Abstract
Severely burned patients are at high risk of local and systemic infections due to skin barrier loss. Their clinical management is complex and requires coordinated intensive care and infection prevention strategies. Diagnosing infective endocarditis (IE) in this population is particularly difficult due to overlapping symptoms and limited diagnostic specificity. Common pathogens include Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii. We conducted a retrospective cohort study on 543 patients with burns affecting >18% of total body surface area (TBSA), admitted to our Burn Intensive Care Unit (BICU) from 2019 to 2024. The incidence of infective endocarditis was 1.47%, involving aortic (75%), mitral (12.5%), and tricuspid (12.5%) valves. Pathogens identified included S. aureus, Klebsiella pneumoniae, A. baumannii, and P. aeruginosa. This incidence is significantly higher than that in the general population. Mortality reached 50%, with an overall 3-month mortality of 75%. The literature on IE in burn patients is scarce, and the role of antibiotic prophylaxis remains controversial. Infective endocarditis in burn patients, although rare, represents a severe complication with high mortality. Early diagnosis and coordinated multidisciplinary care are essential to improve patient outcomes.
1. Introduction
Infective endocarditis represents a severe complication in critically ill patients, with particular relevance in severely burned individuals, where the risk of infection is significantly increased due to both local and systemic infections [1].
In fact, it remains the leading cause of death among burn patients [2,3], likely due to the increased susceptibility to sepsis in severely burned patients due to the loss of the skin barrier and the resulting immune deficiency [4,5].
Extensive skin exposure, the need for invasive devices, and prolonged stays in the burn intensive care unit (BICU) contribute to an increased incidence of nosocomial infections and bacteremia, which are potential predisposing factors for infective endocarditis. Therefore, the management of these patients is an ongoing complex challenge, requiring a multidisciplinary approach involving cooperation between intensive support, advanced surgical treatments, and infection prevention strategies. As a consequence of BICU admission, patients are required to rest in bed for long periods and undergo immobilization, sedation, analgesics, and the use of monitors and invasive devices. This results in the weakening of muscles, including the cardiovascular system and respiratory muscles, including the diaphragm, which progressively weaken [6]. Early rehabilitation, therefore, helps maintain muscle strength and appears to cooperate in reducing inflammation [7]. Another issue is prolonged cardiac stress with tachycardia and increased myocardial oxygen consumption [8]. For early rehabilitation, managing acute pain is necessary to reduce it and allow the patient to regain mobility [9]. However, despite therapeutic advancements, infective endocarditis in severely burned patients remains associated with high mortality, with limited evidence in the literature regarding its incidence and optimal management [10]. The diagnosis of infective endocarditis in this patient population is particularly challenging due to several factors. The generalized systemic inflammatory response, commonly observed in patients with extensive burns, may obscure the classical signs of infection, making timely diagnosis difficult. Additionally, the presence of extensive burns and critical clinical conditions often limit the use of standard diagnostic methods, such as transthoracic and transesophageal echocardiography. The scarcity of epidemiological data in the literature further complicates the characterization of this condition in burn patients, particularly in the presence of additional risk factors, such as substance abuse. From a microbiological perspective, extensive skin exposure may predispose these patients to a higher incidence of infective endocarditis caused by S. aureus, a pathogen known for its high virulence and strong association with increased mortality rates [11]. However, several infections caused by both Gram-positive and Gram-negative bacteria, such as P. aeruginosa, K pneumoniae and A. baumannii, may also occur [12,13]. The evolution of bacterial resistance within BICU settings represents an additional critical factor, potentially impacting the efficacy of conventional antimicrobial therapies. This retrospective study aims to determine the incidence of infective endocarditis in critically ill burn patients, identifying the bacterial populations involved and assessing the clinical implications of these infections. The analysis of collected data will facilitate the development of optimized prevention and treatment strategies, ultimately improving the management of this severe infectious complication in burn patients.
