Urinary Neutrophil Gelatinase-Associated Lipocalin as a Predictor of COVID-19 Mortality in Hospitalized Patients
Abstract
1. Introduction
2. Participants and Methods
2.1. Ethical Approval
2.2. Participants
2.3. Methods
2.3.1. Sampling
2.3.2. Chemiluminescent Microparticle Immunoassay
2.3.3. Radiological Examinations
2.3.4. Patient Data Collection
2.4. Statistical Methods
3. Results
3.1. Clinical Features of Patients
3.2. Factors Associated with Mortality
3.3. Bivariate and Multivariate Logistic Regression with ROC Analysis
4. Discussion
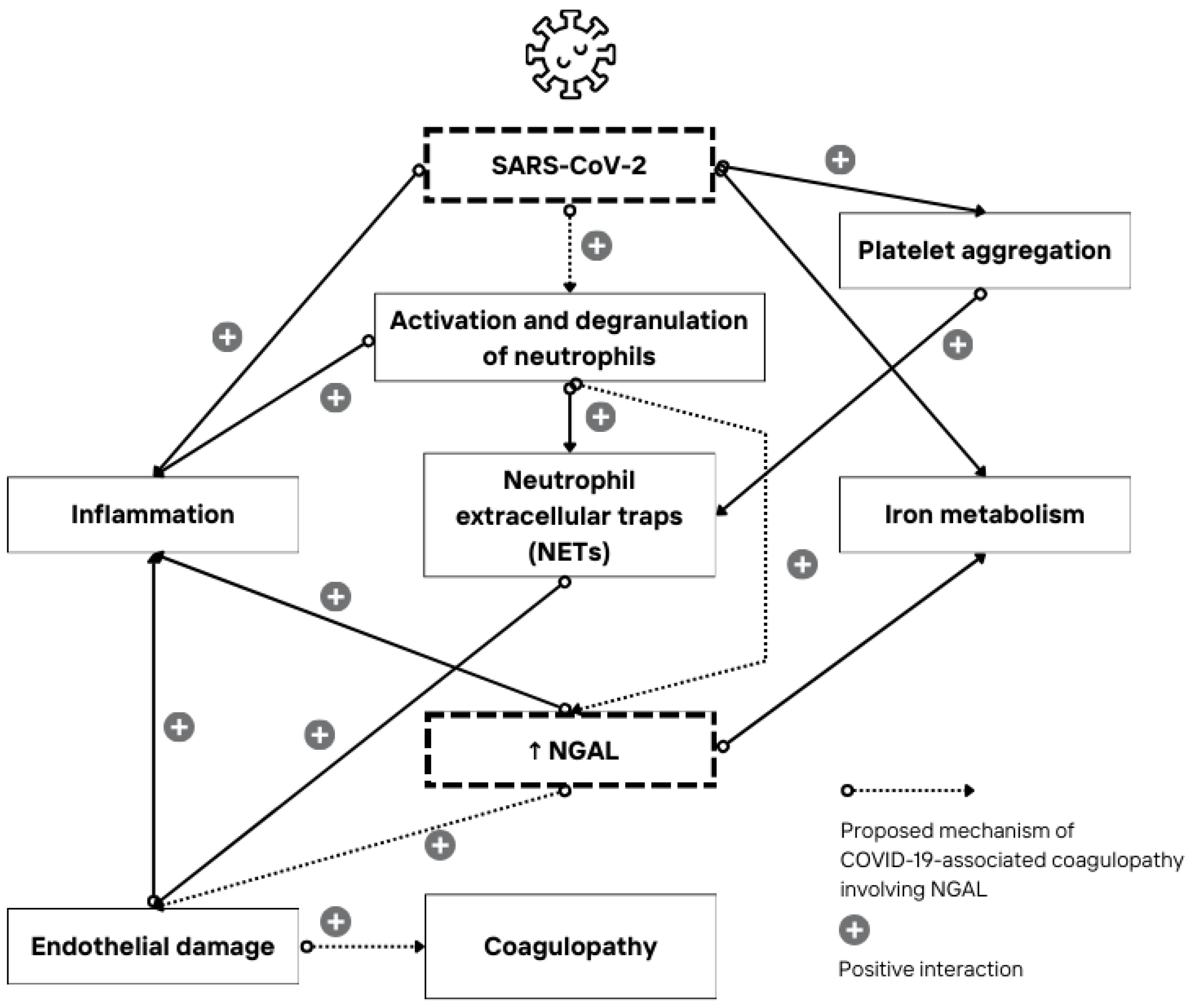
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravi, V.; Saxena, S.; Panda, P.S. Basic virology of SARS-CoV 2. Indian J. Med. Microbiol. 2022, 40, 182–186. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Lentini, P.; De Cal, M.; Clementi, A.; D’Angelo, A.; Ronco, C. Sepsis and AKI in ICU Patients: The Role of Plasma Biomarkers. Crit. Care Res. Pract. 2012, 2012, 856401. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Kim, J.S.; Jeong, K.H.; Kim, S.K. Acute Kidney Injury: Biomarker-Guided Diagnosis and Management. Medicina 2022, 58, 340. [Google Scholar] [CrossRef]
- Buonafine, M.; Martinez-Martinez, E.; Jaisser, F. More than a simple biomarker: The role of NGAL in cardiovascular and renal diseases. Clin. Sci. 2018, 132, 909–923. [Google Scholar] [CrossRef]
- Menez, S.; Moledina, D.G.; Thiessen-Philbrook, H.; Wilson, F.P.; Obeid, W.; Simonov, M.; Yamamoto, Y.; Corona-Villalobos, C.P.; Chang, C.; Garibaldi, B.T.; et al. Prognostic Significance of Urinary Biomarkers in Patients Hospitalized With COVID-19. Am. J. Kidney Dis. 2022, 79, 257–267.e1. [Google Scholar] [CrossRef]
- Singh, M.; Pushpakumar, S.; Zheng, Y.; Smolenkova, I.; Akinterinwa, O.E.; Luulay, B.; Tyagi, S.C. Novel mechanism of the COVID-19 associated coagulopathy (CAC) and vascular thromboembolism. Npj Viruses 2023, 1, 3. [Google Scholar] [CrossRef]
