Abstract
The serendipitous discovery of antiparasitic drugs, such as quinine and artemisinin, of plant origin reveals that searching new chemical pharmacophores from medicinal plants is valuable. The present study sought to explore the antiplasmodial, antileishmanial, and antitrypanosomal activities of Lippia adoensis extracts. Crude extracts of L. adoensis leaves and twigs, which were obtained by extraction using 70% ethanol in water, were assayed for antiplasmodial activity against P. falciparum 3D7 and Dd2 through the SYBR green I-based fluorescence assay; and for antileishmanial, antitrypanosomal, and cytotoxic effects on Leishmania donovani, Trypanosoma brucei brucei, and Vero cells, respectively, using resazurin colorimetric assays. In vitro phytochemical analysis of L. adoensis extracts was performed using standard methods. Moreover, liquid chromatography–mass spectrometry (LC-MS) feature-based detection and molecular networking flow on Global Natural Product Social (GNPS) were also used for the phytochemical screening of L. adoensis extracts. Crude extracts from L. adoensis inhibited the growth of P. falciparum (3D7 and Dd2) (IC50s; (3D7): 10.00 and 97.46 μg/mL; (Dd2): 29.48 and 26.96 μg/mL), L. donovani (IC50s: 22.87–10.52 μg/mL), and T. brucei brucei (IC50s: 2.30–55.06 μg/mL). The extracts were found to be non-cytotoxic to Vero cells, thus yielding median cytotoxic concentrations (CC50s) above 100 μg/mL. In vitro phytochemical analysis of the crude extracts revealed the presence of alkaloids, terpenoids, phenolic compounds, and carbohydrates. The LC-MS tandem molecular networking flow predicted that the extracts contained valsafungin A and bacillamidin in the first cluster, and fatty acids, ketone, and aldehyde derivatives in the second cluster. Overall, the present study demonstrated the antiparasitic effects of L. adoensis extracts, thus justifying the use of this plant in the traditional treatment of fever and malaria conditions. Nevertheless, detailed metabolomic studies and antiparasitic mechanisms of action of the extracts are expected to unveil the potential antiparasitic hit compounds.
1. Introduction
The World Health Organization (WHO) classified malaria, leishmaniasis, and African trypanosomiasis as the triad parasitic diseases that are more prevalent in tropical regions, especially in Sub-Saharan Africa [1]. These diseases claim the lives of a billion people worldwide annually [2]. They are considered neglected and are typically found in tropical and subtropical zones with deprived sanitation and sewage disposal, deficient hygiene practices, as well as low host resistance and environmental changes [3,4]. More than fifty countries have eliminated at least one neglected disease since 2023, thus forming the midway point towards eradication in a hundred countries by 2030. Malaria is triggered by parasites of the genus Plasmodium, the deadliest being caused by Plasmodium falciparum, which is transmitted through bites of infected female Anopheles mosquitoes [5]. In 2022, there were an estimated 249 million malaria cases and 608 000 malaria deaths worldwide [5]. Countries such as Mozambique (4.2%), Uganda (5.1%), the Democratic Republic of the Congo (12.3%), and Nigeria (26.8%) were the most affected by malaria, accounting for over half of all malaria deaths in the world [5]. Malaria is a curable disease, however; early diagnosis and treatment ensure a fast and complete elimination of the parasites from the bloodstream [5]. As per the last malaria report by the WHO, artemisinin-based combination therapies are the recommended treatments for uncomplicated falciparum malaria, whereas the intravenous or intramuscular administration of artesunate is indicated for the severe form, with the use of quinine as an acceptable alternative [5].
Leishmaniasis is caused by parasites of the Leishmania genus and is transmitted through bites by infected female phlebotomine sandflies. This disease manifests into three forms, including cutaneous, mucocutaneous, and visceral forms [6,7]. It mostly affects the poorest regions of the globe and an estimated 1 million new cases occur annually [7]. The arsenal of antileishmanial therapies include pentavalent antimonials, miltefosine, amphotericin B, paromomycin, pentamidine, and others [8]. However, Leishmania parasites have become resistant to the majority of these antileishmanial treatments [9].
