1. Introduction
Since its introduction, oncoplastic surgery has revolutionized the management of breast cancer by integrating oncological radicality with good esthetic outcomes [
1]. This multidisciplinary approach, that combines the expertise of breast surgeons, plastic surgeons, and physical rehabilitation specialists, aims to provide patients with the best possible oncological results while preserving esthetic outcomes. Traditionally, breast cancer surgery, particularly mastectomy, has resulted in suboptimal cosmetic outcomes and significant psychological distress [
2,
3]. Oncoplastic surgery focused the attention between obtaining a satisfactory oncological results and adequate esthetic breast appearance [
4].
To date, the introduction of local perforator flaps, such as the lateral intercostal artery perforator (LICAP) flap and anterior intercostal artery perforator (AICAP) flap, has revolutionized oncoplastic surgery by offering a more refined approach to breast reconstruction with satisfying esthetic results [
5,
6].
These peri-mammary local perforator flaps are based on the intercostal perforator arteries and allow breast reconstruction for patients with small-to-medium-sized breast who do not require extensive tissue transfer. Moreover, unlike fasciocutaneous and musculocutaneous flaps, local perforator flaps involve the exclusive use of skin and subcutaneous tissue, minimizing donor-site morbidity, while also decreasing the risk of damage to underlying nerve and muscle structures [
7].
The aim of this study is to evaluate the clinical experience of patients treated with LICAP and AICAP flaps for immediate breast reconstruction following quadrantectomy for breast cancer. The study analyzes surgical outcomes, esthetic results, and patient satisfaction with these techniques.
2. Materials and Methods
This retrospective, monocentric study of a prospectively maintained anonymous database was conducted from September 2023 to March 2024 at the Breast Unit of ASST Santi Paolo e Carlo Hospital in Milan, Italy. A total of 25 female patients were included.
2.1. Patients’ Data Selection
All patients whose anonymized data were included in the study were managed according to our standard institutional protocol, which incorporated a comprehensive preoperative evaluation and standardized surgical workflow. This included a thorough physical examination, detailed medical history assessment, and multimodal imaging—breast ultrasonography, X-rays, and magnetic resonance imaging—to precisely delineate tumor extent and detect multifocal disease. Histopathological confirmation was obtained via core needle biopsy.
Each case underwent a multidisciplinary evaluation, conducted by plastic surgeons, pathologists, physiatrists, and clinical and radiation oncologists, who also assessed the potential need for adjuvant radiotherapy. The indication for radiotherapy was, for each case, discussed with the patient to evaluate their willingness to undergo treatment and ensure an individualized therapeutic approach. Based on this assessment, the most appropriate treatment plan was devised.
Preoperative planning further involved selecting the optimal perforator flap based on the anticipated volume of tissue resection. Vascular mapping was performed using either contrast-enhanced CT scanning or a handheld Doppler ultrasound device, depending on specific patient-related criteria. Contrast-enhanced CT was preferred in cases requiring precise anatomical visualization, such as in patients with a history of previous abdominal or thoracic surgeries that could alter vascular anatomy, or when deep perforator vessels were suspected. Conversely, a handheld Doppler ultrasound device was utilized in patients with no history of major abdominal or thoracic surgeries, and when superficial perforators were deemed sufficient for flap perfusion. Additionally, patients with contraindications to iodinated contrast agents, such as renal impairment (estimated glomerular filtration rate < 60 mL/min/1.73 m2) or known contrast allergies, were evaluated exclusively with Doppler ultrasound.
Intraoperatively, margin status was assessed through frozen section analysis to ensure complete oncologic resection.
After surgery, patients were routinely evaluated following our outpatient department policy using the BREAST-Q questionnaire both at the immediate postoperative period and at the 3-month follow-up examination, allowing for a standardized assessment of early esthetic and functional outcomes.
2.1.1. Inclusion Criteria
Female patients treated for early-stage breast with unilateral quadrantectomy and immediate breast reconstruction using either LICAP or AICAP flaps.
Absence of evidence of multifocal disease or satellite lesions.
Negative surgical margins.
Total tissue volume resected during surgery between 20% and 30%.
Total breast tumor diameter < 2 cm.
Lower, inner lower, outer superior, or outer lateral tumor location.
Small (250 mL) to medium (500 mL) breast volume with no significant breast ptosis.
BMI between 20 kg/m2 and 32 kg/m2.
