1. Introduction
The Marburg virus (MARV) belongs to the species
Orthomarburgvirus marburgense under the genus
Orthomarburgvirus, a member of the
Filoviridae family [
1]. The virus is known for its aggressive virulence and pathogenicity, which are indicated by the common emergence of the virus in outbreaks including nosocomial and laboratory-originated outbreaks [
2]. The virus is zoonotic in nature and readily infects both humans and animals, transmitting to humans through direct contact with infected people, contaminated items, and the main reservoir for the virus, mainly fruit bats [
2,
3,
4,
5].
The recent outbreak in Rwanda is marked by its emergence as nosocomial, mainly in the intensive care unit (ICU), rapid spread, and mostly affecting healthcare providers and community health workers [
6]. Nonetheless, additional features include the prompt and effective evidence-based and science-guided response [
2,
7]. This included transparent and live communication and sharing of information and enforcing the community adherence to the national prevention and control guidelines including infection prevention and control in public and healthcare facilities [
2,
8].
Due to the highly contagious nature of MARV, it should only be handled in high biocontainment facilities. The aggressive transmissibility of the virus was indicated by its first detection during its involvement in laboratory outbreaks in Serbia and Germany in 1967 [
2,
9,
10]. Additionally, the virus caused three outbreaks in Russia in 1988, 1991, and 1995, all originating from laboratories [
11].
This article outlines the Good Clinical and Laboratory Practices (GCLP) [
12], implemented by the Rwandan National Reference Laboratory (NRL), which successfully prevented contamination and laboratory-acquired infections during the national response to the MVD outbreak in Rwanda.
2. Overview of the Marburg Outbreak in Rwanda
On 27 September 2024, the Ministry of Health in Rwanda declared an outbreak of Marburg virus disease (MVD) in the country [
13]. A rapid response led to a total of 66 confirmed cases. The demographic analysis indicated that males comprised the majority of cases at 68.18%, while females accounted for 31.82%. The age group most affected was 30 to 39 years, with 30 cases reported, followed by 20 to 29 years with 20 cases, 40 to 49 years with 11 cases, 50 to 59 years with 3 cases, and the least impacted group being individuals under 20 years, with only 2 cases, with 49 individuals recovering from the disease. In response to the outbreak, health authorities screened 6099 individuals and administered vaccines to 1629 people [
2,
13]. The outbreak initially spread rapidly within the two main hospitals in Kigali and among the close contacts of the index case. However, due to a swift and robust response by the health authorities, no community transmission has been reported to date. Significantly, nearly 80% of the cases were frontline responders, specifically healthcare workers who were providing emergency care to patients and colleagues [
13]. In response to the crisis, Rwandan health authorities have implemented emergency measures including contact tracing, isolation of suspected cases, and community awareness campaigns [
2]. This outbreak has revealed considerable stress on the healthcare system, highlighting vulnerabilities in infection control practices within hospitals and clinics. Continued vigilance and strong public health measures are essential to contain the outbreak and protect both healthcare workers and the wider community.
3. Transmissibility and High Biocontainment Characteristic of MARV
Human infections with MARV primarily occur through direct contact with the bodily fluids of infected humans or animals including blood, saliva, vomit, and other secretions [
2,
4,
11]. Indirect transmission is also possible if surfaces or medical equipment become contaminated, demonstrating the virus’s ability to spread in both community and healthcare settings [
14]. The virus can enter the body through broken skin or mucosal membranes, leading to potential infections and outbreaks, particularly in environments lacking effective infection control measures [
14,
15]. The significant risk of human-to-human transmission, especially in healthcare settings, has been underscored by historical outbreaks, such as the 1967 outbreaks in Germany and Yugoslavia, which affected laboratory workers handling African green monkeys imported from Uganda [
16]. Similarly, three outbreaks occurred in laboratories in Russia in 1988, 1991, and 1995 [
2,
11]. Other notable outbreaks occurred in 1975 in South Africa, which involved a nurse [
17], and in 1980 in Kenya, where a doctor who treated patients contracted the virus [
14,
18]. These incidents highlight the urgent necessity for stringent hygiene and safety protocols in potentially vulnerable areas.
Due to its high pathogenicity and potential for widespread transmission, MARV is classified as a biosafety level 4 (BSL-4) agent, demanding that laboratories implement exceptionally rigorous biocontainment measures [
19]. These facilities are equipped with advanced safety systems including engineered protection measures and personal protective equipment (PPE) such as positive-pressure suits. These systems provide an additional protective layer for laboratory personnel while ensuring a sterile working environment. Engineered protection measures are designed to minimize exposure risks, enhancing the overall safety of laboratory operations, especially when handling high-risk pathogens [
20,
21]. Moreover, effective air filtration systems and robust decontamination protocols are essential for minimizing the risk of accidental virus release into the environment. The strict application of these containment strategies is crucial not only for the safety of laboratory staff, but also for public health protection. Any breach in containment could result in severe consequences, including outbreaks and increased mortality rates, underscoring the importance of maintaining high safety standards when handling this dangerous pathogen.