2. Materials and Methods
2.1. Study Design and Participants
We conducted a retrospective cohort study to analyze cases of bacterial endocarditis in a cohort of burn patients, in order to evaluate the incidence and microbiological causes. In our retrospective study, we collected cases of bacterial endocarditis occurring in a cohort of 543 burn patients admitted to our intensive care unit at the “A. Cardarelli” hospital in Naples, from 2019 to 2024. The study was approved by the ethics committee with protocol number 17/24 on 4 December 2024. The study population consisted of patients with burns of TBSA > 18%. The inclusion criteria included patients who met the following conditions: (1) admission to intensive care for major burns, (2) TBSA > 18%, and (3) diagnosis of bacterial endocarditis (with echocardiographic evidence and positive blood cultures). The patients were homogeneous in terms of major burn pathology, but heterogeneous regarding pre-existing conditions and comorbidities. Ethical approval was obtained from the relevant ethics committee (reference number: 17/24 from 4 December 2024), which granted a waiver for informed consent since the research was based on secondary data. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. These data were securely stored with a password, ensuring that only the research team had access, and their use was strictly limited to research purposes. We removed any identifying information such as names, addresses, and other personal identifying details.
2.2. Data Collection
The data collection process included the AORN “A. Cardarelli” hospital in Naples. Retrospectively, all patient records from 2019 to 2024 were reviewed. Patients were followed for a total of 3 months after admission to the intensive care unit. The data were collected in a password-protected database, ensuring that only authorized personnel had access. Each patient meeting the inclusion criteria was assigned an anonymous ID, and background variables were collected, including age, gender, admission date, weight, %TBSA, presence of diabetes, the affected valve in the endocarditis, presence of lung injury, devices used, antibiotic therapy, days on mechanical ventilation, presence of septic shock/sepsis, any cardiovascular risk factors, potential predisposing biases for endocarditis, previous drug use, the bacterium responsible for the endocarditis, date of diagnosis, length of BICU stay, and mortality both during the BICU stay and 3 months after discharge.
2.3. Statistical Analysis
The reference incidence data were not obtained from an internal control group but were derived from published epidemiological studies, providing a literature-based benchmark for comparison. Specifically, the expected counts were calculated as λ = n × (incidence rate), yielding λ_min = 543 × (3/100,000) = 0.01629 and λ_max = 543 × (10/100,000) = 0.0543. Given the very low expected counts, the Poisson distribution is an appropriate model to describe the number of events, under the assumption that events occur independently and with a constant average rate. We computed the probability of observing at least 8 cases (k_obs = 8) using the cumulative distribution function of the Poisson model, where the p-value is given by 1 − P(X ≤ 7). In addition, an exact Poisson test was performed using RStudio (version 4.4.3, The R Foundation for Statistical Computing, Wien, Austria) to formally assess the null hypothesis that the true event rate in the burn patient cohort is equal to the literature-based incidence rate. This approach allows us to quantify the discrepancy between the observed and expected counts and to evaluate the statistical significance of the finding.
3. Results
3.1. Baseline Characteristics
The age of the patients ranged from 49 to 79 years, with a mean age of 60 ± 12.43 years. The majority were women (6 out of 8), accounting for 75% female and 25% male. Weight ranged from 60 kg to a maximum of 80 kg, with an average of 73.5 kg and a standard deviation of ±7.03. None of the patients had diabetes. The total body surface area (TBSA) affected by burns ranged from 18% to 50%, with an average of 27.25% ± 13.5%. Five patients (62.5%) required mechanical ventilation for a minimum of 8 days and a maximum of 15 days, with an average duration of 10 days. Patients diagnosed with septic shock accounted for 37.5%, while all patients met the criteria for sepsis according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [13,14]. Only one patient had a history of substance abuse. Regarding cardiac history, 12.5% (1/8) had arterial hypertension and 12.5% (1/8) had ischemic heart disease. The remaining patients had various comorbidities, including aortic thrombosis, atrial flutter, ascending aortic aneurysm, mitral and tricuspid valve insufficiency, and aortic valve insufficiency. The length of stay in the intensive care unit ranged from a minimum of 30 days (1 month) to a maximum of 110 days, with a mean BICU stay of 67 days ± 25.55 (see Table 1).

Table 1.
Baseline characteristics.
3.2. Clinical Characteristics and Outcomes
In a cohort of 543 patients, only 8 cases presented with endocarditis, resulting in an incidence of 1.47%. Specifically, the percentage of affected valves was as follows: 75% aortic valve, 12.5% mitral valve, and 12.5% tricuspid valve (see Figure 1).