2.1.2. Exclusion Criteria
Large breast volume (>1000 mL).
Total breast tumor diameter > 2 cm.
Skin infection or chronic skin diseases that could impair flap healing.
Paget’s disease.
Positive intraoperative surgical margins.
Total tissue volume resected during surgery higher than 30%.
Multifocal breast cancer.
Active smokers.
BMI < 20 kg/m2 or >32 kg/m2.
Patients requiring 2-stage breast reconstruction.
All data used in this study were routinely collected and stored in a fully anonymized database, ensuring patient confidentiality and compliance with data protection regulations. Anonymized clinical data collection was conducted with prior patient acceptance, as routinely undertaken in our surgical department, as part of our department standard clinical practice. As the study involved a retrospective analysis of de-identified data without any direct patient intervention or modification of standard care, it did not require ethical committee approval. Furthermore, all patients had previously provided informed consent for data collection for research use. The study was conducted in full accordance with the Italian and European Ethical Conduct in Human Research guidelines, as well as the principles of the 1964 Helsinki Declaration and its subsequent amendments.
Patient data included demographics, defect location, cancer type, oncological radicality, and the type of flap used for reconstruction. The surgical and esthetic outcomes were evaluated based on the following:
1. Reoperation Rate: Frequency of any complications requiring additional surgery, including flap loss or infection.
2. Oncological Outcomes: Assessment of surgical margins and oncological radicality, including negative margins and the complete resection of the tumor.
3. Esthetic and Functional Outcomes: The BREAST-Q scale was administered in the immediate postoperative period and 3 months postoperatively.
4. Flap Viability and Complications: Flap viability was monitored postoperatively to assess any complications such as necrosis, infection, or hematoma. Flaps were evaluated at regular intervals, including immediate postoperative assessment, daily clinical examinations during hospitalization, and follow-up visits at one week, one month, and three months postoperatively. Partial necrosis was assessed based on clinical signs such as skin discoloration (with or without erythema), delayed capillary refill (>2 s), and tissue breakdown. Fat necrosis was defined as firm, non-tender, or tender nodules within the flap, often with associated induration. Diagnosis was based on palpation and, when necessary, confirmed by ultrasonography to differentiate fat necrosis from seroma or hematoma, eventually revealing hyperechoic areas with posterior shadowing or cystic degeneration.
2.2. Surgical Technique
2.2.1. LICAP Flap
The LICAP flap is based on perforators that arise from the coastal segment of the intercostal arteries, which supply the lateral chest wall. These perforators are typically located between the anterior axillary line and the midline of the chest. The LICAP flap is most often used for reconstructing defects located in the outer (superior and lateral) quadrant of the breast, although it can be adapted to other regions of the breast depending on the location of the volume defect.
