Abstract
This paper provides a comprehensive review of the history of laboratory-acquired infections (LAIs) from a scientific perspective on biosafety risks. It analyzes cases from the late 19th century to the 2020s, whereas the previous research on this topic has primarily focused on social factors. By combining real case studies, this study elucidates the mechanisms of LAI occurrence and development, compares the attribution of risks and mitigation measures, and establishes the scientific patterns of LAIs’ historical evolution. The details of LAI cases are compared to the biosafety risk assessment indices of the World Health Organization (WHO), the United States, and China. These real cases of LAI occurrence risks are now incorporated into biosafety standards and assessments in the modern era. Additionally, factors that pose potential risks of LAIs, even if they have not yet manifested, are also highlighted.
1. Introduction
Laboratory personnel have been exposed to the risk of pathogen infection even before the emergence of microbiology. The Centers for Disease Control and Prevention (CDC) defines a LAI as “an infection acquired by laboratory personnel during laboratory work”. In the 1950s and 1960s, Sulkin and Pike reported over 4000 cases of LAIs between 1949 and 1974, with a death rate of 4.1% [1]. The relative risk of microbiologists acquiring infections ranges from 0.03 to 8000 compared to the general population. In 2018, researchers estimated that the annual incidence rate of LAI in the US was approximately one to five cases per 1000 employees [2].
LAIs have not received the attention they deserve historically, due to the absence of legal requirements and mechanisms for data collection in many countries. Additionally, laboratory heads have been concerned about facing blame, while editors have been reluctant to publish such reports [3]. In 1994, the United Kingdom introduced the “Reporting of Injuries, Diseases and Dangerous Occurrences Regulations”, which mandated the reporting of LAIs; however, the collected information was deemed confidential and not in the public domain. In October 2016, the International Editorial Board of the American Biological Safety Association (ABSA) developed an online searchable database for LAIs, which has effectively reduced the time required to identify relevant biological risks [4]. With the constant emergence of new pathogens and the continuous updating of knowledge on existing pathogens, the research on laboratory biosafety has become increasingly in-depth. In order to better implement the relevant provisions of the Biosafety Law of the People’s Republic of China, the National Health Commission of China has, in 2023, developed a new version of the Catalogue of Human Infectious Pathogenic Micro-organisms by referencing and adopting international and domestic regulations and research achievements.
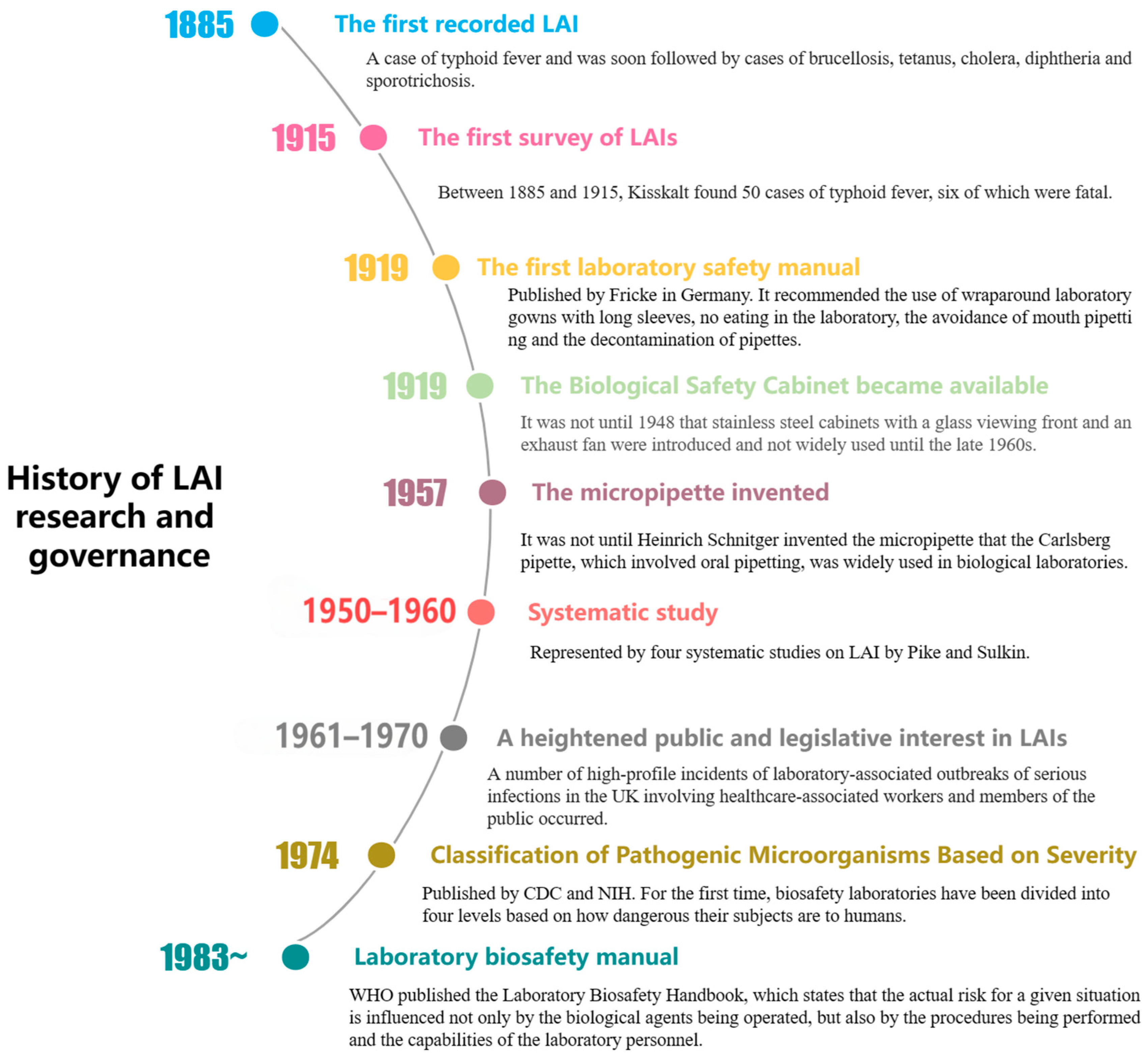
The first recorded LAI was a case of typhoid fever in 1885. Thirty years later, Kisskalt published an investigation, making it the first documented report of LAI [5]. From the 1950s and 1960s, pioneers such as Sulkin and Pike began systematically studying LAIs, and more recent research by K. Byers and L. Harding has involved numerous case investigations and epidemiological analyses of LAIs. Some studies have combined policy research and sociological discussions to view LAIs as occupational diseases or as part of the field of biosafety research. However, there is a lack of research combining the study of LAIs from a historical perspective with real case examples, and an analysis on the intrinsic relationship between LAIs and the assessment of biosafety risks. Based on real-world cases as factual data, this paper analyzes the risk factors and classification of LAIs from the perspective of biosafety science, revealing the historical evolution, key milestones, and biosafety elements of LAIs. Furthermore, the study conducts a comparative analysis of the historical causes of LAI risk factors and the scientific implications of laboratory biosafety, thereby demonstrating the compatibility between modern biosafety concepts and the development path and scientific rationale of the history of technology.
