Inj, Trehalose | Con. Inj. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch No. | 1 | 8 | 22 | 22 | 23 | ||||||||
| Stability Storage Conditions | 25 °C and 60% RH | 25 °C and 60% RH | 25 °C and 60% RH | Not applicable | Not applicable | ||||||||
| Stability Time Point | Initial | 1 M | 3 M | Initial | 1 M | 3 M | Initial | 1 M | 3 M | BL | AL | BL | AL |
| Drug Content (%w/w) | 96.3 | 95.3 | 95.1 | 97.4 | 96.8 | 96.4 | 95.1 | 90.1 | 88.6 | 97.2 | 95.1 | 98.3 | 83.1 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Saeed, S.; Irfan, M.; Naz, S.; Liaquat, M.; Jahan, S.; Hayat, S. Routes and barriers associated with protein and peptide drug delivery system. J. Pak. Med. Assoc. 2021, 71, 2032–2039. [Google Scholar] [CrossRef]
- Ibeanu, N.; Egbu, R.; Onyekuru, L.; Javaheri, H.; Khaw, P.T.; Williams, G.R.; Brocchini, S.; Awwad, S. Injectables and Depots to Prolong Drug Action of Proteins and Peptides. Pharmaceutics 2020, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, S.; Yin, Z. Study in the stabilization of proteins encapsulated in PLGA delivery system: Effects of additives on protein encapsulation, release, and stability. J. Drug Deliv. Sci. Technol. 2022, 73, 103436. [Google Scholar] [CrossRef]
- Matsumoto, A.; Matsukawa, Y.; Nishioka, Y.; Harada, M.; Horikiri, Y.; Yamahara, H. A new method of preparing TRH derivative-loaded poly(dl-lactide-coglycolide) microspheres based on a solid solution system. Drug Discov. Ther. 2008, 2, 45–51. [Google Scholar]
- Li, W.; Chen, J.; Zhao, S.; Huang, T.; Ying, H.; Trujillo, C.; Molinaro, G.; Zhou, Z.; Jiang, T.; Liu, W.; et al. High drug-loaded microspheres enabled by controlled in-droplet precipitation promote functional recovery after spinal cord injury. Nat. Commun. 2022, 13, 1262. [Google Scholar] [CrossRef]
- Parikh, R.H.; Parikh, J.R.; Dubey, R.R.; Soni, H.N.; Kapadia, K.N. Poly(D,L-Lactide-Co-Glycolide) microspheres containing 5-fluorouracil: Optimization of process parameters. AAPS PharmSciTech 2003, 4, 14–21. [Google Scholar] [CrossRef]
- Ding, A.G.; Schwendeman, S.P. Acidic Microclimate pH Distribution in PLGA Microspheres Monitored by Confocal Laser Scanning Microscopy. Pharm. Res. 2008, 25, 2041–2052. [Google Scholar] [CrossRef]
- Park, K.; Otte, A.; Sharifi, F.; Garner, J.; Skidmore, S.; Park, H.; Jhon, Y.K.; Qin, B.; Wang, Y. Formulation composition, manufacturing process, and characterization of poly(lactide-co-glycolide) microparticles. J. Control. Release 2021, 329, 1150–1161. [Google Scholar] [CrossRef]
- Zhang, Y.; Sophocleous, A.M.; Schwendeman, S.P. Inhibition of Peptide Acylation in PLGA Microspheres with Water-soluble Divalent Cationic Salts. Pharm. Res. 2009, 26, 1986–1994. [Google Scholar] [CrossRef][Green Version]
- Kang, F.; Singh, J. Effect of additives on the release of a model protein from PLGA microspheres. AAPS PharmSciTech 2001, 2, 86–92. [Google Scholar] [CrossRef]
- Lin, X.; Yang, H.; Su, L.; Yang, Z.; Tang, X. Effect of size on the in vitro/in vivo drug release and degradation of exenatide-loaded PLGA microspheres. J. Drug Deliv. Sci. Technol. 2018, 45, 346–356. [Google Scholar] [CrossRef]
- Zheng, C.; Liang, W. A one-step modified method to reduce the burst initial release from PLGA microspheres. Drug Deliv. 2010, 17, 77–82. [Google Scholar] [CrossRef]
- Srikanth, S.; Ambrose, J.A. Ambrose Pathophysiology of Coronary Thrombus Formation and Adverse Consequences of Thrombus During PCI. Curr. Cardiol. Rev. 2012, 8, 168–176. [Google Scholar] [CrossRef]
- Iqbal, A.M.; Lopez, R.A.; Hai, O. “Antiplatelet Medications,” StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/30725747/ (accessed on 15 February 2025).