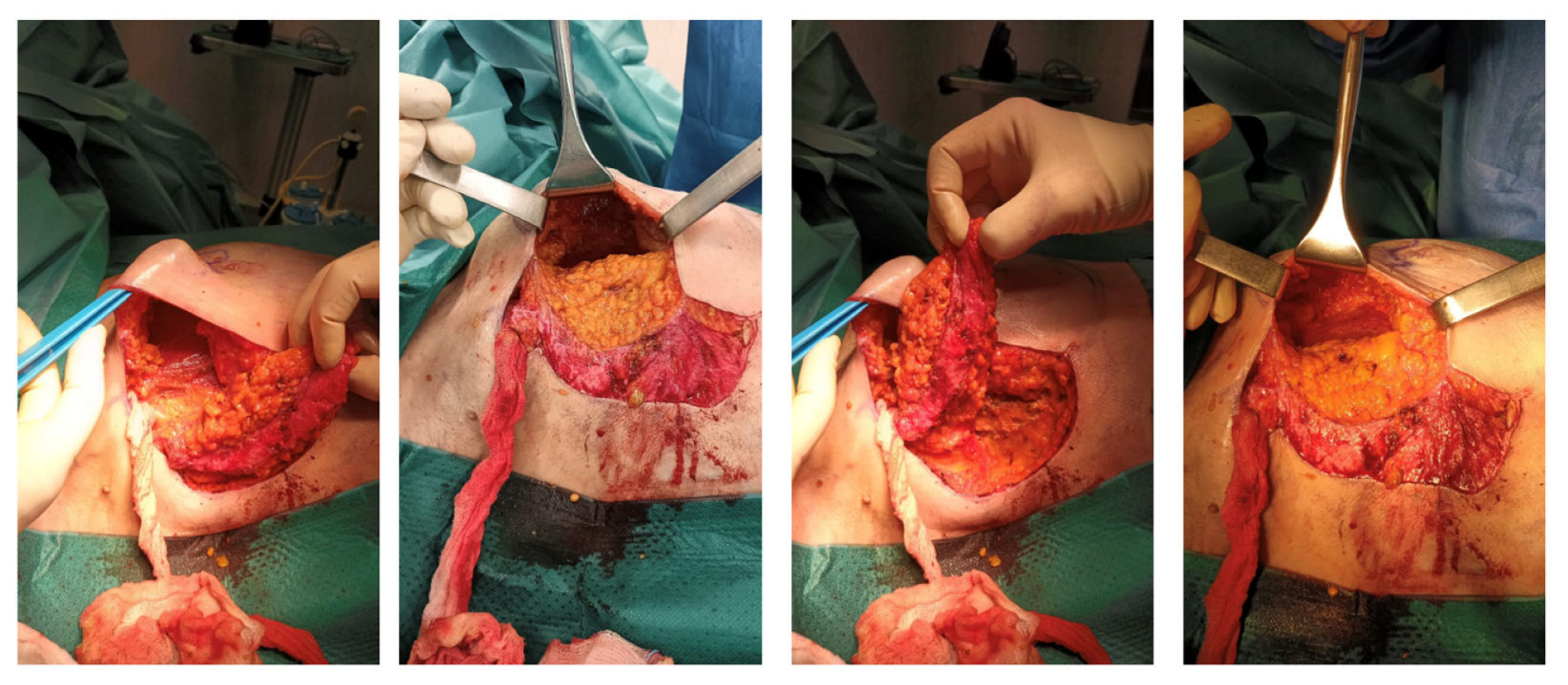
The surgical procedure for harvesting an LICAP flap begins with preoperative mapping of the perforators of the posterior intercostal artery usually located at the 5th intercostal space using CT angiography or, in case of infeasibility of contrast-enhanced imaging, Doppler ultrasound. The patient is positioned supine, with the arm on the affected side elevated at a 90-degree angle to maximize exposure to the lateral chest wall. The skin incision is made along the lateral chest wall behind the posterior axillary line, typically spanning from the second to the sixth intercostal space depending on the extent of the defect and the available tissue, to facilitate primary closure.
The main objective is to intercept the lateral cutaneous branch of the internal costal artery.
Once the incision is made, careful dissection is performed to expose the perforators. The lateral intercostal arteries are identified as they pass through the serratus anterior and latissimus dorsi muscles. The vascular pedicle is preserved by dissecting around the perforators, paying attention to the sensory nerves near the vascular pedicle. The flap is elevated from the chest wall, and the tissue is transposed to the mastectomy defect, where it is carefully shaped to restore the breast’s volume and contour. The donor site is then closed primarily, taking care to minimize tension and avoid potential wound dehiscence.
2.2.2. AICAP Flap
The AICAP flap, based on perforators from the anterior intercostal artery, is typically used for reconstructing defects in the lower and inner lower breast quadrants, such as those involving the inframammary fold or sub-mammary region. The main perforator that arises from the anterior intercostal artery is located about 1 cm lateral to the sternal cartilage, which provides an anatomical advantage for reconstructing the lower portion of the breast.
However, despite anatomical variability, there are multiple smaller-sized perforators along the anterior chest wall that can be used in case of flap design variation.
The surgical technique for harvesting the AICAP flap is similar to the LICAP flap. The patient is positioned supine, and the incision is marked along the inframammary fold, following the natural curve of the breast. Preoperative mapping of the perforators is performed using contrast-enhanced CT scanning or Doppler ultrasound, which allows the surgeon to identify the best vessels to be used for flap elevation. The incision is typically made along the inframammary fold or slightly obliquely, depending on the location and size of the defect. Once the skin incision is made, the underlying tissues are carefully dissected along the fifth to the seventh costal margins to expose the perforators passing through the pectoralis major. The flap is then elevated from the underlying tissues and transposed to the breast defect, where it is reshaped. As with the LICAP flap, the donor site is closed primarily with minimal tension to optimize the cosmetic result (
Figure 1).
2.2.3. Comparison of LICAP and AICAP Flaps
The choice of flap mainly depends on the location and extent of the removed breast tissue.