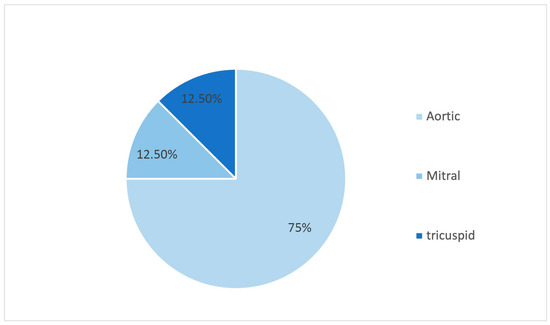
Figure 1.
Valves affected by endocarditis.
The included pathogens were S. aureus (62.50%), K. pneumoniae (37.50%), P. aeruginosa (25%), E. cloacae (25%), A. baumannii (12.50%), E. gallinarum (12.50%), S. auricularis (12.50%), C. parapsilosis (12.50%), E. faecalis (12.50%), E. coli (12.50%) (see Figure 2).
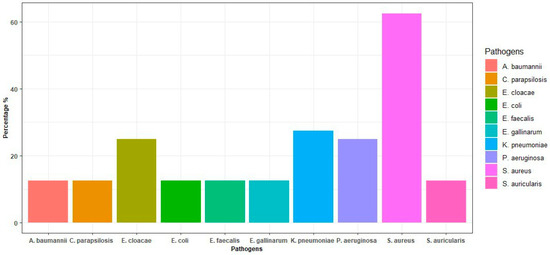
Figure 2.
Incidence of bacteria in blood cultures.
All patients had central venous access, obtained via FICC (Femoral Inserted Central Catheter) or CICC (Centrally Inserted Central Catheter). Two patients had double central venous access for clinical reasons; both died during their BICU stay. None of the patients presented catheter-related bloodstream infections (CRBSI) based on time-to-positivity; therefore, no correlation was observed between the presence of central venous access and the onset of bloodstream infection. The total body surface area (TBSA) burned ranged from 18% to 50%, with a mean of 35 ± 15.5%. Three of these patients (75%) had involvement of the aortic valve, while one (25%) had endocarditis of the tricuspid valve. The in-hospital mortality rate was 50% (4 out of 8 patients); specifically, two were male and two females. Among patients discharged alive, the 3-month follow-up showed a mortality rate of 50% (2 out of 4 patients). Therefore, an in-hospital mortality rate of 50% was observed, with an additional 50% mortality at 3 months follow-up among discharged patients, resulting in an overall mortality rate of 75%. One of the deceased patients had a documented history of intravenous drug use and was also the only case with tricuspid valve endocarditis. The same patient was the only one presenting with lung injury due to smoke inhalation, suggesting a multifactorial burden that could have contributed to the unfavorable outcome. All patients who died in hospital required mechanical ventilation, with durations ranging from 2 to 15 days, and a mean of 10.5 ± 5.4 days. Among the two patients who died during follow-up, one had not required mechanical ventilation, while the other had been ventilated for 8 days, resulting in a mean of 4 ± 4 days. Only one of the in-hospital deceased patients had a history of aortic valve insufficiency, while the other three had no known cardiac history. Among the four in-hospital deaths, three patients (75%) met diagnostic criteria for septic shock, while one did not. In contrast, none of the two patients who died during follow-up were classified as having septic shock.
Patients’ information is shown in Table 2.

Table 2.
Patients’ information.
3.3. Incidence and Analysis
The analysis revealed that the expected number of events in the burn patient cohort is extremely low (λ_min = 0.01629 and λ_max = 0.0543), whereas 8 cases were observed, resulting in an observed event rate of approximately 0.01473. The probability of observing 8 or more events under the null hypothesis was effectively 0 (rounded as < 0.001) for both incidence scenarios. The exact Poisson tests confirmed these results, yielding p-values of <0.001 for both the lower and upper literature incidence rates. The 95% confidence intervals for the event rate, calculated by the exact test, had a lower bound of approximately 0.00733 with the upper bound extending to infinity, which further emphasizes the significant deviation from the expected rate. These findings provide compelling evidence that the incidence of bacterial endocarditis in the cohort of burn patients is significantly higher than that anticipated based on general population data. Such a marked difference suggests that burn patients may be at a substantially elevated risk, warranting further investigation into potential underlying factors contributing to this increased susceptibility.