4. Challenges Faced by Healthcare Providers
The current MVD outbreak in Rwanda revealed that approximately 80% of cases during the current outbreak were doctors, underscoring the dramatic risks faced within clinical settings. Doctors are often at the forefront of patient care, particularly in emergency departments and high-acuity areas where the likelihood of encountering infected individuals is significantly higher. The nature of their work demands close contact with patients, often without the benefit of prior knowledge of a patient’s infectious status.
Environmental factors also played a crucial role in increasing the risk for healthcare providers. Hospitals, particularly those heavily burdened with high patient volumes, often struggle to maintain rigorous infection prevention protocols. The constant influx of patients, combined with the fast-paced nature of emergency medical services, can result in lapses in the strict practices required to safeguard both patients and staff.
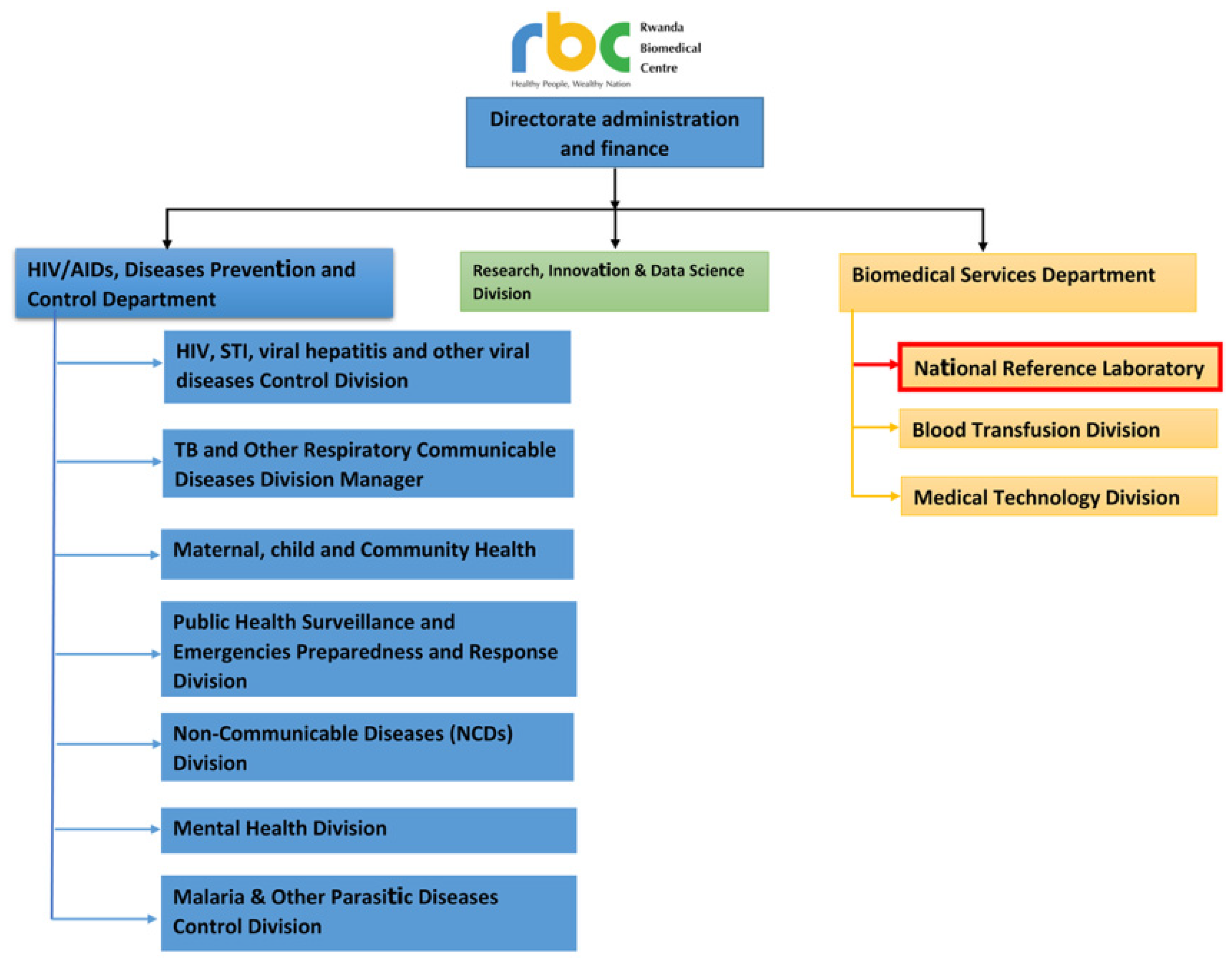
5. Rwandan National Public Health Institute Also Known as Rwanda Biomedical Center (RBC)
The Rwanda Biomedical Center (RBC) is the leading implementing agency for the health system in the country [
22], and leads the provision of healthcare services and implementing public health interventions including national disease control programs (
Figure 1).