One of the key advantages of both flaps is their local nature, meaning that no microsurgical anastomosis is required, which reduces operative time and the risk of complications such as graft failure or thrombosis. Both flaps also have minimal donor-site morbidity when compared to more invasive procedures, such as free flaps from the abdomen or thigh, avoiding the sacrifice of motor nerves or underling nerves. In addition, the use of these local flaps often results in superior esthetic outcomes, particularly when the defect involves the upper or lower quadrants of the breast, where the flap design can be customized preoperatively to closely match the natural breast contour [
8].
Both the LICAP and AICAP flaps offer reliable, esthetic results for oncoplastic breast reconstruction, but they are suited to different types of breast defects. The LICAP flap is typically used for upper outer quadrant defects, where the lateral chest wall provides a reliable vascular source for tissue transfer. Moreover, this flap is suited for patients with a BMI between 20 and 30 kg/m
2 with the need of a large-volume reconstruction, often required in cases of previous mastectomy. LICAP may also be preferred in case of previous lumbar-area radiotherapy treatments, diminishing the risk of suffering from radiation-induced tissue damage or poor healing [
9]. On the other hand, the AICAP flap is more appropriate for lower-quadrant defects, where the perforators from the anterior intercostal arteries provide a direct blood supply to the tissue.
The AICAP flap is also preferred in cases of patients with higher BMIs (>30 kg/m
2) with smaller tissue loss, where smaller amounts of tissue are required to restore the original breast volume [
10].
The main limitation of both LICAP and AICAP flaps is the limited volume of tissue available. For patients with larger defects requiring total breast reconstruction, or in case of defect of the medial breast, these flaps may not provide sufficient coverage or volume, so other techniques, such as implant-based reconstruction, free-flap techniques, or hybrid techniques, may still be necessary [
11] (
Figure 2).
3. Results
A total of 25 patients underwent breast reconstruction using intercostal artery perforator (ICAP) flaps, with a mean age of 45 years (range 32–62 years). The mean BMI of the cohort was 26.1 kg/m2 (range 20–32 kg/m2). Oncologic diagnoses included invasive ductal carcinoma, 20 (80%), invasive lobular carcinoma, 3 (12%), and mixed carcinoma, 2 (8%). Of the 25 patients, 25 (100%) had undergone quadrantectomy as part of their breast cancer treatment. Additionally, six (24%) of the patients had received radiation therapy. Notably, while 12 patients were initially recommended for postoperative radiotherapy based on oncologic guidelines, 6 ultimately declined treatment due to personal reasons, including concerns about potential side effects, logistical difficulties (such as long travel distances to radiotherapy centers, lack of transportation, or conflicts with work and family responsibilities), and personal preferences. All reconstructions were unilateral. Among these, 21 patients (84%) were reconstructed with LICAP flaps, while 4 patients (16%) received AICAP flaps. The distribution of breast defects was as follows: upper outer quadrant—12 patients (48%), lower outer quadrant—9 patients (36%), and lower inner quadrant—4 patients (16%). Oncological outcomes were highly favorable, with negative resection margins achieved in all cases (100%), confirming the completeness of oncological resections. None of the patients required reoperation for oncological reasons during the follow-up period.
All 25 flaps remained viable postoperatively, with no cases of flap necrosis or infection, resulting in a 0% complication rate related to flap loss or infection. There were no reports of hematoma formation, wound dehiscence, or other donor-site complications (
Table 1).
Early esthetic and functional outcomes were quantitatively assessed using the BREAST-Q scale. In the immediate postoperative period and at the 3-month follow-up, the BREAST-Q was administered to assess various esthetic and quality-of-life parameters in patients undergoing immediate breast reconstruction. The results revealed improvements in both esthetic outcomes and patient-reported quality of life over time.
To better analyze the improvement in the analyzed BREAST-Q variables and to enhance the interpretability of the results, we performed a paired statistical analysis. BREAST-Q parameters were found to be normally distributed based on the Shapiro–Wilk test (p > 0.05), and a paired t-test was completed for each variable.
In the immediate postoperative period, the mean score for overall satisfaction with breast was 90.0 (SD 3.5). By 3 months, the mean increased to 95.0 (SD 2.8). At the 3-month follow-up, 80% of patients rated their satisfaction as “Good” to “Excellent,” compared to 60% in the immediate postoperative period.
Using a paired t-test, the mean difference was 5.0 with an estimated standard error of 0.578, yielding a t-value of 8.65 (df = 24, p < 0.001), showing a significant improvement.