Human African trypanosomiasis (HAT, sleeping sickness), which is caused by Trypanosoma brucei, menaces millions of individuals, especially in sub-Saharan Africa, where high transmission occurs due to the presence of tsetse flies [10]. Two parasites that feature the two forms of this disease include Trypanosoma brucei rhodesiense and gambiense, the latter accounting for over 98% of reported cases (WHO, 2024c) [10]. Current medications for human African trypanosomiasis comprise nifurtimox, suramin, pentamidine, eflornithine, melarsoprol, etc.; however, these medicines require prolonged parenteral administration and cause undesirable side effects, such as loss of weight, anorexia, depression of bone marrow, psychic alterations, and articular and muscular pain [11,12].
The continuous use of the same drugs over decades to cure these tropical diseases has led to the development of resistance by the disease-causing parasites [12,13]. Further challenges for trypanosomiasis and leishmaniasis treatment consist of patient compliance with therapy, complex or unknown pathogenic mechanisms, drug instability, and environmental factors that influence the transmission of infection [14].
In addition to the problem of drug resistance, these therapies exhibit a range of toxic effects, thus justifying the pressing need to search for effective antiprotozoal agents.
The serendipitous identification of the two well-known antimalarial drugs quinine and artemisinin from the Cinchona tree and Artemisia annua, respectively, has revolutionized the development of antimalarial therapies from medicinal plants, even though these medications have become less effective due to parasite drug resistance [15]. Moreover, most of the anti-Trypanosomatid or anti-kinetoplastid drugs originate from structural modifications of known natural product scaffolds (nifurtimox: nitrofuran derivative, and pentamidine: diamidine analog), by chemical synthesis (pentavalent antimonials), or repurposed from existing drugs (eflornithine and miltefosine, previous anticancer agents; and amphotericin B, an antifungal agent) [16,17].
Furthermore, the interest in natural product-based drug discovery over high throughput screening of combinatorial libraries [18] justifies the suitability of natural compound pharmacophores in the discovery of antiprotozoal drugs. However, challenges regarding sustainability, changes in the plant’s chemical composition, etc., are some limitations in drug discovery campaigns from plants [19]. In the meantime, accumulated evidence has shown that more than 50% of the currently available synthetic drugs originated from medicinal plants [20,21], thus justifying the need to search for effective antiprotozoal treatments from medicinal plants. Lippia adoensis is an example of a medicinal plant used for the traditional treatment of several diseases, including skin disorders, superficial fungal infections, malaria, and fever conditions [22]. In Ethiopia, the leaves of Lippia adoensis are used to cure toothache, diarrhea, and indigestion [23], whereas the leaf decoction is employed as a remedy for fever and constipation in children, and for the treatment of bronchitis, skin disorders and ophthalmia [24,25]. Other plants from the genus Lippia are used traditionally to relieve cough and colds, wounds, gastrointestinal and respiratory problems, malaria, and fever conditions [26,27]. Before the 1990s, Lippia adoensis was already targeted for its high content of volatile compounds, with linalool (81.30–94.56%) as the highest compound, followed by caryophyllene (5.66%) and 1,8-cineole (3.22%), among others [28]. Research by Abegaz et al. [29] revealed a different composition of essential oils in cultivated and wild-type Lippia adoensis, with the monoterpene ketone ipsdienone found in both types. Linalool, which has been reported by the majority of authors as the main constituent of Lippia adoensis’ essential oil, was found in the cultivated form, but was absent from the wild plant type, even though there was a number of compounds peculiar to the wild type (limonene (3.44–32.73%), perillaldehyde (0.04–26.90%) and piperitenone (0.15–44.48%)) [29]. More than a decade later, Kasali et al. [30] performed GC-MS analysis of the essential oil of Nigerian (Ajara-Badagry, Lagos)-growing Lippia adoensis leaves. As a result, monoterpenes, such as linalool (26.1%), 1,8-cineole (17.4%), and geraniol (19.0%), and the sesquiterpene germacrene D (4.6%), were recorded as the major ingredients. In another location of Nigeria (Ife-Odan, Osun State), Adelani et al. [31] recorded a different chemical composition of the essential oil of Lippia adoensis leaves (major constituents: 1,3,6,10-dodecatetraene (3.74%), 1H-cyclopropa[a]naphthalene (4.25%), α-pinene (5.08%), γ-terpinene (15.24%), α-terpineol (25.99%), and eucalyptol (28.36%)). Fikadu et al. [32] revealed a variability of major compounds of L. adoensis collected in Ethiopia from two different sites viz. Debre Berhan (linalool (86.11%)) and Bishoftu (linalool (66.60%) and caryophyllene (4.28%)). In addition, a phytochemical analysis of water, methanol, petroleum ether, acetone, and aqueous: methanol (20:80, v/v) extracts from L. adoensis leaves revealed the presence of phenolic compounds and flavonoids in this plant [33].