2. Early Lab Safety Challenges and Antibiotic Beginnings (1901–1947)
During the early 20th century, safety hazards were widespread in biological laboratories. The equipment was rudimentary, the environment was poor, and insects were abundant. The staff generally lacked awareness about protective measures, and it was considered normal to smoke, eat, smell cultures, and perform oral pipetting in the laboratory. Among 23 reported cases of LAIs in 1915, 16 cases were attributed to oral pipetting [5]. Although the precursor of the rubber bulb pipette, known as the Pasteur pipette, had been on the market, it was not until Heinrich Schnitger invented the micropipette in 1957 that the Carlsberg pipette, which involved oral pipetting (Figure 1), was widely used in biological laboratories, posing a significant LAI risk to the experimenters [6].

Figure 1.
“Pfc. Johnnie Mae Welton, Negro WAC, laboratory technician trainee, conducts an experiment in the serology laboratory of the Fort Jackson Station Hospital, Fort Jackson, SC”, 20 March 1944.
In addition, the promotion of antibacterial treatment began in 1928 when British bacteriologist Alexander Fleming discovered penicillin, ushering in the “antibiotic era”. A study on 25 cases of jungle typhus and murine typhus LAI between 1931 and 2000 revealed that all 8 cases of mortality occurred before the invention of antibiotics [7]. In the first 40 years of the 20th century, some LAIs were caused by pathologists and technicians during the autopsy process. Even today, histopathologists and forensic pathologists cannot be detached from clinical laboratory activities.
On 3 April 1901, in the laboratory of Dr. F.G. Novy at the University of Michigan, a student who was studying a culture of animal tissues was diagnosed with pulmonary plague. The patient had not been vaccinated but received “protective serum” in the second week of illness [8]. Fortunately, intervention was timely after exposure, and the patient was cured despite the absence of specific drugs. Penicillin was not effective against plague, and, until the discovery of streptomycin in 1943, antibacterial treatment for plague began with sulfa drugs in 1937. In 1947, a laboratory worker in Johannesburg, South Africa, working with a culture of plague-infected tissues, developed symptoms. The doctor recommended a high dose of streptomycin, the patient did not follow the advice and passed away three days later. A postmortem examination confirmed a typical plague infection. A 45-year-old woman had close contact with this patient three days before the onset of illness. The woman started taking streptomycin and received sulfa drugs and two doses of plague bacterium antiserum. She underwent penicillin treatment after a fever relapse and fully recovered thereafter [8].
3. Establishing Initial Awareness of Protection (1948–1972)
With the accumulation of LAI reports, scientists have started to design targeted protective measures. In 1905, Robert Koch first proposed the design of biological safety cabinets (BSCs) to protect workers from aerosol exposure. In 1919, Fricke published the first laboratory safety manual in Germany, recommending the use of long-sleeved laboratory protective clothing, no eating in the laboratory, and avoiding mouth pipetting. However, the improvement of the actual situation often lags behind the formulation of ideas. In 1943, the first Class III BSC was created by Hubert Kaempf and later developed with the introduction of high-efficiency particulate air (HEPA) filters. However, the “aerosol era” is likely to come to an end due to the mandatory rather than voluntary use of BSCs, such as the EU’s Council Directive on the Protection of Workers related to Exposure to Biological Agents at Work, issued in 1990 [9].
As one of the most commonly used laboratory instruments today, the handling of microbial suspensions in these devices, such as centrifuges, pipettes, and stirrers, used for processing and analyzing infectious pathogens, can generate aerosols and potential sources of infection. Insect-borne infections can also be caused by aerosol transmission when pathogens are present in the respiratory secretions of infected individuals or animals. An analysis of 66 papers covering the period of 1930–2008 on LAIs showed that 84% of insect-borne virus infections were caused by microbial aerosols. Infected individuals commonly had only basic personal protective equipment, such as masks, or even no personal protective equipment at all, and the respiratory protection provided by masks was limited. Collins C.’s book “Laboratory-acquired infections: history, incidence, causes and prevention”, published in 1988, referred to the period of 1947–1966 as the “aerosol era” of LAIs [3].
In a laboratory-acquired infection case of Western equine encephalitis virus (WEEV) in 1940, a researcher’s facial oral and nasal mucosa were exposed to a high concentration of chick embryo viral culture fluid and aerosols released due to a centrifuge accident. At the time of the accident, the researcher was not wearing goggles or a protective face mask, and he died from meningitis [10]. In 1924, American surgeon R.R. Spencer and others transformed this highly fatal disease into a preventable, nonlethal form [11]. In 1941, a virologist working with suspensions of Coxiella burnetii in egg yolk sacs in the laboratory accidentally punctured the tip of his ring finger with a needle and syringe containing this micro-organism. After applying iodine, he received a vaccination against Rocky Mountain spotted fever (Lederle).
Numerous cases of LAI occurred in the 1940s during the outbreak of World War II, when there were extensive efforts to develop scrub typhus vaccines to reduce infection rates among the Allied forces in the Asian theater of war. In April 1943, an Australian microbiologist developed a scrub typhus vaccine at the Walter and Eliza Hall Institute of Medical Research in Melbourne. The obituary of this microbiologist stated that they were one of several staff members at the institute who had been infected with murine typhus, providing strong evidence for the lack of cross-immunity between murine typhus and scrub typhus [12]. In 1951, Smadel emphasized the dangers of rickettsial research. He noted that “perhaps the most important measure in preventing and controlling laboratory infections is to raise awareness of the dangers among laboratory personnel and make them realize that minimizing the risk of infection through the adoption of proper techniques is achievable” [13].
Symptoms do not always appear immediately after the exposure to infectious substances, so pathogenic microbiology laboratories usually provide health examinations. After being transferred to the BCG department at the Danish National Serum Institute, a researcher was supposed to undergo a lung examination every 3 months according to the special agreement reached by the chest outpatient department. However, the researcher did not adhere to this plan. In 1966, infiltration was first detected in his lungs, but no anti-tuberculosis treatment was administered. Two weeks later, the lung lesions markedly regressed. Subsequently, the researcher was diagnosed with pulmonary tuberculosis [14].
In August 1967, laboratory workers in Marburg experienced a sudden onset of high fever, diarrhea, vomiting, massive hemorrhage, shock, and circulatory failure. This disease, later known as Marburg hemorrhagic fever, was subsequently identified in Frankfurt and Belgrade, with a total of 31 cases, including 7 fatalities. Among the 31 individuals, 25 were infected through direct contact with monkeys in the laboratories that were contaminated with the Marburg virus. The remaining 6 secondary infections included two doctors, a nurse, an autopsy assistant, and a veterinarian’s wife, all of whom had close contact with the primary patients. The doctors contracted the virus while drawing blood from infected individuals. Three months later, German experts identified the pathogen as a new and dangerous virus with a filamentous, rod-shaped structure, originating from monkeys in Uganda. These monkeys were initially used for poliomyelitis vaccine research and were transported to laboratories in Marburg, Frankfurt, and Belgrade.
On 1 March 1972, a 56-year-old veterinarian died of rabies infection. While working in a commercial laboratory involved in the production of antiviral vaccines, he used a kitchen blender to homogenize the brains of 11 goats infected with rabies. An investigation revealed that, at the time, the patient may have been removed his mask and placed his face directly above the blender for several minutes while transferring equal portions of infectious homogenate into different containers [15].