4. Discussion
This study found an incidence of infective endocarditis of 1.47% in burn patients, with an in-hospital mortality rate of 50%. Additionally, a further 50% mortality was recorded at 3-month follow-up among discharged patients, resulting in an overall 3-month mortality rate of 75%. The literature on this subject is limited, and only a few studies are available [15]; one review even reported a 100% mortality rate [10]. These rates are notably higher than those reported in the general population. For comparison, the 2023 European Society of Cardiology (ESC) Guidelines estimate an IE incidence of 13.8 per 100,000 annually, with global deaths around 66,300 in 2019 [16]. The results of the 2021 Global Burden of Disease Study indicate, however, that in 2021, there was a 40.1% increase in the number of IE cases, with the prevalence rate rising from 4.7 per 100,000 to 5.3 per 100,000. However, the number of deaths did not follow the same trend, showing only a 23.01% increase [17]. Our findings reinforce the hypothesis that burn patients are a high-risk group, likely due to prolonged BICU stays, frequent dressing changes, use of invasive devices, and compromised immunity. All these factors increase the likelihood of bloodstream infections and IE. Such a marked difference suggests that burn patients may be at a significantly higher risk, justifying further investigation into potential additional risk factors, preventive measures, and the importance of proper hand hygiene, early adoption of contact isolation, proper management of devices (central and peripheral intravenous, endotracheal tube), and maintaining aseptic conditions throughout the hospitalization. On the other hand, a severely burned patient remains in intensive care for extended periods and undergoes frequent medicated baths and repeated surgical interventions for skin grafts, in addition to daily dressing changes. However, these precautions are already strictly implemented in our intensive care unit, and patients are housed in isolated rooms. It should be emphasized that daily dressings are performed with 2% Chlorhexidine in a sterile manner, and the assigned staff use sterile gowns and gloves. Therefore, they are carried out under sterile conditions with appropriate personal protective equipment (PPE) for both the patient and healthcare providers. All patients were resuscitated according to the Parkland formula, and medicated baths are performed upon admission to the unit and prior to any skin graft procedures. One possible cause of the increased incidence of endocarditis in severely burned patients admitted to intensive care may be related to bacteremia resulting from frequent dressing changes, which could provide a route for bacterial colonization and infection. The affected valves were 75% aortic valve, 12.5% mitral valve, and 12.5% tricuspid valve, in accordance with the literature [10]. It is reported that one patient had a history of substance abuse, and in fact, the right heart was primarily affected, with involvement of the tricuspid valve. The isolates included S. aureus MRSA, K. pneumoniae KPC, and P. aeruginosa, with a hospitalization of 110 days and, unfortunately, death during the hospitalization. Focusing on the pathogens, in burn patients, bacteremia occurs frequently, especially after manipulation and excision of the wounds [18]. In fact, bacteremia caused by cutaneous surgery, due to probable contamination of the wounds, is ten times more frequent compared to immunocompetent patients [19]. Our study highlights the positive blood cultures for S. aureus, K. pneumoniae, A. baumannii, E. cloacae, and P. aeruginosa, in accordance with the findings of Christie M et al. regarding bloodstream infections (BSIs) caused by S. aureus, E. coli, and P. aeruginosa, which are frequently multidrug-resistant (MDR) [20]. Luo RB et al. analyzed the microbiological characteristics of pathogens isolated in severely burned patients using next-generation genomic sequencing techniques, in relation to the length of hospital stay. Specifically, it was found that bloodstream infections in severely burned patients increased with the length of hospitalization. In the first 7 days, the pathogens involved were mainly Gram-positive bacteria, including Staphylococcus aureus. On the other hand, Gram-negative bacteria, such as K. pneumoniae, P. aeruginosa, and A. baumannii, increased with the length of hospitalization [21,22], in line with our study’s findings. In contrast, the general population shows a different pathogen distribution, with oral streptococci and coagulase-negative staphylococci (CoNS) playing a larger role, as reported by the EURO-ENDO registry and ICE-PCS (Endocarditis-Prospective Cohort Study) [23,24]. These etiological differences highlight the unique risks faced by burn patients. Nevertheless, there is no scientific evidence regarding the use of antibiotic prophylaxis in these patients. Mozingo et al. [25] suggested the use of prophylactic antibiotics in patients with burns >40% TBSA due to the high risk of bacteremia. However, the indication for antibiotic prophylaxis in burn patients remains highly controversial. The goal of antibiotic prophylaxis is to reduce the risk of postoperative local and systemic infections [26]. Unfortunately, however, antibiotic prophylaxis exposes patients to the risk of side effects such as allergic reactions, organ damage, C. difficile colitis, increased costs and hospital stay, and the selection and progression of antibiotic-resistant microorganisms [27,28]. Therefore, to date, no strategy has shown superiority [29], and further studies are needed to clarify the controversy.