Similarly, quality-of-life scores also showed significant improvement. In the immediate postoperative period, 60% of patients rated their quality of life as “Good” to “Very Good,” which increased to 75% at 3 months. The mean score improved from 80.0 (SD 5.5) to 85.0 (SD 4.0), resulting in a mean difference of 5.0 and a t-value of approximately 5.48 (df = 24, p < 0.001).
In terms of emotional well-being, 60% of patients reported satisfaction in the immediate postoperative period, increasing to 72% at 3 months. The mean score for emotional well-being increased from 76.5 (SD 5.5) to 82.0 (SD 4.5), with a mean difference of 5.3 and t-value of approximately 6.03 (df = 24, p < 0.001). Moreover, over 70% of patients indicated that the surgery positively impacted their self-esteem, expressing greater confidence in their appearance.
Regarding the other BREAST-Q domains, for symmetry, 60% of patients rated their breast symmetry as “Good” to “Excellent” in the immediate postoperative period, while at 3 months, this increased to 80%, indicating a marked improvement. The mean score for symmetry in the immediate postoperative period was 88.5 (SD 4.5), while at 3 months, it was 94.0 (SD 3.5), associated with a t-value of 6.92 (df = 29, p < 0.001). In terms of the shape/texture of the reconstructed breast, 65% of patients described the breast as “Natural” in shape and texture in the immediate postoperative period with a mean difference from the immediate postoperative period and the 3-month evaluation of 6.0 and a t-value of 7.56 (df = 25, p < 0.001).
Regarding breast contour, 60% of patients rated the contour as “Acceptable” to “Very Good” in the immediate postoperative period, with a 68% rating, similar to that at the 3-month follow-up. The mean score for breast contour increased from 86.0 (SD 3.7) in the immediate postoperative period to 91.0 (SD 3.2) at 3 months, with a mean difference of 5.0 and a t-value of approximately 6.85 (df = 25, p < 0.001).
Similarly, body image satisfaction showed an increase from 55% of patients reporting being “Satisfied” to “Very Satisfied” in the immediate postoperative period to 75% at 3 months. The mean difference for body image satisfaction was 6.9 with a t-value of approximately 5.4 (df = 29, p < 0.001). Notably, 65% of patients reported a significant improvement in their quality of life, describing a reduction in the psychological distress often associated with breast cancer treatment and the physical changes resulting from quadrantectomy. Furthermore, 60% of patients highlighted that the reconstruction led to an improvement in their emotional state, with several commenting on how the esthetically pleasing results of the surgery helped them regain a sense of normalcy and femininity.
In terms of physical well-being (breast), 65% of patients rated their physical recovery and comfort as “Good” to “Very Good” in the immediate postoperative period, which increased to 72% at the 3-month follow-up. The mean score increased from 74.0 (SD 6.0) to 80.5 (SD 5.57) with a mean difference of 6.5 with a t-value of 4.5 (df = 26, p < 0.001).
To further support patients’ satisfaction with body appearance, satisfaction with scar appearance improved from 60% of patients rating it as “Good” to “Very Good” in the immediate postoperative period to 70% at 3 months. The mean score for scar appearance increased from 80.0 (SD 5.5) to 85.5 (SD 5.0), with a mean difference of 5.5 and t-value of 5.03 (df = 26,
p < 0.001). Satisfaction with the donor site also increased, with 60% of patients rating it as “Good” to “Very Good” in the immediate postoperative period, increasing to 70% at 3 months. The mean score increased from 78.5 (SD 5.0) to 83.0 (SD 4.5), with a mean difference of 4.8 and a t-value of 4.59 (df = 23,
p < 0.001) (
Figure 3).
Physical well-being (general) showed a similar trend, with 65% of patients rating their overall physical well-being as “Good” to “Very Good” in the immediate postoperative period, which increased to 70% at 3 months. The mean score increased from 75.0 (SD 6.0) to 80.0 (SD 5.2), with a t-value of 4.37 (df = 25, p < 0.001).
Finally, psychosocial well-being improved, with 60% of patients rating it as “Good” to “Very Good” in the immediate postoperative period, increasing to 70% at 3 months. The mean score for psychosocial well-being increased from 74.0 (SD 5.0) to 79.5 (SD 4.5), the neat difference was 5.8, and the t-value was 5.53 (df = 29, p < 0.001).