4. Complex Risks and Human Errors in Laboratory Work (1973–2000)
In the 1970s, the occurrence of smallpox outbreaks in the UK and incidents of healthcare workers and LAIs related to kidney dialysis, including hepatitis, increased societal attention toward LAIs. Within one year in 1970, 32 laboratories reported 127 cases of hepatitis, with the majority of infections occurring among technicians specializing in biochemistry or hematology [16]. The rate of infection among biochemical technicians decreased from the previous survey in 1970–1972, indicating an improvement in safety standards within this profession [17]. Vaccination is the primary method for pre-exposure prevention. Even with the strict adherence to biosafety protocols from 1971 to 1976, aerosols containing infectious rickettsiae resulted in nine laboratory-acquired cases of Rocky Mountain spotted fever (RMSF) among individuals who had received both primary and booster doses of the RMSF vaccine. However, newcomers to the laboratory after 1971 did not possess immunity to RMSF like the older individuals because the vaccination plan for low-risk personnel was discontinued [18].
In 1974, CDC and the National Institutes of Health (NIH) introduced the Biosafety Level (BSL) classification system, which categorizes laboratories based on the pathogenicity of the micro-organisms they handle and the corresponding protective measures required. The system comprises four levels: BSL-1 for handling non-pathogenic micro-organisms; BSL-2 for moderately hazardous pathogens requiring the use of biosafety cabinets; BSL-3 for severe respiratory pathogens necessitating stringent air containment; and BSL-4 for high-risk, lethal pathogens requiring completely sealed laboratories and the highest level of protective equipment. This system provides clear guidelines for ensuring laboratory safety. In 1975, the National Institutes of Health (NIH) in the United States established the world’s first comprehensive document on biosafety called the “NIH Guidelines for Laboratory Operations”. Under the leadership of the CDC and NIH, the first edition of “Biosafety in Microbiological and Biomedical Laboratories” (BMBL) was published in 1984. Since 1976, the incidence rate of LAIs has been decreasing due to the improved identification of known sources of exposure, increased awareness of biosafety principles among laboratory personnel, enhanced regulatory capabilities, timely and accurate reporting, and advancements in medical treatment [19]. In 1978, Pike reported a total of 258 laboratory-acquired cases of typhoid fever, including 20 deaths. However, 97% of these cases and all deaths were reported before 1955 [20]. A brief history of LAI research and governance is shown in Figure 2.
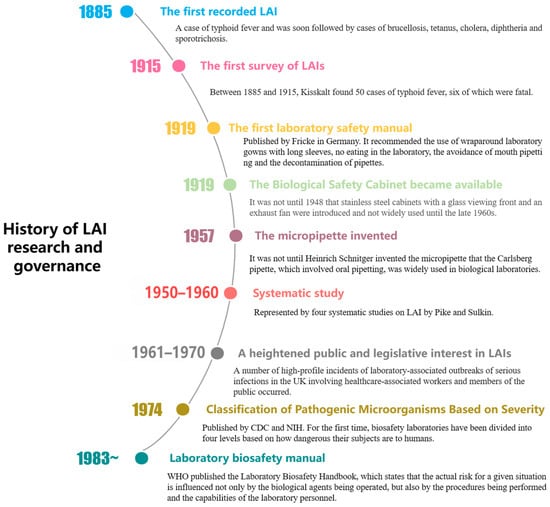
Figure 2.
Brief history of LAI research and governance.
The pathogenicity of many viruses that infect humans has been confirmed only after studying LAIs, and a lack of understanding of the pathogens is one of the main reasons for difficulties in prevention and treatments [21]. In 1978, a 26-year-old female scientist at the German Virus Research Institute became the first documented case of human infection and death from the Semliki Forest virus (SFV) strain [22].
Salmonella typhi is the causative agent of typhoid fever; the potential for lethal infections with S. typhi in clinical or teaching laboratories may not have been fully recognized. Between 1977 and 1980, there was a sudden increase in laboratory-acquired typhoid fever in the US, with 31 reported cases. At least 24 of those cases were attributed to teaching purposes. This investigation led researchers to suggest allowing students to encounter similar micro-organisms in a clinical laboratory setting [23].
The presence of mental disorders among researchers undoubtedly increases the risk of LAIs due to their unpredictable behavior. On 24 February 1982, a 52-year-old female technical expert involved in veterinary vaccine production acquired brucellosis. There were two outbreaks of brucellosis in her laboratory, and she refused testing for the disease. She had a history of three years of personality disorder and urinary incontinence, with a diagnosis of depression. While it cannot be completely ruled out that the brucellosis was intentionally caused or self-injected, it is more likely that the infection occurred through airborne transmission, given the documented outbreaks in her laboratory [24]. The difference in experience leads to varying levels of risk for laboratory personnel, which is why it is crucial for labs to prioritize training and assessment of their workers. In 1986, six newborn calves were infected with mouse-derived Cryptosporidium oocysts. A researcher enlisted the help of five veterinary students, who were new to clinical research and did not have a background in cattle farming, resulting in an infection incident [25].
As a result of accumulating cases of a specific LAI, laboratory personnel often develop routine post-exposure prevention protocols, but this consensus may not necessarily be the optimal choice. In 1988, a laboratory technician, following an accidental inoculation incident, immediately received an intramuscular penicillin injection based on the recognized post-exposure prophylaxis regimen. However, they still contracted leptospirosis. Another technician encountered the same incident and received a doxycycline injection, resulting in neither illness nor seroconversion. Consequently, a post-incident report recommended the use of doxycycline for chemoprophylaxis following exposure as it proved to be more effective in preventing leptospirosis [26].
Shortly after the introduction of a new technology or pathogen into the laboratory, researchers may not fully appreciate the potential risks, resulting in potentially inappropriate experimental procedures. For example, culture media specifically designed for non-surface growth and maximum bacterial dispersion may facilitate the presence of micro-organisms in extremely small droplets, which could penetrate deep into the lungs and cause infection [27]. The use of syringes and pipettes, due to the potential for splashing and spilling, can generate aerosols [28]. In 1988, the incidence rate of acquired Mycobacterium tuberculosis infection in 77 tuberculosis laboratories in Germany, Austria, and Switzerland was 2.63 cases per 100 person-years, which is 100 times higher than the infection rate in the general population. The risk is associated with the number of positive samples isolated by technical staff each year and the lab’s shift system. Some laboratories have implemented broth culture methods for diagnosing tuberculosis, leading to an increased infection rate of 6.74 cases per 100 person-years [29].
In 1994, an individual who had never traveled to Asia and had studied mycology at the Pasteur Institute’s teaching building was infected with the Penicillium marneffei fungus. Since 1956, over 150 cases of infection caused by this fungus have been reported in individuals with human immunodeficiency virus (HIV) infection. Even a small inoculum, such as a few fungal spores, could lead to symptomatic infection in individuals with underlying immune defects [30]. Immunocompromised laboratory personnel need to exercise extra caution when handling pathogenic micro-organisms, as this not only increases the likelihood of infection among the personnel but also restricts the application of commonly used interventions [31]. In 1997, a 30-year-old female laboratory technician accidentally acquired cutaneous leishmaniasis caused by the amastigote form of the Leishmania mexicana parasite through percutaneous inoculation. This occurred 8 months prior to the onset of systemic lupus erythematosus (SLE), which subsequently required immunosuppressive treatment [32].