To unravel the phytochemical composition of plant extracts, recent approaches, such as molecular networking, have become an essential bioinformatics tool to visualize and annotate non-targeted mass spectrometry data [34,35,36]. This tool was released through the Global Natural Product Social (GNPS) Molecular Networking, a web-enabled mass spectrometry knowledge capture and analysis platform (http://gnps.ucsd.edu; accessed on 10 April 2024) [37], and has been widely applied in mass spectrometry-based metabolomics to aid in the annotation of molecular families from their fragmentation spectra [35]. This very metabolomics tool might help to unveil the chemical constituents of Lippia adoensis, which are responsible for its pharmacological effects, such as antibacterial and antifungal [38] and antioxidant [33] activities. Notably, higher (500–2000 mg/kg) repeated oral doses of the ethanolic extract of L. adoensis in Wistar rats for 28 days showed signs of toxicity, even though acute toxicity experiment revealed a median lethal dose (LD50) less than 10,000 mg/kg [27]. To our knowledge, the antiparasitic effects of L. adoensis against pathogens like Plasmodium, Leishmania, and Trypanosoma are still unknown.
Based on the above considerations, the scientific validation of the medicinal plant L. adoensis, which is used for the traditional treatment of malaria and fever conditions, is valuable. Thus, this study sought to investigate the antiparasitic effect of L. adoensis extracts against Plasmodium, Leishmania, and Trypanosoma parasites. The phytochemical screening of L. adoensis extract is also evaluated using a LC-MS feature detection and alignment, and then a molecular networking workflow on GNPS (http://gnps.ucsd.edu; accessed on 10 April 2024).
2. Materials and Methods
2.1. Plant Material
2.1.1. Plant Collection and Identification
The leaves and twigs of Lippia adoensis (Figure 1) were collected at Etoa, Yaounde, Cameroon, in June 2023. The plant material was identified by Mr. NANA Victor, botanist at the Cameroon National Herbarium and a voucher specimen number (HNC-00428FC) was obtained.

Figure 1.
Picture of Lippia adoensis growing in Etoa Village, Yaounde VI, Centre region, Cameroon (photograph by E.A.M.K.).
2.1.2. Plant Extraction
Following plant collection, the organs (leaves and twigs) were dried at room temperature and coarsely powdered. Next, the crude extracts were prepared by maceration of the dried powders in hydroethanol (30/70; v/v). Briefly, 500 g of dried powder was macerated in hydroethanol (1500 mL) for 72 h at room temperature. The resulting solution was then filtered using hydrophilic cotton, and the filtrate was further concentrated using a Buchi rotary evaporator at 55 °C to yield the crude extracts. The extraction yield was calculated using the following formula:
Extraction yield (%) = (mass of the crude extract (g))/(mass of the plant material (g)) × 100
The crude extracts were kept in the refrigerator until further use.
2.2. In Vitro Antiparasitic Activity of Lippia adoensis Extracts
2.2.1. In Vitro Antiplasmodial Test
a. Plasmodium falciparum Culture and Maintenance
To assess the antiplasmodial activity of Lippia adoensis extracts, two strains of Plasmodium falciparum viz. 3D7 (chloroquine-sensitive) and Dd2 (chloroquine-resistant) were used. These parasites were preserved in culture according to a previously described protocol [39], with minor changes [40]. Briefly, a comprehensive RPMI-1640 medium (RPMI-1640 medium at 16.20 g/L containing HEPES (25 mM), Albumax I (0.50%), glucose (11.11 mM), sodium bicarbonate (0.20%), gentamicin (50 μg/mL) and hypoxanthine (45 μg/mL)) was used to culture the parasites in fresh human (healthy volunteers) red blood cells (O-positive blood type) at 4% (v/v) hematocrit, 37 °C, and 5% CO2 atmosphere. Noteworthy, albumax I and gentamicin were obtained from Gibco (Waltham, MA, USA), whereas the remaining reagents were purchased from Sigma Aldrich (Munich, Germany). To propagate the culture, the consumed medium was replenished with fresh complete medium every day. To monitor cell cycle transition and parasitemia, giemsa-stained blood smears were observed microscopically under oil immersion.