- Fischell, T. Eptifibatide: The evidence for its role in the management of acute coronary syndromes. Core Evid. 2009, 4, 49–65. [Google Scholar] [CrossRef]
- King, S.; Short, M.; Harmon, C. Glycoprotein IIb/IIIa inhibitors: The resurgence of tirofiban. Vasc. Pharmacol. 2016, 78, 10–16. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Hirsh, J. Combined antiplatelet and anticoagulant therapy: Clinical benefits and risks. J. Thromb. Haemost. 2007, 5, 255–263. [Google Scholar] [CrossRef]
- Malanga, M.; Szemán, J.; Fenyvesi, É.; Puskás, I.; Csabai, K.; Gyémánt, G.; Fenyvesi, F.; Szente, L. ‘Back to the Future’: A New Look at Hydroxypropyl Beta-Cyclodextrins. J. Pharm. Sci. 2016, 105, 2921–2931. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.D.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.D.; De Lima, A.N. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Basu, P.; Narendrakumar, U.; Arunachalam, R.; Devi, S.; Manjubala, I. Characterization and Evaluation of Carboxymethyl Cellulose-Based Films for Healing of Full-Thickness Wounds in Normal and Diabetic Rats. ACS Omega 2018, 3, 12622–12632. [Google Scholar] [CrossRef] [PubMed]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Paolino, D.; Celia, C.; Mero, A.; Joseph, A.S.; Wolfram, J.; Cosco, D.; Schiavon, O.; Shen, H.; Fresta, M. Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. J. Control. Release 2015, 199, 106–113. [Google Scholar] [CrossRef]
- Péan, J.; Boury, F.; Venier-Julienne, M.; Menei, P.; Proust, J.; Benoit, J. Why does PEG 400 co-encapsulation improve NGF stability and release from PLGA biodegradable microspheres? Pharm. Res. 1999, 16, 1294–1299. [Google Scholar] [CrossRef]
- Chirife, J.; Herszage, L.; Joseph, A.; Bozzini, J.P.; Leardini, N.; Kohn, E.S. In vitro antibacterial activity of concentrated polyethylene glycol 400 solutions. Antimicrob. Agents Chemother. 1983, 24, 409–412. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]
- Kyatanwar, A. Development of Controlled Release Parenteral Formulation of An Antiplatelet Drug Eptifibatide. Ph.D. Thesis, Narsee Monjee Institute of Management Studies, Mumbai, India, June 2023. [Google Scholar]

| Batch Size | Quantity for a Batch of 10 Vials; Solids in (g); Liquids in (mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients/Trials | mg/vial | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Additives | NA | HPβCD | Trehalose | Na-CMC | ||||||||
| Drug–Additive Ratio | - | 1:0.5 | 1:1 | 1:2 | 1:1 R | 1:0.5 | 1:1 | 1:2 | 1:0.5 | 1:1 | 1:2 | |
| Eptifibatide (g) | 12.96 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| Additives | 6.5 to 26 | - | 0.07 | 0.13 | 0.26 | 0.13 | 0.07 | 0.13 | 0.26 | 0.07 | 0.13 | 0.26 |
| DMFA (mL) | - | 1 | 1 | 1.5 | 2 | 1.5 | 1 | 1.5 | 2 | 1 | 1.5 | 2.5 |
| PLGA 75:25 (g) | 60 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| DCM (mL) | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1.5 |
| PVA (g) | - | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Water (mL) | - | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 |
| D-Mannitol (g) | 7.04 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Water (mL) | - | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Batch Size | Quantity for a Batch of 10 Vials; Solids in (g); Liquid in (mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients/Trials | mg/vial | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| Additives | mPEG-2000-DMPE, Na | PVA | PEG 400 | ||||||||
| Polymer–Additive Ratio | 1:0.5 | 1:0.5 | 1:1 | 1:2 | 1:0.5 | 1:1 | 1:2 | 1:0.5 | 1:1 | 1:2 | |