In a review of exposure incidents from 1989 to 2002, only five cases of infection were attributed to bioagent exposure. Vaccination (such as anthrax and yellow fever vaccines) approved by the Food and Drug Administration (FDA) have contributed to a decrease in LAIs caused by these pathogens. Since 1990, some laboratories have also implemented safer needle systems, resulting in a low annual frequency of needlestick injuries. The number of LAIs has steadily declined since 1965. A UK-based study found that the annual incidence of infections decreased from 82.7 cases per 100,000 people in 1988–1989 to 16.2 cases per 100,000 people in 1994–1995 [33]. This reflects the improved awareness of laboratory safety among personnel, the implementation of higher-level laboratory biosafety management, and the increased efficacy of vaccines, which have gradually reduced some of the factors that previously led to LAIs. However, the complexity of experimental procedures and research subjects has also introduced new hidden risks, with human errors becoming the main cause of present-day LAIs.
Between 1971 and 2000, laboratory personnel were infected with pathogens such as Rocky Mountain spotted fever, typhoid fever, Semliki Forest virus, brucellosis, cryptosporidiosis, Creutzfeldt–Jakob disease, leptospirosis, Mycobacterium tuberculosis, Penicillium infection, and leishmaniasis. These incidents highlighted the importance of vaccination, using attenuated strains, addressing the mental health of laboratory personnel, providing professional training, assessing potential risks, chemoprophylaxis, and limiting the exposure of immunocompromised individuals. These lessons have helped improve laboratory safety management systems, enhancing the protective awareness and skills of laboratory personnel. The third part of LAI cases’ information summary is shown in Table 1.

Table 1.
LAI cases’ information summary.
5. Ongoing Improvements and Emerging Recombinant Microbial Hazards (2001–2023)
In 2000, Harding and Byers conducted an analysis of 1267 cases of LAIs that occurred after 1978. The analysis indicated a significant decrease in LAIs over the past 20 years [34]. In 2006, Harding and Byers conducted a statistical analysis and found a total of 5527 reported cases of LAIs in the literature, resulting in 204 deaths. They argued that the lack of a centralized reporting system for infections and regular evaluation of staff exposure risks makes it difficult to assess the actual occurrence of LAI events. Historically, only 18% of LAIs have been associated with specific accidents or incidents with known sources of exposure [35].
As the research on high-risk but rare pathogens advances, the demand for diagnostic laboratories familiar with such organisms becomes increasingly important. In 2002, a 43-year-old male patient died rapidly after being infected with laboratory-acquired tularemia. The patient’s healthcare facility failed to notify the microbiology and autopsy departments of the suspected clinical information regarding tularemia, resulting in a delay in the identification of the micro-organism, and 11 microbiology laboratory employees as well as 2 staff members involved in the patient autopsy were exposed to the risk. It is important to enable the quicker identification and isolation of bacteria for the referral to higher-level secure laboratories for final identification, thus improving laboratory safety [36].
Laboratory animals should be procured in accordance with the national standards and regulations outlined in GB14922.2-2011 [37], ensuring that they are free of Hantaan virus. Furthermore, regular virus screening and testing should be conducted to detect infected animals as early as possible. Despite the implementation of strict regulations and standards, there was a reported infection incident in 2004 caused by a Wistar rat that was infected at the time of purchase [38].
In November 2004, the Boston Public Health Commission (BPHC) received reports of three cases of tularemia. Laboratory researchers suspected the presence of a live attenuated strain (LVS) of Francisella tularensis, which was previously thought to be incapable of causing human disease, in their work environment [39]. In October 2004, a case of ocular vaccinia infection was reported in an unvaccinated laboratory worker in Philadelphia, USA. The patient was an immunology graduate student who was born after the cessation of routine smallpox vaccination in the early 1970s. Some institutions have eliminated vaccine requirements due to the limited efficacy of available vaccines and the challenges in maintaining consistent vaccination programs. For instance, as discussed in the context of laboratory-acquired leptospirosis, traditional prophylactic measures such as penicillin have shown limited effectiveness, leading to recommendations for alternative chemoprophylaxis with doxycycline [40]. Consequently, there are no specific recommendations on the level of preventive measures for individuals who are unvaccinated, highlighting the need for the ongoing evaluation and adaptation of laboratory safety protocols.
The study of recombinant micro-organisms emerged in the latter half of the 20th century, but the associated risks have not received sufficient attention. Some argue that laboratory personnel have a lower likelihood of falling ill after exposure to recombinant micro-organisms compared to non-recombinant ones, based on the characteristics of Escherichia coli K12. The regulatory oversight of DNA recombinant materials has been under the purview of the National Institutes of Health (NIH) in the United States from 1976 to 2010. In 2004, the first documented case of human exposure to recombinant raccoonpox virus (RCN) occurred. Due to the partial cross-reactivity with vaccinia hemagglutinin antigen in animals with RCN virus antibodies, the lab considered administering a smallpox vaccine to workers handling RCN [41]. Additionally, gain-of-function (GoF) experiments, which involve altering micro-organisms to enhance their properties (such as increased transmissibility or pathogenicity), have raised significant biosecurity and biosafety concerns. These experiments necessitate stringent regulatory oversight and risk assessment to prevent potential laboratory-acquired infections and broader public health implications.
In April 2004, a graduate student from a university in central Taiwan was infected with local dengue fever serotype 1 while researching anti-bacterial protein genes in harassing Aedes mosquitoes. The Department of Disease Control under the Ministry of Health and Welfare believed that the improper design of the mosquito breeding area in the laboratory failed to completely isolate virus-carrying mosquitoes from healthy mosquitoes. Additionally, the entrance to the mosquito-handling room did not have double screens to prevent dengue-virus-carrying mosquitoes from entering. After this incident was disclosed, the laboratory immediately halted all related research, and authorities compiled the publication “Guidelines for the Management and Operation of Invertebrate Laboratories” for all similar laboratories in the region [42].
In April 2008, a 23-year-old microbiology student in Australia had been handling live Vibrio cholerae during laboratory practical classes for four weeks. Two days before the onset of symptoms, an open triangular flask containing approximately 300 mL of overnight culture of V. cholerae was accidentally knocked over, spilling the culture onto a nearby laboratory shaker where the student had been working. As a result of this laboratory incident, the laboratory replaced the shaker plate from one without clamps to a traditional one with mechanical clamps [43].
In 2013, two pathologists in California contracted tuberculosis. Both individuals had prepared frozen sections for a lung nodule that was removed due to suspected cancer. Subsequently, it was confirmed that the nodule was a tuberculoma infested with Mycobacterium tuberculosis. As aerosolization of the TB bacilli is a common mode of transmission for healthcare workers, caution must be exercised when using handheld compressed gas coolants to freeze tissue sections. Extreme care should be taken if this technique is employed [44].
In 2014, a male experimenter in a laboratory in Hisar, India sustained an accidental cut on his right palm from fragments of a shattered ampoule while isolating BPXV strains through freeze-drying. The glass ampoule was precooled to −80 °C, causing hairline cracks in the glass, which subsequently ruptured when placed in the freeze-drying manifold. Following this incident, the laboratory conducted a review of the freeze-drying process and lowered the precooling temperature to −60°C. Measures were also taken to ensure the use of higher-quality ampoules [45].
Between 2015 and 2017, the city of New York reported 11 confirmed cases of brucellosis, resulting in 10 instances of exposure to Brucella in clinical laboratories. In four of the brucellosis incidents, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) technology was used. However, this technique has limited capabilities in identifying biological threat agents (BTAs). Clinical laboratories are required to adhere to strict safety protocols, such as handling and manipulating all slow-growing micro-organisms within a biosafety cabinet. MALDI-TOF MS technology should only be used for identification purposes once the exclusion of all biological threat agents has been confirmed [46].