- Racovitan, D.; Hogeweg, M.; Doevelaar, A.A.; Seidel, M.; Rohn, B.; Bettag, S.; Rieckmann, S.; Babel, N.; Seibert, F.S.; Westhoff, T.H. Urinary biomarkers to predict acute kidney damage and mortality in COVID-19. Clin. Nephrol. 2023, 99, 161–171. [Google Scholar] [CrossRef]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; Almeida de Jesus, A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight 2021, 6, e144455. [Google Scholar] [CrossRef]
- He, L.; Zhang, Q.; Li, Z.; Shen, L.; Zhang, J.; Wang, P.; Wu, S.; Zhou, T.; Xu, Q.; Chen, X.; et al. Incorporation of Urinary Neutrophil Gelatinase-Associated Lipocalin and Computed Tomography Quantification to Predict Acute Kidney Injury and In-Hospital Death in COVID-19 Patients. Kidney Dis. 2021, 7, 120–130. [Google Scholar] [CrossRef]
- Larcher, R.; Bargnoux, A.S.; Badiou, S.; Besnard, N.; Brunot, V.; Daubin, D.; Platon, L.; Benomar, R.; Amalric, M.; Dupuy, A.-M.; et al. Acute kidney injury in critical COVID-19 patients: Usefulness of urinary biomarkers and kidney proximal tubulopathy. Ren. Fail. 2023, 45, 2292152. [Google Scholar] [CrossRef]
- Paranjpe, I.; Jayaraman, P.; Su, C.-Y.; Zhou, S.; Chen, S.; Thompson, R.; Del Valle, D.M.; Kenigsberg, E.; Zhao, S.; Jaladanki, S.; et al. Proteomic characterization of acute kidney injury in patients hospitalized with SARS-CoV2 infection. Commun. Med. 2023, 3, 81. [Google Scholar] [CrossRef]
- Casas-Aparicio, G.; Alvarado-de la Barrera, C.; Escamilla-Illescas, D.; León-Rodríguez, I.; Del Río-estrada, P.M.; Calderón-Dávila, N.; González-Navarro, M.; Olmedo-Ocampo, R.; Castillejos-López, M.; Figueroa-Hernández, L.; et al. Role of Urinary Kidney Stress Biomarkers for Early Recognition of Subclinical Acute Kidney Injury in Critically Ill COVID-19 Patients. Biomolecules 2022, 12, 275. [Google Scholar] [CrossRef]
- Filev, R.; Lyubomirova, M.; Hristova, J.; Bogov, B.; Kalinov, K.; Svinarov, D.; Rostaing, L. Serum and Urinary Biomarkers in COVID-19 Patients with or without Baseline Chronic Kidney Disease. J. Pers. Med. 2023, 13, 382. [Google Scholar] [CrossRef]
- Lablad, Y.; Vanhomwegen, C.; De Prez, E.; Antoine, M.H.; Hasan, S.; Baudoux, T.; Nortier, J. Longitudinal Follow-Up of Serum and Urine Biomarkers Indicative of COVID-19-Associated Acute Kidney Injury: Diagnostic and Prognostic Impacts. Int. J. Mol. Sci. 2023, 24, 16495. [Google Scholar] [CrossRef]