Therapeutic drug monitoring (TDM) could, however, lead to the optimization of antibiotic therapy, Macheda et al. [30] states that 46.3% of patients treated with antibiotics were overdosed and showed an altered creatinine clearance.
Antibiotic resistance (AMR), arising from both Gram-positive and Gram-negative species, also poses a significant clinical challenge and highlights the need to discover new antibiotics targeting specific bacterial structures and/or metabolites, such as metallophores, or to develop new strategies for delivering antibiotics into bacterial cells, such as the Trojan horse technique [31,32].
Another limitation is diagnostic feasibility: echocardiographic exams are often impractical due to extensive burns, and classic Duke criteria may be unreliable in these patients, where the localization and extent of the lesions, along with the presence of dressings covering almost the entire body, often make it impossible to perform a transthoracic or transesophageal echocardiogram for the diagnosis of endocarditis, even though it is essential for diagnosis, so diagnostic clues were minimal [10,33,34,35]. The same issue applies to the duke’s criteria, where it may be difficult to identify minor criteria such as Osler’s nodes and Janeway lesions, which are often covered by the burns in severely burned patients.
5. Limitations
Our study has some limitations: it is a single-center study, and it would be interesting to know the other incidences of endocarditis. Furthermore, due to the small number of burn subjects, our results do not have sufficient statistical power to convincingly reveal the incidence and the mortality rate of endocarditis in patients with severe burns.
6. Conclusions
This study identified a 1.47% incidence of infective endocarditis in patients with severe burns, with an in-hospital mortality rate of 50%, substantially higher than that in the general population. Additionally, a further 50% mortality was recorded at 3-month follow-up among discharged patients, resulting in an overall 3-month mortality rate of 75%. These patients present a complex clinical challenge for healthcare professionals, requiring a multidisciplinary approach that integrates intensive care support, advanced surgical treatments, and infection prevention strategies, as well as the need for early identification and tailored interventions.
Extensive skin exposure, the primary barrier to infection, the necessity for invasive devices, and prolonged stays in the burn intensive care units (BICUs) all contribute to a higher incidence of nosocomial infections and bacteremia, which are potential predisposing factors for infective endocarditis. It would be of interest to compare these findings with those of burn patients treated with new dermal substitutes, which may reduce the incidence of endocarditis, particularly from S. aureus. The literature on this topic is extremely limited; therefore, further research is needed to clarify the most effective diagnostic pathways and to assess the role of antibiotic prophylaxis. Innovative imaging techniques and standardized protocols may improve early detection in cases where standard echocardiography is not feasible.