In 2016, during routine experiments involving the production of HIV-1 pseudovirus, a recombinant clone was mistakenly introduced into a BSL-2 laboratory. Although BSL-2 labs are equipped with safety hoods and other protective measures, an infection still occurred. The laboratory staff member, who was only supposed to handle noninfectious vectors, inadvertently worked with a recombinant clone capable of producing infectious particles. Previous research indicated that HIV-1 produced by VSV-G-infected cells could generate phenotypically mixed virus particles with an expanded host range, potentially containing both VSV-G and full-length HIV-1 genomes. This broader virus tropism and increased infectivity could lead to accidental, unnoticed infections [47]. The incident highlighted the critical need for stringent biosafety protocols and rigorous training to prevent such occurrences.
China Animal Husbandry Group resulted in the formation of a bacterial aerosol carrying fermentation fluid. During vaccine production, the prevailing winds in the area were mainly southeast, blowing toward the Lanzhou Veterinary Research Institute, which is located downwind from the biopharmaceutical factory. The factory was ordered to conduct a comprehensive inspection and implement corrective measures, with a focus on the brucellosis vaccine workshop. The brucellosis vaccine workshop was prohibited from resuming production without prior acceptance by the industry regulatory authorities [48].
In November 2022, routine wastewater monitoring at the Bilthoven vaccine production facility in Utrecht Science Park, the Netherlands, detected the presence of wild poliovirus type 3 (WPV3) in a sample collected. This sample originated from an active WPV3-infected individual. Following detection, all employees who had contact with this wild poliovirus strain were tested, and one employee was found to be infected and subsequently isolated. The infected individual, who was asymptomatic, had received vaccination. However, they resided in a community with a vaccination coverage of less than 90%. The production facility had implemented biosecurity measures, although it remains unclear how the employee was infected [49].
Between 2001 and 2022, laboratory infection incidents involved pathogens such as Francisella tularensis, hantavirus, buffalopox virus, recombinant raccoonpox virus, rubella, Vibrio cholerae, Mycobacterium bovis, HIV-1 recombinant clone, and poliovirus. These incidents highlighted several important lessons: incorporating the identification of highly infectious pathogens into standard operating procedures, adhering to national standards for purchasing laboratory animals, vaccinating or adopting higher levels of protective measures, designing laboratories appropriately, improving equipment quality, and strengthening routine monitoring. A summary of LAI cases’ information in the fourth part is laid out in Table 2.

Table 2.
LAI cases information summary.
6. Discussion
6.1. Four Periods of LAIs’ Historical Evolution
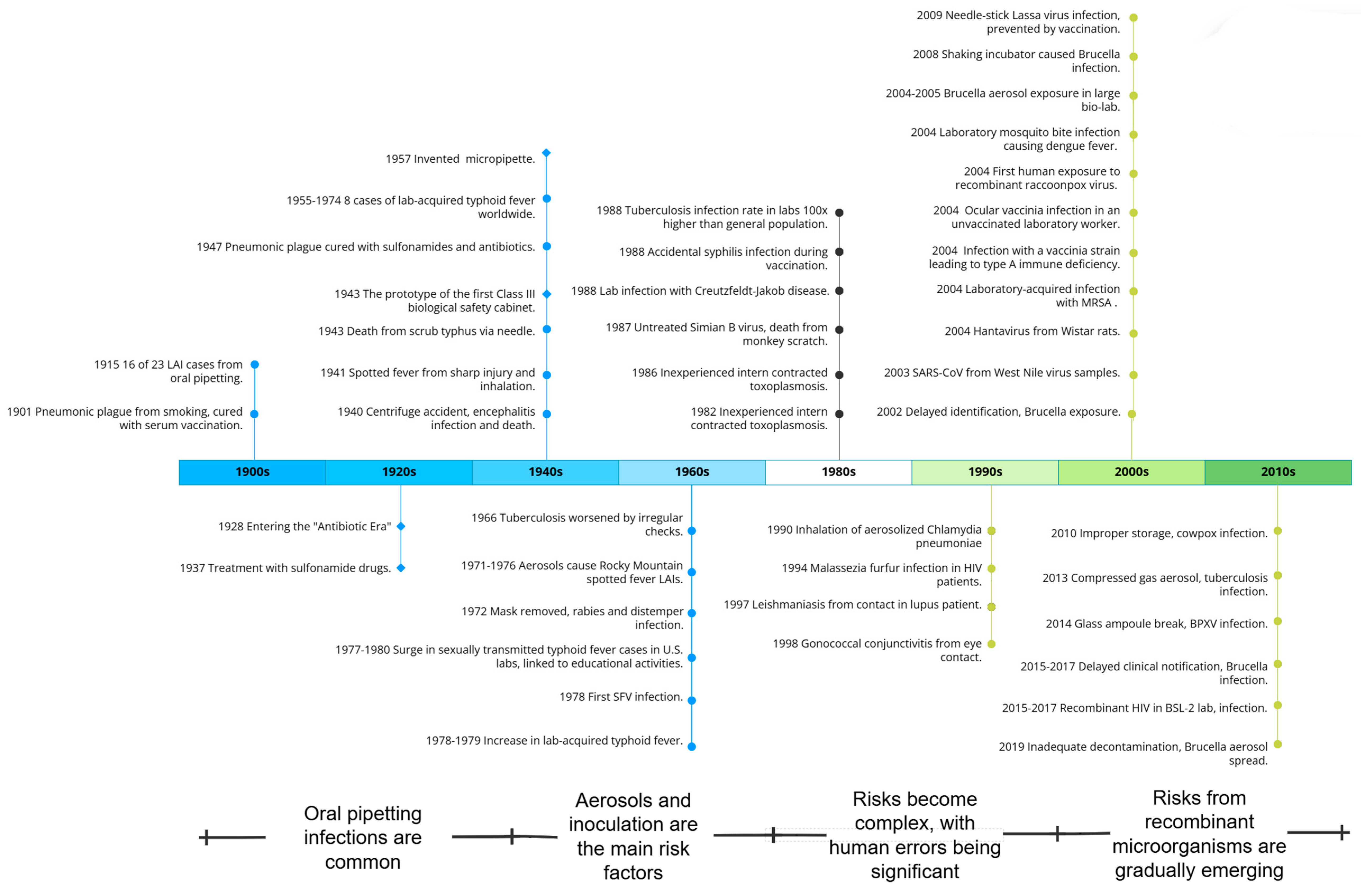
This study mainly divides the historical evolution of LAIs into four distinct periods, each characterized by different factors, including laboratory environment, researcher awareness, techniques used, protective and therapeutic measures, and study subjects. The schedule of LAI historical periods is shown in Figure 3.
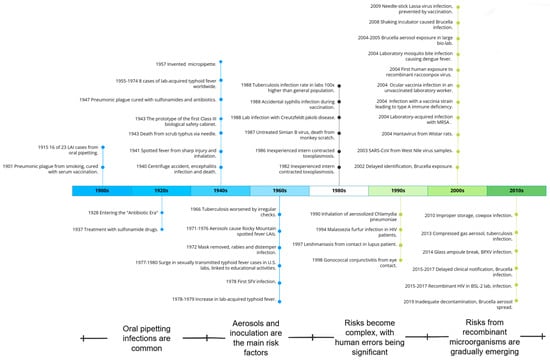
Figure 3.
LAI historical period schedule.