More importantly, within its departments and division, RBC contains and leads the operation of the National Reference Laboratory (NRL), which operates centrally but also coordinates, oversees, and supports a national network of province- and district-based laboratories that include over 45 laboratories distributed throughout the country [
23,
24], both regional reference laboratories and major hospital laboratories. Additionally, it provides the diagnostic and pathogen characterization services for routine and different programs’ surveillance systems [
23]. Appreciating the crucial need for a highly functioning national reference laboratory for safeguarding the national and regional health security, since its establishment in 2014, RBC has invested continuously and effectively in building the capacity of the NRL as part of a holistic approach to safeguard the global health security nationally and regionally [
25].
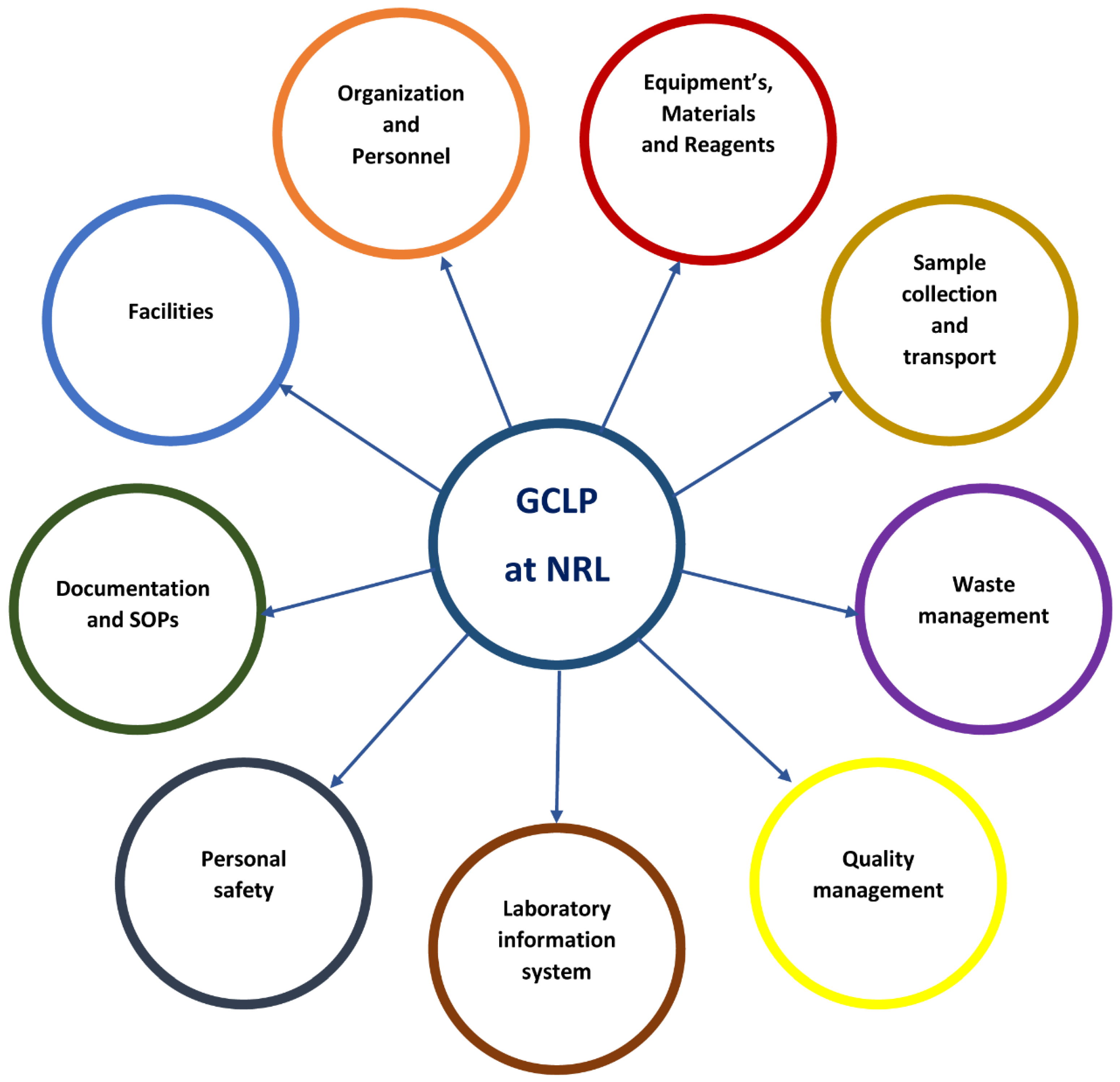
6. Best Practices of Biosafety and Biosecurity at the Rwandan National Reference Laboratory (NRL)
The Rwandan National Reference Laboratory (NRL) plays a vital role in ensuring the safety and security of biological materials, particularly in the context of emerging infectious diseases such as MARV infection. By adhering to the principles and components of Good Clinical Laboratory Practice (GCLP) (
Figure 2), the NRL has established a robust framework that encompasses various components essential for biosafety and biosecurity. This framework not only safeguards laboratory personnel and facilities, but also enhances the reliability of laboratory results. The following sections delve into each component of GCLP, illustrating how the NRL utilizes these practices to mitigate the risks associated with laboratory operations and protect against infectious agents like MARV infection.