Author Contributions
Conceptualization, F.C. and A.S.; methodology, M.R.C., G.N. and P.R.; software, R.A.; validation, M.M.; formal analysis, R.A.; investigation, T.A.; resources, I.M.; data curation, A.S.; writing—original draft preparation, F.C., A.S. and P.R.; writing—review and editing, M.M. and R.A.; visualization, F.S., A.T. and C.S.; supervision, R.V.; project administration, C.P. and A.P.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee Campania 3, in accordance with the appropriate institutional review board. Reference number: 17/24, dated 4 December 2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting the results of this study can be obtained from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BICU | Burn Intensive Care Unit |
| TBSA | Total Body Surface Area |
| FICC | Femoral inserted catheter central |
| CICC | Centrally inserted catheter central |
| ESC | European Society of Cardiology |
| IE | Infective endocarditis |
| PPE | Personal protective equipment |
| MRSA | Methicillin-Resistant Staphylococcus Aureus |
| KPC | Klebsiella pneumoniae carbapenemase |
| BSIs | Bloodstream infections |
| MDR | Multidrug-resistant |
| ICE-PCS | Endocarditis-Prospective Cohort Study |
| CoNS | Coagulase-negative staphylococci |
| TEE | Transesophageal echocardiography |
| TTE | Transthoracic echocardiography |
References
- Branski, L.K.; Al-Mousawi, A.; Rivero, H.; Jeschke, M.G.; Sanford, A.P.; Herndon, D.N. Emerging infections in burns. Surg. Infect. 2009, 10, 389–397. [Google Scholar] [CrossRef]
- Muller, M.J.; Herndon, D.N. The challenge of burns. Lancet 1994, 343, 216–220. [Google Scholar] [CrossRef]
- Saffle, J.R.; Davis, B.; Williams, P. Recent outcomes in the treatment of burn injury in the United States: A report from the American Burn Association Patient Registry. J. Burn. Care. Rehabil. 1995, 16, 219–232. [Google Scholar] [CrossRef]
- Richards, C.; Emori, T.G.; Edwards, J.; Fridkin, S.; Tolson, J.; Gaynes, R. Characteristics of hospitals and infection control professionals participating in the National Nosocomial Infections Surveillance System 1999. Am. J. Infect. Control. 2001, 29, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Schwacha, M.G. Macrophages and post-burn immune dysfunction. Burns 2003, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cartotto, R.; Johnson, L.; Rood, J.M.; Lorello, D.; Matherly, A.; Parry, I.; Romanowski, K.; Wiechman, S.; Bettencourt, A.; Carson, J.S.; et al. Clinical Practice Guideline: Early Mobilization and Rehabilitation of Critically Ill Burn Patients. J. Burn. Care. Res. 2023, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Adams, V.; Möbius-Winkler, S.; Linke, A.; Erbs, S.; Yu, J.; Kempf, W.; Schubert, A.; Schuler, G.; Hambrecht, R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am. Coll. Cardiol. 2003, 42, 861–868. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Suman, O.E.; Lee, J.O.; Norbury, W.B.; Branski, L.K.; Mlcak, R.P.; Jeschke, M.G. Changes in cardiac physiology after severe burn injury. J. Burn. Care. Res. 2011, 32, 269–274. [Google Scholar] [CrossRef]
- Coletta, F.; Pirolli, R.; Annunziata, R.; Nugnes, M.; Tommasello, A.; Villani, R.; Giaccari, L.G.; Passavanti, M.B.; Pace, M.C.; Sansone, P. Efficacy and Adverse Effects of IV Morphine for Burn Pain Management in the Emergency Department: An Observational Study. Pain. Ther. 2024, 13, 857–864. [Google Scholar] [CrossRef]
- Regules, J.A.; Glasser, J.S.; Wolf, S.E.; Hospenthal, D.R.; Murray, C.K. Endocarditis in burn patients: Clinical and diagnostic considerations. Burns 2008, 34, 610–616. [Google Scholar] [CrossRef]
- Tak, T.; Reed, K.D.; Haselby, R.C.; McCauley, C.S.; Shukla, S.K. An update on the epidemiology, pathogenesis and management of infective endocarditis with emphasis on Staphylococcus aureus. WMJ 2002, 101, 24–33. [Google Scholar] [PubMed]
- Khaledi, M.; Afkhami, H.; Nezhad-Matouri, R.; Dezfuli, A.A.Z.; Bakhti, S. Effective Strategies to Deal With Infection in Burn Patient. J. Burn. Care Res. 2022, 43, 931–935. [Google Scholar] [CrossRef]