During the first period of LAIs, laboratory activities were in their early stages and there was a lack of awareness and treatment protocols regarding protection. Cases of infection through oral ingestion were common. The lab was poorly equipped for a long time, with no protective equipment. Practices such as eating, smoking, and oral pipetting led to significant exposure. The lack of treatment drugs and vaccines poses challenges, but prompt treatment can cure some severe infections. For example, some plague patients recovered after receiving injections of a protective serum. Over time, self-protection measures such as face masks, pipettes, and bio-security cabinets have been gradually optimized and promoted.
The second period of LAIs began with the invention and application of experimental instruments and equipment, such as high-speed centrifuges. Researchers are beginning to build an initial awareness of protection, with aerosols and injections being the main risk factors. Aerosol exposure has resulted in cases of infection with pathogens such as tuberculosis and Rocky Mountain spotted fever, and even rabies. However, the treatment of pathogen infections in humans has entered the antibiotic era, with various vaccines being developed, and significantly improved post-exposure prophylaxis capabilities.
In the third period of LAIs, the experimental activities become more diverse, the risks more complex, and there are significant human errors. Since 1978, LAIs have decreased significantly due to improved laboratory regulations, increased awareness of conservation, and equipment optimization. However, as laboratory and personnel sizes expand, work intensities increase, modern technologies are applied, and research subjects become more complex, laboratory workers face increasingly complex risks, including prominent human factors. Many inexperienced individuals, those at risk of host-specific immunity, and even those with mental disorders engage in pathogen-related work, leading to cases of previously unrecognized pathogens such as hantaviruses and Epstein–Barr virus infections. Some labs have stopped preventive vaccinations for diseases such as cowpox and Salmonella typhi, resulting in a secondary increase in LAIs from certain pathogens.
In the fourth period of LAIs, laboratory safety protection and intervention continue to be optimized, with the threat from recombinant micro-organisms increasing. In the 21st century, studies have shown a significant decrease in LAIs due to improved equipment and technology. However, with advances in recombinant micro-organism technology and synthetic biology, LAIs caused by natural pathogens such as recombinant raccoonpox virus and recombinant HIV-1 clones have been reported. Cases of the mismanagement of sample handling and cultivation of different viruses have been observed due to the expansion of laboratory scales and the diversity of subjects.
6.2. Scientific Attribution of Biosafety Risk Assessment
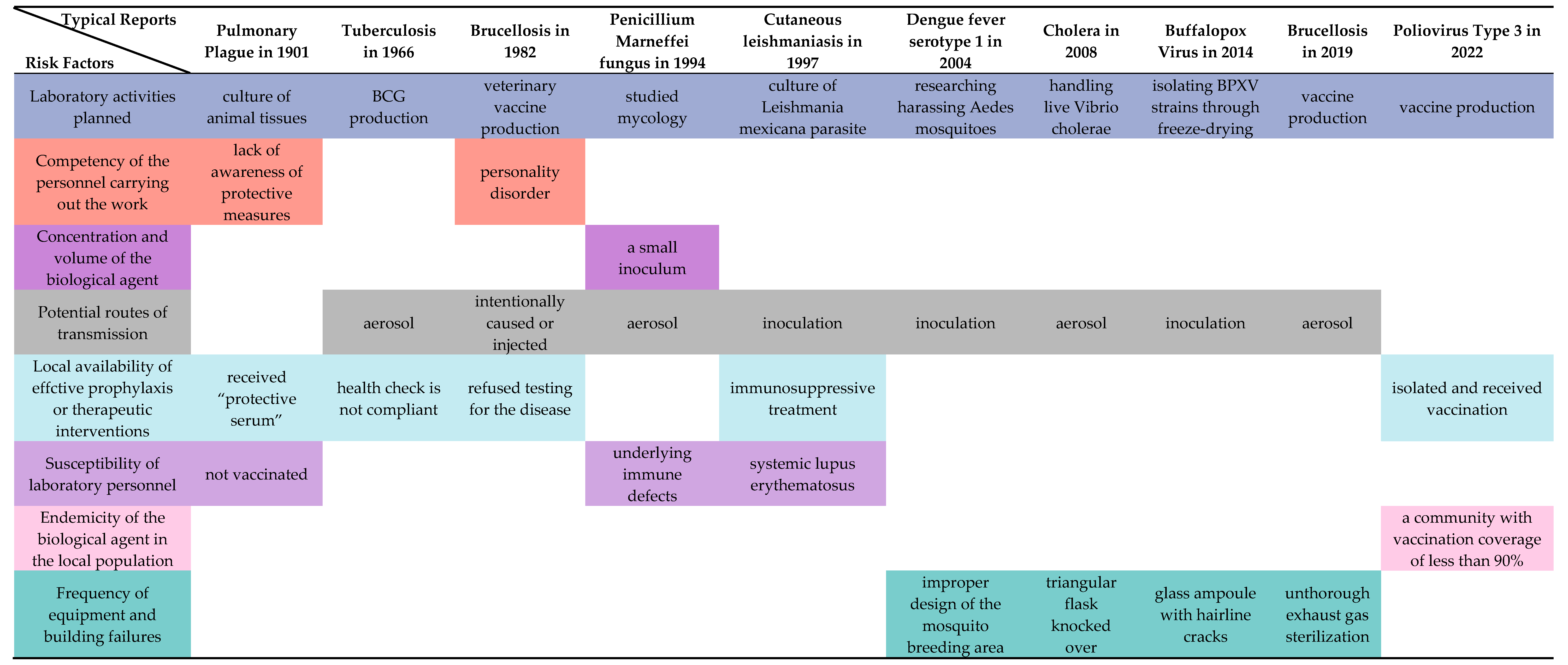
Representative risk factors in the historical development of LAIs in the technological domain are now considered key core factors for risk assessment in modern laboratory biosafety systems. The “Laboratory Biosafety Manual” (LBM) published by the WHO, the Biosafety Management Level (BMBL), and the Biosecurity Law of the People’s Republic of China (BLPRC) all emphasize the need to strengthen the management of biosafety in laboratories handling pathogenic micro-organisms. The fourth edition of the 2020 LBM comprehensively addresses the 13 major risk factors that contribute to overall risk in biological labs. These factors align with the scientific context and evolution of LAI’s historical development (Figure 4). They include considerations of the characteristics of the micro-organisms being handled, such as the concentration and volume of potentially infectious substances, the infectious dose of biological agents, the transmissibility of the biological agents, the severity of infection, the stability of the biological agents in the laboratory and external environment, and the local prevalence of the biological agents among the population. In addition, the manual highlights potential hazards related to operating procedures and the behavior of personnel.
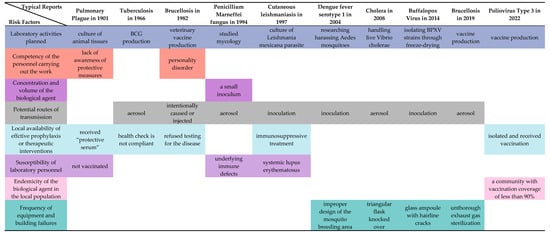
Figure 4.
Risk factors reflected in typical LAI reports. Infectious dose of the biological agent, communicability of the biological agent, severity of infection with the biological agent, range of hosts of the biological agent and Stability of the biological agent in the laboratory and external environment are related to the nature of pathogen. References are [8,14,24,30,32,42,43,48,49].