b. In Vitro Assay on P. falciparum
The SYBR green I-based colorimetric method was used to appraise the antiplasmodial activity of crude extracts of L. adoensis leaves and twigs [41]. To this end, 25 mg of each extract was dissolved in 1 mL of dimethylsulfoxide (DMSO). Stock solutions of chloroquine and artemisinin were prepared at a concentration of 1 mM. The as-prepared solutions were introduced to 96 well plates and further diluted with RPMI-1640 medium to obtain suitable concentrations (for extracts: from 0.10 to 100 μg/mL; for chloroquine and artemisinin: 1 µM) needed for the experiment. Noteworthy, the extract preparation was 0.4% DMSO concentrated in each well, a concentration that was found to be harmless to the test parasite. Overall, the tests were performed in duplicate. In brief, the culture of P. falciparum parasites was synchronized by sorbitol treatment [42]. Next, 100 μL of the as-prepared P. falciparum culture containing parasitemia (1%) and hematocrit (2%) was introduced to 96-well plates (flat bottom), followed by the addition of crude extracts or positive (chloroquine and artemisinin) or negative (0.4% DMSO) controls. After incubation of the plates for 48 h at 37 °C and 5% CO2 atmosphere, 100 μL of SYBR green I solution in lysis buffer (20 mM Tris (pH 7.5), 0.008%, w/v saponin, 5 mM ethylenediaminetetraacetic acid (EDTA) and 0.08, v/v Triton X-100) was added to each well and then incubated for an additional 1 h at 37 °C under darkness. Afterward, the fluorescence intensity of each well was quantified using an Infinite M200 fluorescence well plate reader (Tecan, Austria GmbH, Grödig Flachgau, Austria) at wavelengths of 485 and 530 nm for excitation and emission, respectively. The half-maximal inhibitory concentrations (IC50s) were determined by analysis of dose–response curves obtained by plotting fluorescence counts versus concentrations of extracts or drugs (chloroquine or artemisinin) using the GraphPad Prism 8.0 software (San Diego, CA, USA). The results were further validated microscopically by examining Giemsa-stained smears of treated (extracts or drug) or untreated parasite cultures.
2.2.2. Antileishmanial Screening
a. Parasite Culture and Maintenance
Leishmania donovani (1S MHOM/SD/62/1S) was used to evaluate the antileishmanial activity of extracts from Lippia adoensis twigs and leaves. This parasite was graciously granted by Bei Resources (Biodefense and Emerging Infections Research Resources Repository). From cryopreserved vials, L. donovani promastigotes were routinely cultured in the Laboratory for Phytobiochemistry and Medicinal Plants Studies (University of Yaoundé I, Cameroon) using Sigma’s Medium 199 supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, and Sigma’s heat-inactivated fetal bovine serum (10%). Cell culture flasks (dimension: 75 cm2) were used to maintain parasite culture at 28 °C with a daily check for growth, whereas parasite sub-culturing was conducted every 3 days [43].
b. Inhibitory Assay Against L. donovani Promastigotes
As previously described by Siqueira-Neto et al. [44], the resazurin colorimetric assay was used to evaluate the antileishmanial activity of Lippia adoensis crude extracts against cultured L. donovani promastigotes.