- Clinical Management of COVID-19: Living Guideline. Available online: https://pubmed.ncbi.nlm.nih.gov/35917394/ (accessed on 25 September 2024).
- EQUATOR Network|Enhancing the QUAlity and Transparency of Health Research. Available online: https://www.equator-network.org/ (accessed on 7 July 2024).
- Vahey, G.M.; McDonald, E.; Marshall, K.; Martin, S.W.; Chun, H.; Herlihy, R.; Tate, J.E.; Kawasaki, B.; Midgley, C.M.; Alden, N.; et al. Risk factors for hospitalization among persons with COVID-19-Colorado. PLoS ONE 2021, 16, e0256917. [Google Scholar] [CrossRef]
- Velásquez García, H.A.; Adu, P.A.; Harrigan, S.; Wilton, J.; Rasali, D.; Binka, M.; Sbihi, H.; Smolina, K.; Janjua, N.Z. Risk factors for COVID-19 hospitalization after COVID-19 vaccination: A population-based cohort study in Canada. Int. J. Infect. Dis. 2023, 127, 116–123. [Google Scholar] [CrossRef]
- Gao, Y.-d.; Ding, M.; Dong, X.; Zhang, J.-j.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.-l.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Bezerra, G.F.; Meneses, G.C.; Albuquerque, P.L.; Lopes, N.C.; Santos, R.S.; Da Silva, J.C.; Mota, S.M.; Guimarães, R.R.; Guimarães, F.R.; Guimarães, Á.R.; et al. Urinary tubular biomarkers as predictors of death in critically ill patients with COVID-19. Biomark. Med. 2022, 16, 681–692. [Google Scholar] [CrossRef]
- Xu, K.; Shang, N.; Levitman, A.; Corker, A.; Kudose, S.; Yaeh, A.; Neupane, U.; Stevens, J.; Sampogna, R.; Mills, A.M.; et al. Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19. Kidney Int. Rep. 2021, 6, 2979–2992. [Google Scholar] [CrossRef]
- Volbeda, M.; Jou-Valencia, D.; van den Heuvel, M.C.; Knoester, M.; Zwiers, P.J.; Pillay, J.; Berger, S.P.; van der Voort, P.H.J.; Zijlstra, J.G.; van Meurs, M.; et al. Comparison of renal histopathology and gene expression profiles between severe COVID-19 and bacterial sepsis in critically ill patients. Crit. Care 2021, 25, 202. [Google Scholar] [CrossRef]
- Battaglini, D.; Lopes-Pacheco, M.; Castro-Faria-Neto, H.C.; Pelosi, P.; Rocco, P.R.M. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front. Immunol. 2022, 13, 857573. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef]