7. Organization and Personnel
Organizational structure and well-defined roles are fundamental for ensuring biosafety and biosecurity [
26]. The NRL maintains a cohesive team of qualified personnel who undergo regular training on the biosafety protocols and emergency response procedures. This structured approach aids in assigning responsibilities for monitoring compliance with safety practices. By fostering a culture of safety, personnel are empowered to recognize potential hazards and act promptly to mitigate risks, thereby minimizing the likelihood of MARV exposure.
8. Facilities
The design and structure of laboratory facilities are crucial for streamlining operations while enhancing the safety and security [
26,
27]. This was foreseen at the establishment of the NRL facilities, which were prospectively engineered to enhance both biosafety and biosecurity. Key features of these facilities include tightly controlled access to prevent unauthorized entry, advanced ventilation systems designed to maintain negative pressure, and employ high-efficiency particulate air (HEPA) filtration to effectively capture airborne pathogens. Additionally, comprehensive decontamination protocols are integral to ensuring the safety and integrity of laboratory environments, alongside real-time monitoring and alarm systems that respond to deviations in safety parameters. The layout was strategically planned to prevent cross-contamination and facilitate effective workflow management. By optimizing physical spaces for safety, the NRL minimizes risks, creating an environment where staff can perform their work competently while mitigating potential exposure to infectious agents. For molecular biology laboratories at the NRL, a one-way flow design implemented in molecular biology laboratories serves as a crucial strategy to minimize the risk of cross-contamination and ensure the reliability of experimental results. This design entails a systematic workflow where materials, samples, and personnel move in a single direction, beginning from areas with the highest contamination risk to the least. At the entry zone, all incoming materials and personnel undergo decontamination to prevent the introduction of contaminants into the main laboratory space. As samples are processed, they move through distinct zones dedicated to specific tasks, such as the preparation, amplification, and detection of nucleic acids, ultimately reaching a clean area reserved for post-processing activities and data analysis. The segregation of tasks coupled with strict personnel movement protocols ensures that those entering sensitive areas adhere to safety measures, further preventing contamination. Upon receiving samples, they are immediately labeled with a unique laboratory number before being sent to the inactivation room. From there, samples progress to the extraction room, and subsequently to the PCR room, ensuring that the flow of materials between these areas strictly adheres to one-directional protocols. To safeguard against infection, all personnel working in these spaces are required to wear full personal protective equipment (PPE). Access to the laboratory is restricted to individuals who have undergone rigorous training provided by the NRL and are qualified molecular biologists. Moreover, the implementation of advanced containment practices is essential, particularly in light of the MARV and its high transmissibility and lethality [
28]. Laboratories handling MARV samples adhere to strict protocols for sample transportation and biological waste management, all designed and validated by the National Research Laboratory (RBC-NRL). These measures are critical for ensuring that all potentially infectious materials are handled and disposed of appropriately, thereby minimizing the risk of environmental contamination and further transmission.
9. Personnel Safety
Personnel safety is a major component of GCLP as they are the main guard for biosafety, biosecurity, and effective operation of the lab [
29]. Ensuring the safety of personnel is a top priority at the NRL. The facility offers thorough training on biosafety practices and the correct use of personal protective equipment (PPE). Additionally, regular drills and simulations of emergency situations, like exposure to infectious agents, enhance staff preparedness.
Regular drills and simulations of emergency scenarios, such as exposure to infectious agents, bolster staff preparedness. By prioritizing personnel safety, the NRL minimizes the risk of laboratory-acquired infections, including those caused by highly infectious organisms such as dengue, Rift Valley fever, Ebola, MARV, and others. Furthermore, the laboratory personnel who work with highly infectious agents are required to wear personal protective equipment (PPE) including gowns, gloves, masks, and face shields [
30]. This layered approach to protection is vital for creating a safe working environment. The correct and consistent use of PPE significantly reduces the risk of contamination and transmission, enabling staff to focus on their crucial tasks without compromising their health [
30].
In addition to PPE, laboratory personnel follow stringent cleaning protocols to maintain a safe and sterile environment. Each day begins with trained laboratory staff cleaning the floors with a 0.5% bleach solution (chlorine) and emptying dustbins while fully dressed in their protective gear. At the end of the day, laboratory operators meticulously disinfect the interior of glove boxes and workbenches using a 0.5% bleach solution or other disinfectants in accordance with the NRL’s standard procedures. These cleaning and decontamination activities are carefully documented in laboratory registers, maintenance logs, and decontamination sheets, ensuring that all procedures are tracked and adhered to.