Firstly, L. adoensis extracts were dissolved with 100% DMSO to afford the extract stock solutions, which underwent serial dilutions in incomplete (not supplemented) culture medium. Secondly, promastigotes (90 μL) of L. donovani (4 × 105/mL/well), obtained from an exponential growth culture, were seeded in a 96-well plate; then, 10 μL of various concentrations of L. adoensis extracts (0.16, 0.8, 4, 20, and 100 μg/mL) were added to the plates and subsequently incubated for 72 h at 28 °C. The final concentration of DMSO in each well was not higher than 1%. After incubation of the plates for 28 h at 28 °C, a resazurin (0.15 mg/mL in Sigma Dulbecco’s phosphate-buffered saline; DPBS) solution was added at 1 mg/mL, followed by an additional incubation for 44 h. The percentage cell viability corresponded to the quantity of the highly fluorescent resorufin (pink color) obtained as a result of the interaction of living parasites with resazurin (blue color). In this experiment, amphotericin B, which was obtained from Sigma-Aldrich (Darmstadt, Germany), was used as a positive control at concentrations varying between 10 and 0.016 μg/mL, while DMSO (0.1%) was considered as the negative control. When the incubation time elapsed, the fluorescence intensity of the as-prepared plates was measured with a Tecan Magelan Infinite M200 fluorescence microplate reader (Tecan, Austria GmbH, Grödig Flachgau, Austria) at wavelengths of 530 and 590 nm for excitation and emission, respectively. For each sample, growth percentages were calculated and dose–response curves were plotted to determine the half-maximal inhibitory concentration (IC50) using GraphPad Prism 8.0 software (San Diego, CA, USA).
2.2.3. Antitrypanosomal Screening
a. Parasite Growth Conditions
An antitrypanosomal test was performed in trypomastigote forms of Trypanosoma brucei subsp. Brucei (strain Lister 427 VSG 221), a parasite that was gracefully received from the BEI resource. Complete Hirumi’s modified Iscove’s medium 9 (HMI-9) (Gibco, Waltham, MA, USA) was used to carry out an axenic culture of T. brucei parasites in sterile cell culture flasks and incubated at 37 °C and 5% CO2 atmosphere. It is worth noting that HMI-9 medium encompassed 500 mL of Gibco’s IMDM (Iscove’s modified Dulbecco’s medium) (Gibco, Waltham, MA, USA) accompanied by serum plus (10% v/v), heat-inactivated fetal bovine serum (HI FBS; 10% v/v), and HMI-9 supplement (1 mM hypoxanthine, 50 µM bathocuproine disulfonic acid, 1.25 mM pyruvic acid, 1.5 mM cysteine, 0.16 mM thymidine, 0.2 mM 2-mercaptoethanol, and 1% (v/v) penicillin/streptomycin). Thereafter, an inverted fluorescence microscope (Lumascope LS520, Etaluma, San Diego, CA, USA) was used to monitor parasite density in culture every 72 h, and viable T. brucei parasites were subsequently passaged with fresh complete medium so that the cell density did not exceed 2 × 106 cells/mL [45].
b. Inhibition Test Against Trypanosoma brucei Brucei
The in vitro inhibitory activity of Lippia adoensis extracts was evaluated against bloodstream forms of Trypanosoma brucei brucei using the resazurin-based test as previously described [46]. In brief, parasites at their mid-logarithmic growth phase were counted, and the cell density was adjusted with fresh complete HMI-9 medium to 2 × 105 trypanosomes per mL. Next, 90 µL of parasite suspension was distributed into 96-well flat-bottomed plates containing 10 µL of extracts, followed by a serial dilution at 5-point concentrations (0.16, 0.8, 4, 20, and 100 μg/mL). In each plate, the first column served as a negative (cells with 0.1% DMSO) control, whereas the last column was employed as a positive (cells with 10 µM pentamidine isethionate) control. Afterward, the plates were incubated for 68 h at 37 °C and 5% CO2, followed by the addition of a Sigma resazurin (0.15 mg/mL in DPBS) solution. After incubation of the as-prepared plates for an additional 4 h, a Tecan fluorescence microplate reader was used to measure the fluorescence intensity of each well at excitation and emission wavelengths of 530 and 590 nm, respectively. Each experiment was repeated twice and executed in two replicates. The percentage of parasite inhibition by the extract or drug (positive control) was obtained from the measured fluorescence data in relation to the mean fluorescence of negative control wells using Microsoft Excel (version 2013). A concentration–response curve was plotted using GraphPad Prism 8.0 software and the half-maximal inhibitory concentrations (IC50s) were subsequently calculated using the following formula:
where H is the slope factor or hill coefficient [47].