| Eptifibatide (g) | 12.96 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| DMFA (mL) | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| PLGA75:25(g) | 60 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Additives | 30 to120 | 0.3 | 0.3 | 0.6 | 1.2 | 0.3 | 0.6 | 1.2 | 0.3 | 0.6 | 1.2 |
| DCM (mL) | - | 2 | 2 | 2 | 3 | 2 | 2 | 2.5 | 2 | 2 | 2.5 |
| PVA (g) | - | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Water (mL) | - | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 |
| D-Mannitol (g) | 7.04 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Water (mL) | - | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Details of Additives | MSP | HPβCD | Trehalose | Na CMC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Drug–Additive Ratio | NA | 1:0.5 | 1:1 | 1:2 | 1:1 | 1:0.5 | 1:1 | 1:2 | 1:0.5 | 1:1 | 1:2 | |
| Total Drug (%w/w) | 95.40 | 95.2 | 97.6 | 96.1 | 98.2 | 89.1 | 93.2 | 90.5 | 95.3 | 94.8 | 95.1 | |
| Free Drug (%w/w) | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | |
| PSD d 50 (µm) | 25.12 | 14.15 | 18.34 | 19.81 | 18.11 | 14.28 | 19.20 | 18.21 | 17.25 | 16.14 | 15.21 | |
| PSD d 90 (µm) | 47.22 | 46.87 | 48.30 | 43.46 | 47.23 | 47.32 | 48.67 | 46.12 | 43.21 | 45.18 | 45.11 | |
| In vitro release in lactic acid buffer pH 2.2 (% cumulative release) Time points (h) | 0.25 | 13.21 | ND | ND | 3.21 | 2.31 | ND | ND | ND | ND | ND | ND |
| 0.5 | 14.55 | ND | ND | 4.01 | 2.93 | ND | ND | ND | ND | ND | ND | |
| 1 | 17.43 | ND | ND | 5.44 | 4.33 | ND | ND | ND | ND | ND | ND | |
| 8 | 34.67 | 24.21 | 15.13 | 10.23 | 10.41 | 35.67 | 33.12 | 38.51 | 37.23 | 35.33 | 36.56 | |
| 12 | 38.60 | 27.18 | 31.10 | 30.15 | 31.33 | 39.42 | 40.13 | 53.11 | 40.41 | 43.11 | 42.11 | |
| 24 | 58.10 | 64.12 | 56.14 | 55.34 | 55.45 | 61.03 | 56.33 | 64.34 | 59.45 | 61.02 | 58.65 | |
| 48 | 74.30 | 94.47 | 90.21 | 91.35 | 91.34 | 87.56 | 88.78 | 90.12 | 83.45 | 88.32 | 84.34 | |
| Details of Additives | MSP | mPEG-2000-DMPE, Na | PVA | PEG-400 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch No. | 1 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| Polymer–additive | NA | 1:0.5 | 1:0.5 | 1:1 | 1:2 | 1:0.5 | 1:1 | 1:2 | 1:0.5 | 1:1 | 1:2 |
| Total drug (%w/w) | 95.40 | 94.23 | 95.1 | 93.44 | 92.31 | 92.3 | 93.23 | 92.13 | 94.4 | 94.56 | 93.34 |
| Free drug (%w/w) | 0.02 | 0.01 | 0.02 | 0.01 | 0.0.2 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 |
| PSD d 50 (µm) | 25.12 | 12.34 | 13.45 | 14.33 | 13.22 | 13.2 | 13.51 | 14.12 | 14.21 | 16.01 | 19.43 |
| PSD d 90 (µm) | 47.22 | 43.12 | 44.56 | 45.18 | 44.45 | 44.1 | 44.17 | 45.12 | 45.11 | 44.23 | 45.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyatanwar, A.; Prabhakar, B. A Study of the Impact of Additives on the Physicochemical Properties of Eptifibatide-Loaded Microspheres for Drug Delivery. J. Pharm. BioTech Ind. 2025, 2, 8. https://doi.org/10.3390/jpbi2020008
Kyatanwar A, Prabhakar B. A Study of the Impact of Additives on the Physicochemical Properties of Eptifibatide-Loaded Microspheres for Drug Delivery. Journal of Pharmaceutical and BioTech Industry. 2025; 2(2):8. https://doi.org/10.3390/jpbi2020008
Chicago/Turabian StyleKyatanwar, Anand, and Bala Prabhakar. 2025. "A Study of the Impact of Additives on the Physicochemical Properties of Eptifibatide-Loaded Microspheres for Drug Delivery" Journal of Pharmaceutical and BioTech Industry 2, no. 2: 8. https://doi.org/10.3390/jpbi2020008
APA StyleKyatanwar, A., & Prabhakar, B. (2025). A Study of the Impact of Additives on the Physicochemical Properties of Eptifibatide-Loaded Microspheres for Drug Delivery. Journal of Pharmaceutical and BioTech Industry, 2(2), 8. https://doi.org/10.3390/jpbi2020008