10. Documentation and Standard Operating Procedures (SOPs)
Comprehensive documentation and standardized operating procedures (SOPs) are essential for ensuring safety and consistency in laboratory operations [
31]. The NRL has established rigorous SOPs covering all aspects of laboratory work including the handling of high-risk infectious samples such as those associated with the MARV, Ebola, Rift Valley fever, and dengue. Additionally, specific SOPs are in place for the operation and maintenance of each piece of laboratory equipment. Personnel are thoroughly trained to adhere to these written protocols with precision, ensuring that all procedures are executed safely and effectively. This adherence to SOPs is particularly critical when working with dangerous pathogens like MARV, as it helps mitigate potential risks and promotes a safe working environment.
11. Equipment
High quality equipment and advanced cost-effective tools for laboratory diagnoses and investigations are key components of efficient lab operation and functionality [
32]. The NRL is outfitted with cutting-edge technology to ensure the safe handling and analysis of infectious samples [
23]. Key equipment in the laboratory includes a range of biosafety cabinets classified from Class I to Class III as well as autoclaves, both of which are essential for maintaining a secure laboratory environment [
33]. To further mitigate exposure risks and ensure the safety of laboratory personnel while preventing environmental contamination, samples suspected of containing Marburg virus (MARV), a hazard group 4 pathogen, are processed under stringent biosafety level 4 (BSL-4) conditions to ensure maximum safety. This includes the use of air-fed suits, Class III biological safety cabinets (BSCs), and additional safety measures such as negative-pressure glove boxes and restricted laboratory access [
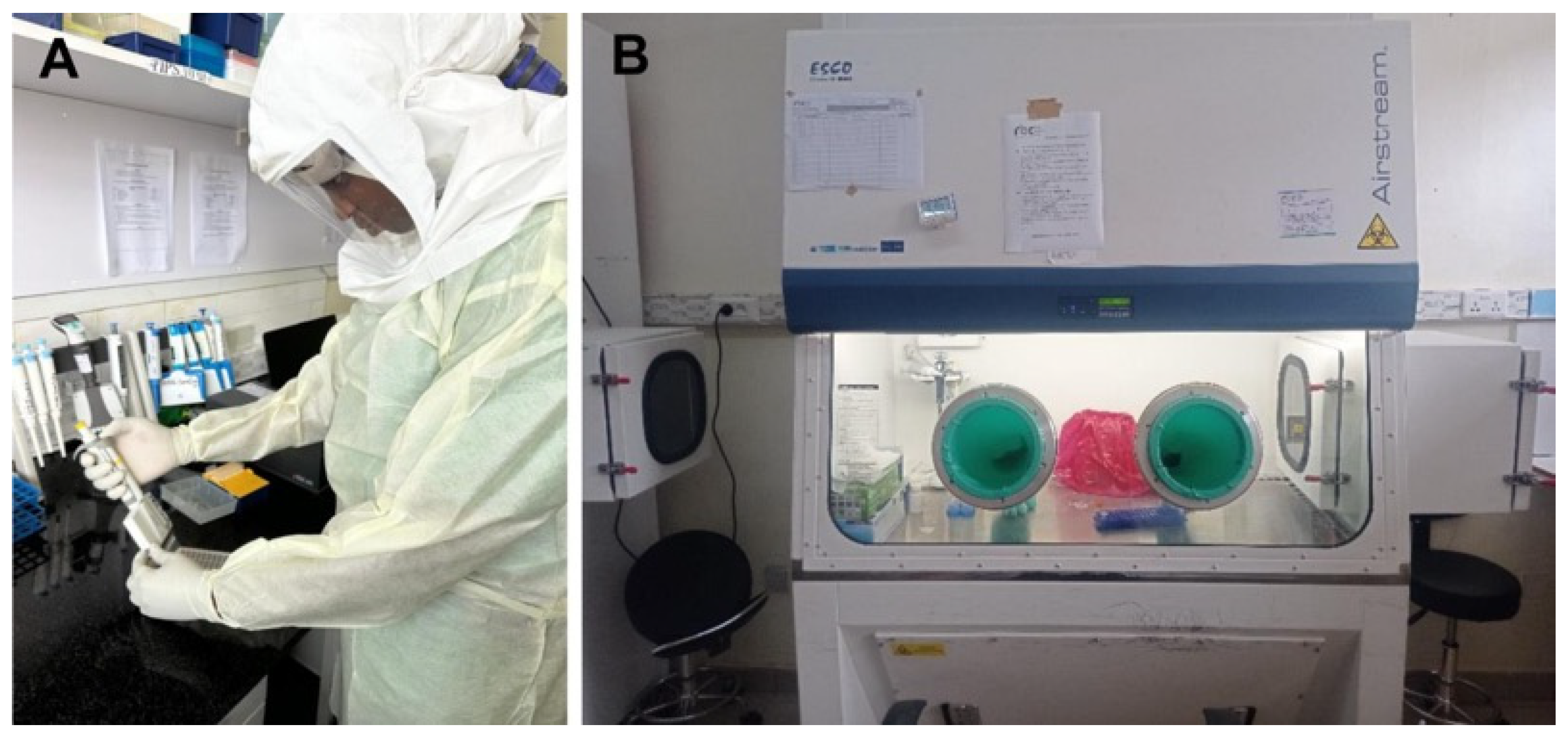
33]. For the inactivation of MARV-suspected samples, the NRL employs Class III BSCs (
Figure 3). This ensures that all procedures are conducted with the highest safety standards in place. These meticulous protocols are designed to minimize any potential exposure to the virus, thereby safeguarding both the personnel and the laboratory environment.