In addition to these positive outcomes, pain scores showed a marked reduction. The median pain score decreased from 5 (range 2–8) in the immediate postoperative period to 2 (range 0–5) at the 3-month follow-up, reflecting an improvement in physical recovery and comfort over time.
Overall, these results indicate that esthetic and quality-of-life outcomes, as measured by the BREAST-Q, improved during the first 3 months after partial breast reconstruction. Both esthetic satisfaction and psychosocial well-being were notably enhanced over time, suggesting that LICAP and AICAP flaps provide substantial benefits in terms of both cosmetic outcomes and overall patient quality of life (
Table 2).
4. Discussion
The validity of breast-conserving surgery (BCS) as an effective treatment for early-stage breast cancer has been well established since its introduction in 1981 by Veronesi [
12]. However, over time, increasing awareness of the potential esthetic deformities that may follow BCS has led to the integration of oncoplastic surgery into the standard care pathway for breast cancer patients [
13]. Oncoplastic surgery is not merely a reconstructive approach but rather an integrated care plan designed to balance optimal cancer control with the restoration of an esthetically pleasing breast appearance. The goal is to achieve both oncologic radicality and an acceptable esthetic outcome, ultimately enhancing the patient’s postoperative quality of life [
14].
A personalized approach is essential, as no single reconstructive method is universally applicable. Key factors, such as tumor localization, breast size, symmetry, and scarring, must be carefully considered during the surgical decision-making process to ensure the approach aligns with the patient’s specific concerns [
15].
The success of oncoplastic surgery is influenced not only by technical outcomes but also by the patient’s subjective satisfaction with the esthetic results. For example, in cases involving larger breasts, achieving an esthetically favorable outcome may require a comprehensive assessment of the patient’s body morphology and preferences regarding breast size and shape post-surgery. This individualized, patient-centered approach ensures that the goals of surgery are congruent with the patient’s expectations, ultimately leading to improved postoperative satisfaction.
Oncoplastic surgery techniques can generally be categorized into two major approaches: volume displacement and volume replacement. Volume displacement reshapes existing breast tissue to fill defects, while volume replacement relies on autologous tissue flaps to replace lost volume [
16]. The introduction of pedicled perforator flaps, including LICAP and AICAP flaps for partial breast reconstruction in 2004 [
7], which fall under the volume replacement category, represented a significant advancement in oncoplastic surgery. These flaps offer a highly effective solution for women with small-to-medium-sized breasts requiring immediate breast reconstruction, especially those needing volume restoration following quadrantectomy or lumpectomy. The main advantage of these flaps lies in their ability to repair small breast defects (less than 20% of the total breast volume) located in various segments of the breast, including both the upper and lower quadrants, without sacrificing major donor-site muscles, thus preserving muscle function and minimizing postoperative complications [
17]. This not only reduces operative time but also makes these techniques feasible for patients with significant comorbidities, as the donor-site morbidity is minimized compared to traditional muscle-based flaps like the transverse rectus abdominis myocutaneous (TRAM) flap or latissimus dorsi flap both in terms of seroma formation and wound healing issues [
18,
19].
One other significant advantage of the pedicled flap for IBR is the shorter recovery time following the procedure along with the reduced need for postoperative rehabilitation. A study by Rindom et al. [
20] demonstrated that patients who underwent reconstruction with perforator-based flaps had a reduced need for postoperative physical therapy compared to those who underwent muscle-based reconstructions [
21]. This is because the donor-site tissue is taken from the lateral or anterior chest wall, areas that do not involve large muscle groups, resulting in less postoperative pain and quicker recovery. The reduced recovery time is particularly important for patients with preexisting health conditions, as it allows them to return to their normal activities sooner.
In terms of esthetic outcomes, LICAP and AICAP flaps have demonstrated excellent results. Research consistently shows that LICAP and AICAP flaps provide a natural breast shape, resulting in high patient satisfaction [
22,
23,
24,
25].
Muktar et al. [
26] reported that up to 66% of patients who underwent reconstruction with chest wall perforator flaps were pleased with the overall esthetic outcomes, including breast symmetry and volume.
A retrospective study by Hamdi et al. [
7] demonstrated that patients who underwent reconstruction with perforator flaps had significantly less scarring in the donor site compared to those who underwent TRAM or latissimus dorsi flap reconstructions.
Pedicled flaps have also been shown to obtain satisfactory results even when compared to implant-based techniques (IBTs) [
27].