- Hedayati-Ch, M.; Ebrahim-Saraie, H.S.; Bakhshi, A. Clinical and immunological comparison of COVID-19 disease between critical and non-critical courses: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1341168. [Google Scholar] [CrossRef]
- Krishna Reddy, C.H.; Achari, P.K.; Nisha, B.; Radha, A.R. Significance of Laboratory Markers in Predicting the Severity of COVID-19 in the Central Reserve Police Force Front-line Workers with a Review of Literature. Indian J. Public Health 2022, 66, 512–515. [Google Scholar] [CrossRef]
- Goyal, A.; Daneshpajouhnejad, P.; Hashmi, M.F.; Bashir, K.; John, B.K. Acute Kidney Injury (Nursing); StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sikora, J.P.; Karawani, J.; Sobczak, J. Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS). Int. J. Mol. Sci. 2023, 24, 13469. [Google Scholar] [CrossRef]
- Sciaudone, A.; Corkrey, H.; Humphries, F.; Koupenova, M. Platelets and SARS-CoV-2 During COVID-19: Immunity, Thrombosis, and Beyond. Circ. Res. 2023, 132, 1272–1289. [Google Scholar] [CrossRef]
- Suriawinata, E.; Mehta, K.J. Iron and iron-related proteins in COVID-19. Clin. Exp. Med. 2023, 23, 969–991. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin—From iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef]
- Macdonald, S.P.J.; Bosio, E.; Neil, C.; Arendts, G.; Burrows, S.; Smart, L.; Brown, S.G.A.; Fatovich, D.M. Resistin and NGAL are associated with inflammatory response, endothelial activation and clinical outcomes in sepsis. Inflamm. Res. 2017, 66, 611–619. [Google Scholar] [CrossRef]
- McKenna, E.; Wubben, R.; Isaza-Correa, J.M.; Melo, A.M.; Mhaonaigh, A.U.; Conlon, N.; O’Donnell, J.S.; Cheallaigh, C.N.; Hurley, T.; Stevenson, N.J.; et al. Neutrophils in COVID-19: Not Innocent Bystanders. Front. Immunol. 2022, 13, 864387. [Google Scholar] [CrossRef]
- Liu, J.T.; Song, E.; Xu, A.; Berger, T.; Mak, T.W.; Tse, H.-F.; Law, I.K.; Huang, B.; Liang, Y.; Vanhoutte, P.M.; et al. Lipocalin-2 deficiency prevents endothelial dysfunction associated with dietary obesity: Role of cytochrome P450 2C inhibition. Br. J. Pharmacol. 2012, 165, 520–531. [Google Scholar] [CrossRef]