In addition to these safety measures, the laboratory is equipped with advanced diagnostic technologies, including real-time polymerase chain reaction (PCR) machines and conventional thermocyclers. The NRL also boasts sequencing facilities with high-capacity sequencers, all of which enable precise and timely diagnostics. This comprehensive suite of technological resources is vital in facilitating an effective and rapid response to the ongoing MARV outbreak affecting the country.
12. Materials and Reagents
Compliance with biosafety measures is critical in the sourcing and handling of materials and reagents [
34]. The NRL implements stringent criteria for selecting reagents, ensuring that they are sourced from reputable suppliers and are properly labeled. This comprehensive approach not only reduces the risk of accidents, but also enhances the laboratory’s overall safety protocols. Staff undergo thorough training to safely handle materials and reagents, equipping them with the knowledge of associated risks. The NRL maintains an inventory log to track all supplies meticulously. Furthermore, by ensuring a robust supply of properly sourced and handled materials, the NRL can consistently perform timely analytical testing (TAT) and maintain the capability to conduct necessary tests and investigations. This proactive approach supports the laboratory’s mission to deliver accurate and reliable results while safeguarding the health of both staff and the wider community. The National Reference Laboratory (NRL) relies on several critical reagents to enhance its response capability, particularly in the context of diagnosing viral and other pathogenic infections. Key reagents include nucleic acid extraction kits, PCR reagents, and specialized enzymes, all essential for ensuring the swift and accurate detection of pathogens. In our laboratory, we utilize a variety of diagnostic kits, such as those from Qiagen (Hilden, Germany), which are pivotal for rapid DNA and RNA extraction processes. Additionally, we have an extensive selection of consumables and kits designed for various applications, including rapid detection assays and confirmatory testing, allowing us to efficiently respond to public health needs and outbreaks. These resources are crucial in supporting our mission to maintain high standards of diagnostic accuracy and reliability in pathogen surveillance and response. Additionally, we use numerous types of glove boxes to enhance safety during procedures including Cleatech HEPA filtered units and germ free units.
13. Sample Collection and Transport
Proper protocols for sample collection and transport are critical for maintaining the integrity of biological specimens and ensuring safety [
27]. The NRL has established clear SOPs for the collection, labeling, and transport of samples to minimize contamination and exposure risks. Personnel are trained in using appropriate biosafety containers and following protocols that prioritize safety, thereby reducing the likelihood of infection transmission within the laboratory.
14. Waste Management
Effective waste management procedures are implemented at the NRL to safely handle potentially infectious waste. This includes the segregation, treatment, and disposal of biological waste according to local and international regulations [
35]. To ensure the highest standards of safety, the laboratory has validated its waste management protocols and trains its personnel to meticulously follow these guidelines. This ensures that all waste, including samples suspected of containing Marburg virus (MARV), is disposed of in a manner that effectively prevents any risk of exposure to infectious agents.
15. Laboratory Information System
The NRL employs a laboratory information management system (LIMS) to enhance data management and the traceability of samples. This system allows for the secure documentation of all laboratory activities including sample processing and results reporting. By maintaining accurate records, personnel can quickly trace any issues that arise.
16. Quality Management
Quality management systems at the NRL ensure that laboratory processes meet high standards of accuracy and reliability [
31,
36]. Regular audits and proficiency testing are conducted to evaluate laboratory performance. Personnel are trained to adhere to quality management principles, which help in identifying deviations from established protocols and implementing corrective actions promptly, thereby maintaining operational integrity and safety during MVD investigations.
17. Continuous Training
Training programs are also a fundamental component of the laboratory personnel’s role. Ongoing education and regular drills are conducted not only in response to the MARV outbreak, but also draw from experiences during previous and ongoing public health challenges such as the COVID-19, Ebola, and Mpox outbreaks [
37]. This continuous training ensures that all staff members are proficient in sample handling, laboratory procedures, and emergency responses [
34]. By fostering a culture of safety and preparedness, these programs empower laboratory personnel to respond swiftly and effectively in the event of accidental exposure or other emergencies.
Overall, the role of laboratory personnel in diagnosing and managing MARV cases is multifaceted and intricately linked to robust biosafety practices. Their unwavering commitment to stringent safety standards, effective utilization of protective equipment, ongoing training, and adherence to waste and transport protocols highlights their crucial role in the fight against high-risk pathogens like MARV.