- Ioannou, P.; Miliara, E.; Baliou, S.; Kofteridis, D.P. Infective endocarditis by Klebsiella species: A systematic review. J. Chemother. 2021, 33, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ajmone-Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Lin, L.; Xu, J.; Chai, Y.; Wu, W. Global, regional, and national burden of infective endocarditis from 2010 to 2021 and predictions for the next five years. BMC Public Health 2025, 25, 1115. [Google Scholar] [CrossRef]
- Ramos, G.E.; Resta, M.; Durlach, R.; Patiño, O.; Bolgiani, A.; Prezzavento, G.; Canigia, L.F.; Benaim, F. Peri-Operative Bacteraemia in Burn Patients. What Does it Mean? Ann. Burn. Fire Disasters 2006, 19, 130–135. [Google Scholar] [PubMed]
- Baskin, J.; Rosenthal, A.; Pruitt, B. Acute bacterial endocarditis: A silent source of sepsis in the burns patient. Ann. Surg. 1976, 184, 618–621. [Google Scholar] [CrossRef]
- Christie, M.; Avenant, T.; Nembudani, M.; Mnqandi, A.; Muller, C.; de Villiers, M.; Bhikhoo, Z. Insights into bloodstream infections in South African paediatric burn patients. Infect. Dis. 2025, 25, 362. [Google Scholar] [CrossRef]
- Luo, R.B.; Huang, M.; Hu, H.; Zhang, R.; Han, C.M. Microbiological characteristics of patients with severe burns caused by blast and application of metagenomics next-generation sequencing in the detection of pathogenic microorganisms. Zhonghua Shao Shang Za Zhi 2021, 37, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Yehya, A.; Ezzeddine, Z.; Chakkour, M.; Dhaini, Z.; Saba, M.S.B.; Saba, A.S.B.; Nohra, L.; Nassar, N.B.; Yassine, M.; Bahmad, H.F.; et al. The intricacies of Acinetobacter baumannii: A multifaceted comprehensive review of a multidrug-resistant pathogen and its clinical significance and implications. Front. Microbiol. 2025, 16, 1565965. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO registry. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Mozingo, D.; McManus, A.; Kim, S.; Pruitt, B.A. Incidence of bacteraemia after wound manipulation in the early post-burn period. J. Trauma 1997, 42, 1006–1011. [Google Scholar] [CrossRef]
- Dépret, F.; Farny, B.; Jeanne, M.; Klouche, K.; Leclerc, T.; Nouette-Gaulain, K.; Pantet, O.; Rémerand, F.; Roquilly, A.; Rousseau, A.-F.; et al. The A2B trial, antibiotic prophylaxis for excision-graft surgery in burn patients: A multicenter randomized double-blind study. Trials 2020, 21, 973. [Google Scholar] [CrossRef]
- Ugburo, A.O.; Atoyebi, O.A.; Oyeneyin, J.O.; Sowemimo, G. An evaluation of the role of systemic antibiotic prophylaxis in the control of burn wound infection at the Lagos University Teaching Hospital. Burns 2004, 30, 43–48. [Google Scholar] [CrossRef]
- Crabtree, S.J.; Robertson, J.L.; Chung, K.K.; Renz, E.M.; Wolf, S.E.; Hospenthal, D.R.; Murray, C.K. Clostridium difficile infections in patients with severe burns. Burns 2011, 37, 42–48. [Google Scholar] [CrossRef]
- Barajas-Nava, L.A.; López-Alcalde, J.; Roqué i Figuls, M.; Wolf, S.E.; Hospenthal, D.R.; Murray, C.K. Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database Syst. 2013, 6, CD008738. [Google Scholar] [CrossRef]
- Macheda, G.; El Helali, N.; Péan de Ponfilly, G.; Kloeckner, M.; Garçon, P.; Maillet, M.; Tolsma, V.; Mory, C.; Le Monnier, A.; Pilmis, B. Impact of therapeutic drug monitoring of antibiotics in the management of infective endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1183–1190. [Google Scholar] [CrossRef]
- Ghssein, G.; Ezzeddine, Z. The Key Element Role of Metallophores in the Pathogenicity and Virulence of Staphylococcus aureus: A Review. Biology 2022, 11, 1525. [Google Scholar] [CrossRef]
- Tillotson, G.S. Trojan Horse Antibiotics-A Novel Way to Circumvent Gram-Negative Bacterial Resistance? Infect. Dis. 2016, 11, 45–52. [Google Scholar] [CrossRef]
- Kawtharani, I.; Ghssein, G.; Srour, O.; Chaaban, A.A.; Salameh, P. Molecular Epidemiology of Different Bacterial Pathogens and Their Antimicrobial Resistance Genes Among Patients Suffering from Surgical Site Infections in Lebanon. Microbiol. Res. 2025, 16, 216. [Google Scholar] [CrossRef]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.-U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef]
- Chambers, J.; Sandoe, J.; Ray, S.; Prendergast, B.; Taggart, D.; Westaby, S.; Arden, C.; Grothier, L.; Wilson, J.; Campbell, B.; et al. The infective endocarditis team: Recommendations from an international working group. Heart 2014, 100, 524–527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).