- Song, E.; Fan, P.; Huang, B.; Deng, H.-B.; Cheung, B.M.Y.; Félétou, M.; Vilaine, J.-P.; Villeneuve, N.; Xu, A.; Vanhoutte, P.M.; et al. Deamidated lipocalin-2 induces endothelial dysfunction and hypertension in dietary obese mice. J. Am. Heart Assoc. 2014, 3, e000837. [Google Scholar] [CrossRef]
| Number (%) of Patients | |
|---|---|
| Disease outcome | |
| Recovery | 58 (67) |
| Death outcome | 28 (33) |
| Admission to the ICU | |
| ICU | 21 (24) |
| Non-ICU | 65 (76) |
| Number (%) of Patients Regarding Outcome | p * | |||
|---|---|---|---|---|
| Survived | Death Outcome | Total | ||
| Sex | ||||
| Male | 26 (45) | 11 (39) | 37 (43) | 0.63 |
| Female | 32 (55) | 17 (61) | 49 (57) | |
| Disease severity | ||||
| Moderate | 5 (9) | 0 | 5 (6) | <0.001 |
| Severe | 52 (90) | 11 (39) | 63 (73) | |
| Critical | 1 (1) | 17 (61) | 18 (21) | |
| Comorbidities | ||||
| Type 2 diabetes mellitus | 14 (24) | 5 (18) | 19 (22) | 0.51 |
| Hypertension | 38 (66) | 24 (86) | 62 (72) | 0.05 |
| Cardiomyopathy | 10 (17) | 11 (39) | 21 (24) | 0.03 |
| Atrial fibrillation | 3 (5) | 5 (18) | 8 (9) | 0.11 † |
| Chronic lung disease | 5 (9) | 3 (11) | 8 (9) | 0.75 |
| Chronic kidney disease | 2 (3) | 1 (4) | 3 (4) | >0.99 † |
| Median (Interquartile Range) | Difference | 95% CI | p * | ||
|---|---|---|---|---|---|
| Survived | Death Outcome | ||||
| Age (years) | 71 (62–80) | 80 (76–86) | 8 | 4–14 | 0.001 |
| Length of hospitalization (days) | 8 (6–12) | 11 (7–15) | 2 | 0–4 | 0.10 |
| MAP (mmHg) | 83.3 (80–93.3) | 81.7 (73.3–88.3) | −3.3 | −10–0 | 0.12 |
| BMI (kg/m2) | 26.14 (24.6–28.7) | 28.6 (23.8–32.2) | 0.97 | −2.85–4.99 | 0.55 |
| Median (Interquartile Range) | Difference | 95% CI | p * | ||
|---|---|---|---|---|---|
| Survived | Death Outcome | ||||
| At admission | |||||
| uNGAL (ng/mL) | 21.2 (10.6–41.3) | 27.6 (15.4–91.8) | 5.2 | −2–17.2 | 0.21 |
| Follow-up sampling | |||||
| uNGAL (ng/mL) | 15.6 (9–40.7) | 34.5 (19.9–103.2) | 17.7 | 6.4–36.9 | 0.001 |
| β | Wald | p Value | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|