18. Laboratory Staff Continuity and Sample Flow During Outbreaks
Despite the significant challenges posed by the current MARV outbreak in Rwanda [
2], the laboratory’s routine sample processing workflow and supportive services to the national disease control programs have remained uninterrupted. All routine samples for chemical pathology, hematology, parasitology, microbiology, toxicology, immunology, and virology continue to flow smoothly, allowing laboratory personnel to carry out their critical work without delay. The commitment and resilience of the staff have ensured that diagnostic and management processes for MARV cases and other diseases proceed efficiently. This enables the rapid turnaround times for test results, ultimately facilitating timely care and support for the community.
Since the beginning of 2024, the National Reference Laboratory in Rwanda has dealt with many high-risk suspected samples for various infectious diseases. The laboratory has handled 923-suspected cases of Rift Valley fever, 560 cases of dengue, 3704 cases of Mpox, and 6626 cases each of Ebola and MARV, among other infectious samples. Remarkably, throughout this period, there have been no reports of laboratory-acquired infections.
This continuity highlights the effectiveness of the laboratory’s biosafety protocols and the quality of personnel capacity and performance. It reinforces the essential role that laboratories play in outbreak management, even amidst significant public health threats.
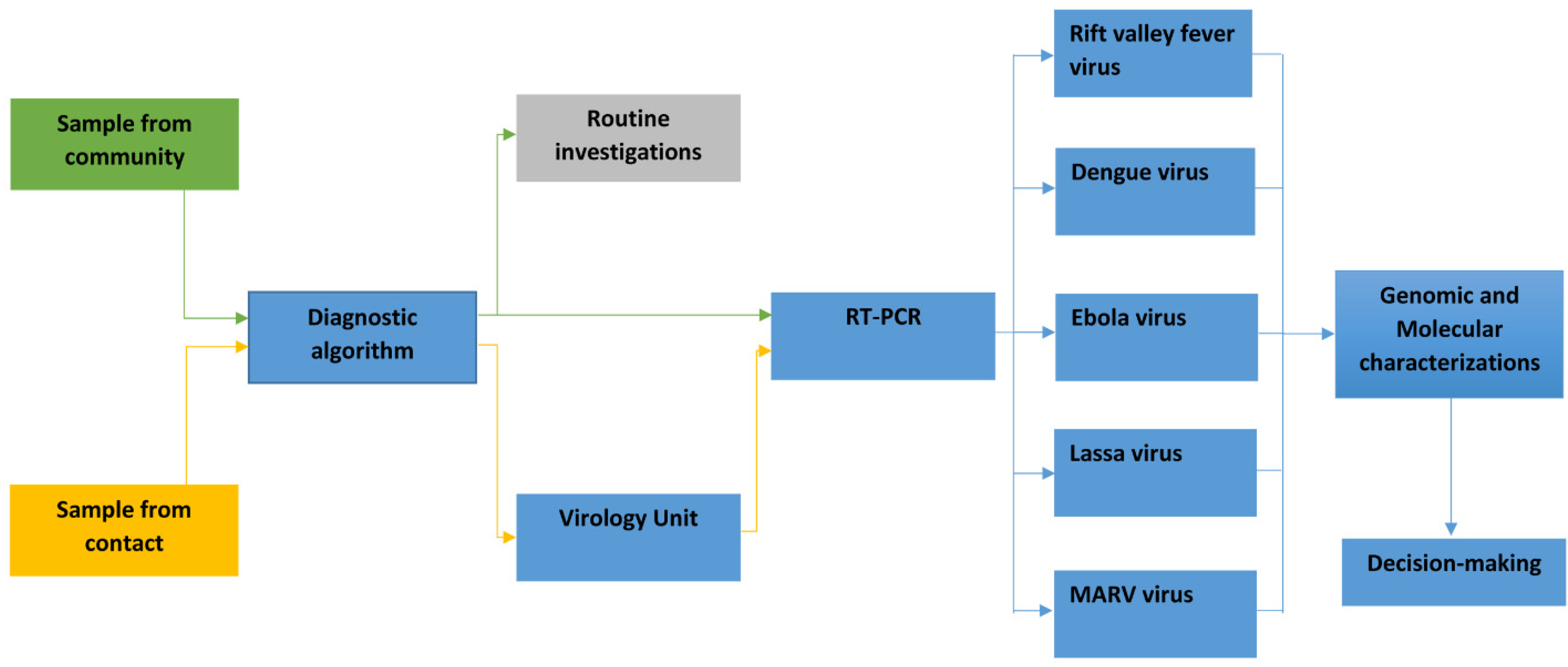
Diagnostic algorithm for prioritizing samples for specific tests and characterization:
The NRL has successfully established a diagnostic algorithm to prioritize samples, playing a pivotal role in managing public health responses to infectious diseases, particularly MARV (
Figure 4).