Although IBTs may offer a quicker and less invasive option, in terms of operative time, hospital stay, and donor-site morbidity, the implants may have long-term complications linked to the potential need for future revision, capsular contraction, and a less natural feel when compared to autologous tissue [
28].
In addition to the esthetic and functional benefits, the psychological impact of achieving a natural and esthetically pleasing breast reconstruction should not be underestimated. Breast symmetry and the restoration of a natural appearance are crucial factors in enhancing emotional recovery and quality of life following breast cancer surgery.
A visually satisfactory breast reconstruction can significantly improve body image, reduce anxiety, and lower the incidence of depression in breast cancer survivors [
29]. The psychological benefits of restoration are largely attributed to the increased sense of self-esteem and improved self-perception that accompanies a natural breast appearance [
30]. Furthermore, patients who are satisfied with their reconstructive outcomes are more likely to experience a smoother emotional transition, with reduced feelings of disfigurement or loss. This, in turn, can contribute to better long-term mental health and a greater sense of overall well-being. Given the substantial evidence linking esthetic satisfaction to psychological outcomes, achieving optimal esthetic results with techniques like LICAP and AICAP flaps is critical not only for physical reconstruction but also for enhancing the patient’s emotional and psychological recovery.
5. Limitations and Future Directions
Although our research yielded encouraging results, several limitations must be considered when evaluating the data. Firstly, the small sample size of 25 patients limits the ability to generalize the findings to a broader population. A larger cohort would offer more robust data and allow for a more comprehensive understanding of the techniques’ applicability across diverse patient groups.
Furthermore, another potential limitation of our study is the absence of preoperative BREAST-Q evaluations. Including preoperative data would have allowed for a more direct comparison of patient-reported outcomes before and after surgery, offering deeper insights into the impact of the procedure on quality of life. In our case, the decision not to include preoperative BREAST-Q assessments was mainly due to the inconsistent availability of preoperative data and logistical constraints. Collecting and managing additional patient-reported health-related quality-of-life measures before surgery would have required substantial resources, staff involvement, and patient compliance, which was not feasible in our clinical setting. Furthermore, the study was specifically designed to focus on postoperative outcomes and patient satisfaction at defined time points rather than baseline comparisons. Nevertheless, incorporating preoperative BREAST-Q assessments in future research would provide a more comprehensive evaluation of changes in patient well-being and expectations over time.
In addition, the relatively short follow-up period of 3 months also restricts our ability to assess the long-term impact of the reconstruction, particularly in terms of sustained esthetic outcomes, breast symmetry, and patient satisfaction. It is crucial to determine whether the observed esthetic improvements are maintained over time, especially given that changes in skin quality, breast contour, and symmetry can occur as patients age or undergo additional treatments such as radiation or chemotherapy.
A specific concern regarding the 3-month follow-up period is whether it is sufficient to detect meaningful changes following adjuvant radiotherapy. Radiation therapy can lead to progressive tissue fibrosis, skin retraction, and volume loss, which often manifest several months or even years after treatment. The early postoperative period may not capture the full extent of radiation-induced changes in breast shape, tissue pliability, or surgical flap perfusion. Longer follow-up intervals, including assessments at 6 months, 1 year, and beyond, may be necessary to evaluate delayed complications, such as fat necrosis, contour irregularities, or compromised flap viability.
Moreover, an additional limitation of this study is that only 6 out of 12 patients who were advised to undergo adjuvant radiotherapy received treatment, while the remaining 6 declined due to personal reasons, including concerns about potential side effects, logistical difficulties, or personal preferences. This discrepancy introduces a potential selection bias, as the patients who refused radiotherapy may have different oncologic and reconstructive outcomes compared to those who completed the treatment. The lower-than-expected number of irradiated patients limits our ability to fully assess the long-term impact of radiotherapy on surgical outcomes. Future studies should not only extend follow-up durations but also ensure a more comprehensive evaluation of patients who decline adjuvant treatments to better understand their long-term outcomes and the reasons behind their treatment decisions. Additionally, strategies to improve patient counseling regarding the benefits and risks of adjuvant radiotherapy may help reduce the rate of refusal and provide a more balanced dataset for analysis.