| Bivariate regression | |||||
| Age | 0.07 | 8.48 | 0.004 | 1.07 | 1.02–1.12 |
| Cardiomyopathy | 1.09 | 4.57 | 0.03 | 2.97 | 1.09–8.6 |
| IMV | 4.60 | 18.4 | <0.001 | 99.8 | 12.2–817.5 |
| uNGAL (at admission) | 0.001 | 0.63 | 0.43 | 1.001 | 0.99–1.003 |
| uNGAL (follow-up sampling) | 0.01 | 4.30 | 0.03 | 1.01 | 1.001–1.03 |
| Multivariate regression | |||||
| Age | 0.11 | 6.33 | 0.01 | 1.12 | 1.03–1.22 |
| uNGAL (follow-up sampling) | 0.1 | 3.88 | 0.04 | 1.01 | 1.001–1.03 |
| IMV | 5.07 | 17.4 | <0.001 | 159.4 | 14.7–1728.5 |
| Constant | −141.05 | 9.01 | 0.003 |
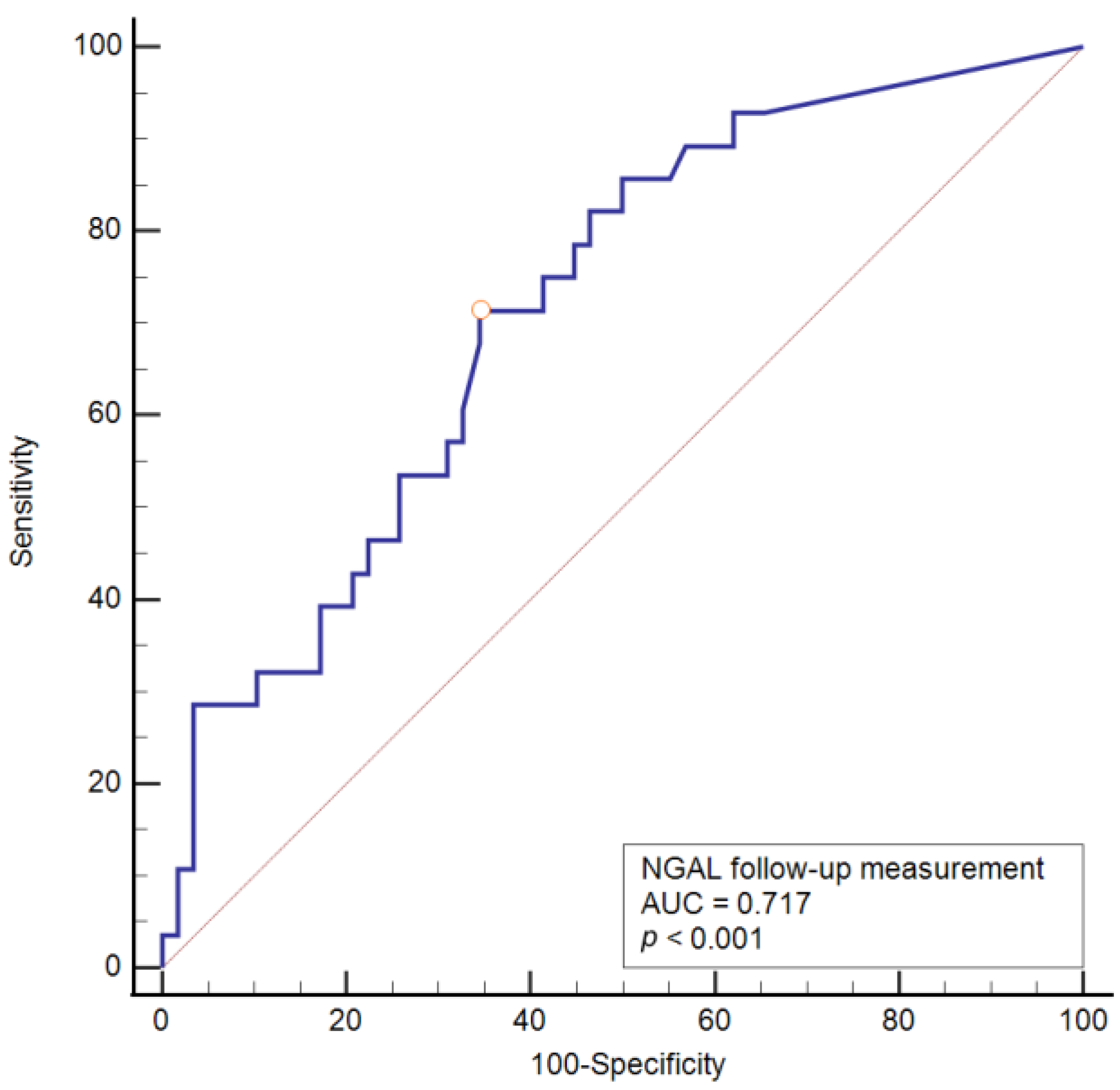
| AUC | 95% CI | Sensitivity | Specificity | Cut-Off | Youden Index | p Value | |
|---|---|---|---|---|---|---|---|
| uNGAL (follow-up sampling) (ng/mL) | 0.717 | 0.610–0.809 | 71.4 | 65.5 | >23.8 | 0.370 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švitek, L.; Zlosa, M.; Grubišić, B.; Kralik, K.; Perić, N.; Berišić, B.; Lišnjić, D.; Mandić, S. Urinary Neutrophil Gelatinase-Associated Lipocalin as a Predictor of COVID-19 Mortality in Hospitalized Patients. Acta Microbiol. Hell. 2024, 69, 224-235. https://doi.org/10.3390/amh69040021
Švitek L, Zlosa M, Grubišić B, Kralik K, Perić N, Berišić B, Lišnjić D, Mandić S. Urinary Neutrophil Gelatinase-Associated Lipocalin as a Predictor of COVID-19 Mortality in Hospitalized Patients. Acta Microbiologica Hellenica. 2024; 69(4):224-235. https://doi.org/10.3390/amh69040021
Chicago/Turabian StyleŠvitek, Luka, Mihaela Zlosa, Barbara Grubišić, Kristina Kralik, Nora Perić, Bernarda Berišić, Dubravka Lišnjić, and Sanja Mandić. 2024. "Urinary Neutrophil Gelatinase-Associated Lipocalin as a Predictor of COVID-19 Mortality in Hospitalized Patients" Acta Microbiologica Hellenica 69, no. 4: 224-235. https://doi.org/10.3390/amh69040021
APA StyleŠvitek, L., Zlosa, M., Grubišić, B., Kralik, K., Perić, N., Berišić, B., Lišnjić, D., & Mandić, S. (2024). Urinary Neutrophil Gelatinase-Associated Lipocalin as a Predictor of COVID-19 Mortality in Hospitalized Patients. Acta Microbiologica Hellenica, 69(4), 224-235. https://doi.org/10.3390/amh69040021