The risks associated with laboratory activities representing different stages of LAI development are adequately reflected in this study. Activities such as pipetting, centrifugation, and the use of sharp objects regularly expose laboratory personnel. As technology has advanced, different risk factors have emerged. For example, aerosols can be generated by the manual compression of a gas coolant to facilitate pathological slicing, and advanced cryogenic techniques require more intense glass equipment. The competence of the personnel involved in the performance of the task is an issue that manifests itself early on, as violations and non-compliance with medical treatment instructions can result in delayed treatment and even death. Problems with sample management, the cultivation of viruses, and human errors became major concerns as the laboratory scale grew. Another effect of the expansion of laboratory scales is the increased involvement of high-risk individuals in laboratory work, whose sensitivity becomes an individual risk factor to be considered. Take the case of the first human SFV infection and the case of an HIV patient infected with Malassezia furfur as lessons learned. The lack of cross-immunity to a source pathogen in individuals already exposed to a particular pathogen highlights the importance of studying the host range of biological agents, such as potential zoonotic diseases. In addition, as laboratory biosafety increasingly relies on equipment and facilities, failure to implement these protections can result in large-scale exposures that can even affect the surrounding community and environment.
Additionally, documents such as the LBM, the Biosafety Management Level (BMBL), and the Biosecurity Law of the People’s Republic of China highlight factors that pose potential risks of LAIs even if they have not yet manifested. For instance, LBM mentions the review of novel biological agents. The Canadian Biosafety Handbook (2nd Edition) discusses biotechnology, which involves the creation of transgenic organisms by inserting, deleting, replacing, or altering genes or gene fragments. The technique could be used to create new disease-causing organisms or enhance the virulence of existing ones. In cell lines and cell cultures, growth conditions (such as pH, temperature, and culture media supplements) can lead to changes in the expression of oncogenes, latent virus expression, interaction between recombinant genomic fragments, or alterations in cell surface protein expression, thus introducing additional risks. The Biosecurity Law of the People’s Republic of China emphasizes that relevant departments of the State Council shall trace and evaluate biotechnology application activities in accordance with the law.
7. Summary
This paper takes a historical approach, presenting historical context, providing detailed descriptions of real-world cases, and summarizing commonalities among iconic cases at different stages of development. It identifies the four stages of development and their main characteristics by analyzing the scientific and conceptual risks associated with LAIs. The development of LAIs has been intertwined with advances in biological experimental techniques, ideas, and protective measures, and has also been influenced by societal factors and healthcare standards. A study of the technological history of LAIs reveals typical cases and the evolution of risk factors in laboratory biosafety, and how it has evolved into an innovative theory for constructing a modern framework for risk assessment in this area. The evolution of LAI serves as a microcosm of the dual nature of technology, as humanity explores the unknown while also exposing itself to risks, gaining breakthroughs and facing new challenges. In response to increasingly complex and dangerous LAI incidents, more effective response measures have emerged. Behind what is now considered standard theoretical knowledge lies the experience gained through the painstaking efforts of previous researchers. Thus, the evolutionary history of LAIs is of scientific interest and merits further investigation.
Author Contributions
Conceptualization: K.Z., Z.W. and H.L.; methodology: K.Z. and H.L.; investigation: K.Z. and Z.W.; visualization: K.Z. and Z.W.; funding acquisition: H.L.; project administration: H.L. and C.Z.; supervision: H.L. and C.Z.; writing—original draft: K.Z.; writing—review and editing: H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “National Key R&D project granted by the Ministry of Science and Technology, grant number 2018YFA0902400”, “Chinese Center for Disease Control and Prevention research project, grant number BB2110240075”, and “A World History of Medicine Research Project, grant number 2023YCJC24”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gugnani, H.C.; Harbans, S.R. Laboratory-Acquired Fungal Infections—A Review. Arch. Microbiol. Immunol. 2020, 4, 51–56. [Google Scholar] [CrossRef]
- Roy, M.C.; Stevens, M.; Fidsa, F. Guide to infection control in the healthcare setting. Int. Society Infect. Dis. 2018, 2, 1–20. [Google Scholar]
- Collins, C.H. Laboratory-Acquired Infections: History, Incidence, Causes and Prevention; Butterworth & Co. Publishers Ltd.: London, UK, 1988. [Google Scholar]
- Gillum, D.; Krishnan, P.; Byers, K. A Searchable Laboratory-Acquired Infection Database. Appl. Biosaf. 2016, 21, 203–207. [Google Scholar] [CrossRef]
- Kisskalt, K. Laboratory infections with typhoid bacilli. Z. Hyg. Infekt. 1915, 80, 145–162. [Google Scholar] [CrossRef]
- Wedum, A.G. History & Epidemiology of Laboratory-Acquired Infections (in relation to the cancer research program). J. Am. Biol. Saf. Assoc. 1997, 2, 12–29. [Google Scholar]
- Blacksell, S.D.; Robinson, M.T.; Newton, P.N.; Day, N.P.J. Laboratory-acquired Scrub Typhus and Murine Typhus Infections: The Argument for a Risk-based Approach to Biosafety Requirements for Orientia tsutsugamushi and Rickettsia typhi Laboratory Activities. Clin. Infect. Dis. 2019, 68, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, R.W.; Tigertt, W.D.; Overholt, E.L. Laboratory-acquired pneumonic plague: Report of a case and review of previous cases. Ann. Intern. Med. 1962, 56, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Kiley, M.P. Clinical Laboratory Safety, Biohazard Surveillance, and Infection Control; Clinical Laboratory Medicine; Mosby-Year Book, Inc.: St. Louis, MI, USA, 1992; pp. 13–24. [Google Scholar]
- Pedrosa, P.B.S.; Telma, A.O.C. Viral infections in workers in hospital and research laboratory settings: A comparative review of infection modes and respective biosafety aspects. Int. J. Infect. Dis. 2011, 15, e366–e376. [Google Scholar] [CrossRef] [PubMed]
- Wolbach, S.B. Studies on Rocky Mountain spotted Fever. J. Med. Res. 1919, 41, 1–198. [Google Scholar]
- Anonymous. Obituary for Dora Lush. Lancet 1943, 241, 726. [Google Scholar] [CrossRef]
- Smadel, J.E. The hazard of acquiring virus and rickettsial diseases in the laboratory. Am. J. Public Health Nations Health 1951, 41, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Engbaek, H.C.; Vergmann, B.; Bunch-Christensen, K. Pulmonary tuberculosis due to BCG in a technician employed in a BCG laboratory. Bull. World Health Organ. 1977, 55, 517. [Google Scholar] [PubMed]