Additionally, the study was conducted in a single-center setting, which may introduce selection bias and limit the external validity of the results. Future studies should aim for multicenter designs to increase the diversity of the patient population and improve the generalizability of the findings. Furthermore, the absence of a comparative group (such as patients receiving implant-based or muscle-based reconstructions) makes it difficult to definitively evaluate the advantages of LICAP and AICAP flaps over alternative methods.
While our study includes some psychological assessments, a more detailed evaluation of the emotional and psychosocial impacts of partial breast reconstruction is needed. Understanding the long-term effects of these procedures on body image, self-esteem, and quality of life is essential, as is assessing the impact on daily functioning, including physical activity, clothing choices, and social interactions. Future research should include validated psychological assessments and longer follow-up periods to provide more comprehensive data on the psychosocial benefits of these reconstructive techniques and their long-term outcomes. Larger-scale studies with more diverse patient populations and longer observation periods will be essential to validate and expand upon the findings of this study.
Moreover, while the current study provides promising insights into the technical and esthetic benefits of LICAP and AICAP flap procedures, there is a pressing need for further research into their oncological safety. Specifically, longer-term data on breast-cancer-specific survival (BCSS) and overall survival (OS) are critical to confirming the oncological safety of the chest wall perforator flap (CWPF) techniques. Previous studies have emphasized the importance of evaluating the long-term oncological outcomes of breast reconstruction techniques, particularly those involving autologous tissue transfer, as complications such as local recurrence could potentially affect survival rates [
31]. In their analysis, Nuyten et al. noted that while autologous breast reconstruction is associated with favorable esthetic outcomes, comprehensive oncological follow-up data are essential to ensure that these procedures do not interfere with cancer management or survival.
However, while studies on larger autologous flaps like DIEP or TRAM have shown significant potential negative impact on BCSS or OS [
32], the long-term oncological safety of LICAP and AICAP, which involve more localized tissue transfer with different vascular patterns, remains inadequately studied. Therefore, future studies should incorporate longer follow-up periods and direct comparison with alternative reconstructive strategies, especially in terms of recurrence rates and long-term oncological outcomes. These data are necessary to conclusively determine the safety of CWPF techniques in the context of breast cancer reconstruction.
There is a growing recognition that the oncological safety of breast reconstruction should be rigorously evaluated. A key concern is the potential for local recurrence in the reconstructed area, which could affect both BCSS and OS. In patients undergoing partial breast reconstruction, such as those treated with LICAP or AICAP flaps, the risk of recurrence may be influenced by factors such as the adequacy of cancer resection, the quality of the remaining tissue, and the impact of adjuvant therapies like radiation. Therefore, confirming that the reconstructive procedure does not contribute to increased risk of recurrence is essential for validating its use.
The absence of long-term follow-up in the present study limits the ability to assess whether LICAP and AICAP flaps have any impact on local recurrence rates or distant metastasis, both of which directly affect BCSS. Additionally, while OS encompasses all-cause mortality, it is important to consider that complications arising from the flap procedure itself, such as flap failure or infection, may indirectly impact overall survival by requiring additional interventions or prolonging recovery times.
To definitively establish the safety of LICAP and AICAP flaps, future studies must incorporate comprehensive oncological follow-up, ideally extending over 5 to 10 years, to assess both BCSS and OS outcomes. Such follow-up should include tracking recurrence rates at the site of reconstruction and distant metastasis, as well as evaluating the effect of adjuvant therapies on the reconstructed breast. The inclusion of these long-term data will be essential for confirming whether the observed esthetic benefits of LICAP and AICAP flaps are achieved without compromising the patient’s cancer outcomes. Prospective studies with multi-institutional participation would also help to ensure that findings are generalizable to a wider patient population, further validating the oncological safety of these techniques.
6. Conclusions
Both LICAP and AICAP flaps represent reliable and esthetic options for oncoplastic breast reconstruction, offering a safe and effective solution for patients requiring partial breast reconstruction following oncological resection. The anatomical advantages of these flaps, combined with their local nature and ability to provide adequate tissue for breast volume restoration, make them highly suitable for the reconstruction of upper- and lower-quadrant defects. The minimal donor-site morbidity, reduced operative time, and favorable esthetic outcomes further enhance their appeal in oncoplastic surgery. However, their limitations, including the restricted volume of available tissue, suggest that careful patient selection is crucial to achieving optimal outcomes. When used appropriately, both the LICAP and AICAP flaps can significantly improve the cosmetic and functional outcomes in breast reconstruction, helping patients achieve a natural-looking breast with minimal impact on their overall quality of life.