This algorithm enables the NRL to systematically evaluate samples obtained from various sources including community health facilities and individuals identified through the contact tracing of MARV cases. Upon receipt, each sample undergoes rigorous testing for multiple viral infections [
38], guided by factors such as epidemiological characteristics, clinical manifestations, and geographical areas with a high incidence of MARV cases. If any samples yield positive results, they are promptly forwarded to the genomic laboratory within the NRL for detailed characterization [
24,
39]. The insights gained from these analyses are vital for informing public health decisions, allowing for rapid communication to decision-makers and the Head of Department (HOD). This timely information facilitates effective intervention strategies and optimal resource allocation. A critical component of the NRL’s response capability includes ensuring the availability of reagents and diagnostic kits alongside a well-trained workforce. These elements are essential for comprehensive investigation and preparedness efforts [
23,
40]. By prioritizing samples that are most likely to indicate active transmission, the NRL enhances disease surveillance and strengthens outbreak response strategies in at-risk communities. In this way, the NRL plays an essential role in safeguarding public health and controlling the spread of infectious diseases, ultimately contributing to the preparedness and resilience of the healthcare system in the face of emerging health threats.
19. Key Lessons Learned
The MVD outbreak in Rwanda has provided several key lessons that can enhance future responses to similar health crises. Firstly, it is critical to ensure a reliable supply chain of adequate personal protective equipment (PPE) in healthcare settings. Access to quality PPE is essential for reducing the infection rates among frontline staff, making regular assessments of PPE availability crucial in preemptively addressing potential shortages.
20. Infection Prevention, Control, and Enforcing the Use of Proper PPE
The challenges faced by healthcare providers also underscore the necessity of reinforcing infection prevention and control protocols, especially in emergency and high-pressure situations. Hospitals must prioritize training for all staff on strict adherence to guidelines, even during chaotic conditions, to minimize the risks of transmission. Ongoing training programs for healthcare workers on emerging infectious diseases, the proper use of PPE, and the safe handling of potentially infectious patients are vital. Incorporating lessons learned from previous outbreaks into training modules can help ensure staff are well-prepared.
21. Building the Community Trust in the Health System and Leadership
Effective communication and the dissemination of information are equally important. The rapid spread of information regarding MARV dynamics emphasizes the need for efficient communication channels to provide timely updates on treatment protocols and infection prevention practices. This enhances the healthcare workers’ ability to respond appropriately to emerging threats.
22. Improving Healthcare Facilities Structure and Operation to Reduce Occupational Hazards
The outbreak has also revealed the necessity for hospitals to improve their environmental controls. Enhancing safety features, increasing staffing during crises, and streamlined patient flow management can prevent overcrowding and support adherence to infection control measures. Additionally, the mental and physical toll of working long hours under stressful conditions impacts compliance with safety protocols. Therefore, institutions should prioritize mental health resources and support systems for healthcare workers to help them cope with the demanding nature of their roles and reduce fatigue.
23. Streamlining Evidence-Based Decision Making
Fostering cooperation and the timely sharing of information between laboratory and clinical teams is beneficial. The contrast in outcomes for laboratory personnel and healthcare providers shows the value of joint training sessions and protocol development, enhancing their understanding of risks and safe practices across different settings. Community engagement is another vital aspect. Public health measures, including awareness campaigns, play a crucial role in fostering cooperation and compliance with health guidelines during outbreaks. Educating the community about the disease, its transmission, and the importance of seeking care could help reduce stigma and improve the outbreak response.
24. Having a Ready Preparedness and Emergency Response Action Plan
Developing robust emergency response frameworks is essential. The swift actions taken by health authorities in Rwanda highlight the need for well-defined frameworks that can be activated immediately in the event of outbreaks. These frameworks should encompass action plans for containment, resource allocation, and stakeholder coordination.
25. Flexibility in the Science-Guided Response According to Emerging Evidence
Finally, ongoing research into MARV and other high-risk pathogens is crucial for adapting response protocols as new information becomes available. Public health policies should remain flexible and responsive to the evolving understanding of disease transmission and management. By reflecting on these lessons learned, the healthcare community can better prepare for future outbreaks, thereby enhancing the safety of both healthcare providers and patients in the face of emerging infectious diseases.
26. Conclusions
The establishment and adherence to advanced standards of good laboratory practices at the Rwandan National Reference Laboratory have empowered the lab and its personnel to champion biosafety and biosecurity. This was evident by its outstanding performance during the response to the Marburg virus outbreak in the country. During this outbreak, the NRL was able to establish and coordinate with six regional labs distributed throughout the country to expand testing for the virus. Meanwhile, the routine surveillance, testing, and characterization of samples, including the implementation of diagnostic services for endemic and emerging infections such as the national disease control programs, continued conducting business as usual. A diagnostic algorithm was deployed to prioritize the testing of samples from the community for hemorrhagic fevers including the Marburg virus, Mpox, other emerging diseases, and/or endemic diseases in the country.