- Winkler, W.G.; Fashinell, T.R.; Leffingwell, L.; Howard, P.; Conomy, J.P. Airborne rabies transmission in a laboratory worker. JAMA 1973, 226, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Grist, N.R. Hepatitis in clinical laboratories: A three-year survey. J. Clin. Pathol. 1975, 28, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Grist, N.R. Hepatitis in clinical laboratories 1973–74. J. Clin. Pathol. 1976, 29, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Oster, C.N.; Burke, D.S.; Kenyon, R.H.; Ascher, M.S.; Harber, P.; Pedersen, C.E., Jr. Laboratory-acquired Rocky Mountain spotted fever: The hazard of aerosol transmission. N. Engl. J. Med. 1977, 297, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Muhammad, B.; Hafiz, M.I. Improved biosafety and biosecurity measures and/or strategies to tackle laboratory-acquired infections and related risks. Int. J. Environ. Res. Public Health 2018, 15, 2697. [Google Scholar] [CrossRef] [PubMed]
- Pike, R.M. Past and present hazards of working with infectious agents. Arch. Pathol. Lab. Med. 1978, 102, 333–336. [Google Scholar] [PubMed]
- Gaidamovich, S.Y.; Butenko, A.M.; Leschinskaya, H.V. Human laboratory acquired arbo-, arena-, and hantavirus infections. J. Am. Biol. Saf. Assoc. 2000, 5, 5–11. [Google Scholar] [CrossRef]
- Willems, W.R.; Kaluza, G.; Boschek, C.B.; Bauer, H.; Hager, H.; Schütz, H.-J.; Feistner, H. Semliki Forest Virus: Cause of a Fatal Case of Human Encephalitis. Science 1979, 203, 1127–1129. [Google Scholar] [CrossRef]
- Hoerl, D.; Rostkowski, C.; Ross, S.L.; Walsh, T.J. Typhoid Fever Acquired in a Medical Technology Teaching Laboratory. Lab. Med. 1988, 19, 166–168. [Google Scholar] [CrossRef][Green Version]
- Montes, J.; Rodriguez, M.A.; Martin, T.; Martin, F. Laboratory-Acquired Meningitis Caused by Brucella abortus Strain 19. J. Infect. Dis. 1986, 154, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Pohjola, S.; Oksanen, H.; Jokipii, L.; Jokipii, A.M.M. Outbreak of Cryptosporidiosis among Veterinary Students. Scand. J. Infect. Dis. 1986, 18, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Gilks, C.F.; Lambert, H.P.; Broughton, E.S.; Baker, C.C. Failure of penicillin prophylaxis in laboratory acquired leptospirosis. Postgrad. Med. J. 1988, 64, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Long, E.R. The hazard of acquiring tuberculosis in the laboratory. Am. J. Public Health Nations Health 1951, 41, 782–787. [Google Scholar] [CrossRef]
- Phillips, G.B.; Bailey, S.P. Hazards of mouth pipetting. Am. J. Med. Technol. 1966, 32, 127–129. [Google Scholar] [PubMed]
- Müller, H.E. Laboratory-acquired mycobacterial infection. Lancet 1988, 332, 331. [Google Scholar] [CrossRef]
- Hilmarsdottir, I.; Coutellier, A.; Elbaz, J.; Klein, J.M.; Datry, A.; Guého, E.; Herson, S. A French case of laboratory-acquired disseminated Penicillium marneffei infection in a patient with AIDS. Clin. Infect. Dis. 1994, 19, 357–358. [Google Scholar] [CrossRef]
- Malhotra, R.; Karim, Q.; Acheson, J. Hospital-acquired adult gonococcal conjunctivitis. J. Infect. 1998, 37, 305. [Google Scholar] [CrossRef]
- Knobloch, J.; Demar, M. Accidental Leishmania mexicana infection in an immunosuppressed laboratory technician. Trop. Med. Int. Health 1997, 2, 1152–1155. [Google Scholar] [CrossRef]
- Singh, K.; Weinstein, R.A. Laboratory-Acquired Infections. Clin. Infect. Dis. 2009, 49, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.L.; Karen, B.B. Epidemiology of laboratory-associated infections. Biol. Saf. Princ. Pract. 2006, 4, 53–77. [Google Scholar]
- Campbell, M.J. Characterizing Accidents, Exposures, and Laboratory-Acquired Infections Reported to the National Institutes of Health’s Office of Biotechnology Activities (NIH/OBA) Division under the NIH Guidelines for Work with Recombinant DNA Materials from 1976–2010. Appl. Biosaf. 2015, 20, 12–26. [Google Scholar] [CrossRef]
- Shapiro, D.S.; Donald, R.S. Exposure of laboratory workers to Francisella tularensis despite a bioterrorism procedure. J. Clin. Microbiol. 2002, 40, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- GB14922.2-2011; Laboratory Animal—Microbiological Standards and Monitoring; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of China: Beijing, China, 2011.
- Liu, X.Y.; Xue, K.N.; Rong, R.; Zhao, C.H. Fault tree analysis: Investigation of epidemic hemorrhagic fever infection acquired in animal laboratories in China. Biomed. Environ. Sci. 2016, 29, 690–695. [Google Scholar] [PubMed]
- Barry, M.A. Report of Pneumonic Tularemia in Three Boston University Researchers; Boston, Communicable Disease Control, Boston Public Health Commission: Boston, MA, USA, 2005. [Google Scholar]
- Lewis, F.M.; Chernak, E.; Goldman, E.; Li, Y.; Karem, K.; Damon, I.K.; Henkel, R.; Newbern, E.C.; Ross, P.; Johnson, C.C. Ocular Vaccinia Infection in Laboratory Worker, Philadelphia, 2004. Emerg. Infect. Dis. 2006, 12, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Rocke, T.E.; Dein, F.; Fuchsberger, M.; Fox, B.C.; Stinchcomb, D.T.; Osorio, J.E. Limited infection upon human exposure to a recombinant raccoon pox vaccine vector. Vaccine 2004, 22, 2757–2760. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-S.; Wu, W.-C.; Kuo, H.-S. A Three-Year Experience to Implement Laboratory Biosafety Regulations in Taiwan. Appl. Biosaf. 2009, 14, 33–36. [Google Scholar] [CrossRef]
- Huhulescu, S.; Leitner, E.; Feierl, G.; Allerberger, F. Laboratory-acquired Vibrio cholerae O1 infection in Austria, 2008. Clin. Microbiol. Infect. 2010, 16, 1303–1304. [Google Scholar] [CrossRef]
- Duray, P.H.; Flannery, B.; Brown, S. Tuberculosis Infection from Preparation of Frozen Sections. N. Engl. J. Med. 1981, 305, 167. [Google Scholar]
- Riyesh, T.; Karuppusamy, S.; Bera, B.C.; Barua, S.; Virmani, N.; Yadav, S.; Vaid, R.K.; Anand, T.; Bansal, M.; Malik, P.; et al. Laboratory-acquired Buffalopox Virus Infection, India. Emerg. Infect. Dis. 2014, 20, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Ackelsberg, J.; Liddicoat, A.; Burke, T.; Szymczak, W.A.; Levi, M.H.; Ostrowsky, B.; Hamula, C.; Patel, G.; Kopetz, V.; Saverimuttu, J.; et al. Brucella exposure risk events in 10 clinical laboratories, New York City, USA, 2015 to 2017. J. Clin. Microbiol. 2020, 58, e01096-19. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Alteri, C.; Scarlatti, G.; Bertoli, A.; Tolazzi, M.; Balestra, E.; Bellocchi, M.C.; Continenza, F.; Carioti, L.; Biasin, M.; et al. Occupational HIV infection in a research laboratory with unknown mode of transmission: A case report. Clin. Infect. Dis. 2017, 64, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, Z.; Wang, C.; Chen, H.; Wang, M.; Wei, K.; Li, Z.; Liu, Z. Laboratory-acquired Brucella infection and S2 vaccine infection events in China. Med. Environ. Sci. 2020, 1–8. [Google Scholar] [CrossRef]
- Ryerson, A.B.; Lang, D.; Alazawi, M.A.; Neyra, M.; Hill, D.T.; George, K.S.; Fuschino, M.; Lutterloh, E.; Backenson, B.; Rulli, S.; et al. Wastewater Testing and Detection of Poliovirus Type 2 Genetically Linked to Virus Isolated from a Paralytic Polio Case-New York, March 9–October 11, 2022. MMWR Morb. Mortal Wkly Rep. 2022, 71, 1418–1424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).