y = 100/[1 + 10 (logIC50/99 − x)H]
2.3. Cytotoxicity Assay
2.3.1. Maintenance of Mammalian Cells
The African green monkey kidney Vero cell line (ATCC CRL-1586), which was generously received from the Centre Pasteur of Cameroon (CPC), was cultured in complete Dulbecco’s modified Eagle’s medium (DMEM) complemented with fetal bovine serum (FBS, 10%), nonessential amino acids (1%), and penicillin/streptomycin (1% v/v) at 37 °C and 5% CO2 using T-25 ventilated cap tissue culture flasks. After medium renewal on the third day, an inverted microscope (Lumascope LS520) was used to monitor cell growth. When the cells reached percentage confluence between 80 and 90%, they were sub-cultured by treatment with 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) to allow cell detachment. After cell detachment, the preparation was centrifuged at 1800 rpm for 5 min and the resulting pellet was resuspended for further microscopic cell estimation. Next, a Neubauer chamber was used to count the cells in the pellet solution in the presence of trypan blue, which excluded cells that were incapable of growth. At a density of 104 cells/well, cultured cells were processed for the experiment.
2.3.2. Cytotoxic Effect of Extracts
The cytotoxicity of L. adoensis extracts was determined in a 96-well tissue culture-treated plate as per a previously described protocol [46]. Briefly, Vero cells (density: 104 cells/well) were cultured in complete DMEM medium (100 µL) overnight in order to promote the detachment of cells. Cell adherence, integrity, and sterility were monitored using the inverted fluorescence microscope Lumascope LS520. Afterward, the excess medium was removed from the wells and replaced by freshly prepared complete medium (90 µL). Next, 10 µL of various concentrations of L. adoensis extracts (1.6, 8, 40, 200, and 1000 μg/mL) and podophyllotoxin (positive control) (0.016, 0.08, 0.4, 2, and 10 μg/mL) were added to the plates. The negative control wells were treated with 0.5% DMSO, which further yielded 100% cell viability. Thereafter, the as-prepared plates were incubated for 48 h in a 5% CO2 atmosphere. For the revelation of the plates, a freshly prepared solution of resazurin was added to each well at a concentration of 0.15 mg/mL in Sigma Dulbecco’s phosphate-buffered saline (DPBS) and subsequently incubated for an extra 4 h. After the incubation time elapsed, the plate was subjected to a Tecan Magelan Infinite M200 fluorescence plate reader for fluorescence measurements at excitation and emission wavelengths of 530 nm and 590 nm, respectively. The obtained fluorescence readouts aided in the calculations of the percentages of cell viability. The concentration–response curves were subsequently plotted using GraphPad Prism 8.0 software in order to determine the median cytotoxic concentration (CC50) for each extract and podophyllotoxin. The sample selectivity was estimated by calculation of the selectivity index (SI) for each extract using the following formula: SI = CC50 (Vero cells)/IC50 (parasite).
2.3.3. Buildout of Feature-Based Molecular Networking
The phytochemical screening of extracts from L. adoensis twigs and leaves was performed using a two-step process (first step: LC-MS feature detection and alignment; second step: molecular networking workflow on GNPS) as previously reported by Quinn et al. [35]. According to stage 1, two files, including (i) the MS spectral summary (.MGF format) and (ii) the feature quantification table (.TXT format), were exported [35]. The mapping information between both files allowed for establishing a link between LC-MS information and the molecular network nodes. The feature-based molecular networking (FBMN) workflow was integrated into the GNPS (Global Natural Product Social) system (available on the GNPS link: https://gnps.ucsd.edu/; accessed on 10 April 2024) and thus benefitted from the connection with other GNPS features. Therefore, the automatic MS spectral library search could be performed by a direct addition and curation of library entries, then, a search of a spectrum against public datasets with mass spectrometry search tool (MASST) [48], and ultimately a visualization of molecular networks through the web browser [49] or by using Cytoscape [50]. Detailed guidelines used for FBMN research with required tools are fully provided in the GNPS documentation (https://ccms-ucsd.github.io/GNPSDocumentation/featurebasedmolecularnetworking, Version 1.3.16-GNPS; accessed on 1 April 2024).
2.3.4. In Vitro Phytochemical Screening
A phytochemical analysis of hydroethanol extracts of Lippia adoensis leaves and twigs was conducted to identify different constituents, such as triterpenoids and phenolic compounds [51], carbohydrates [52], and alkaloids [53], which could be responsible for the antiparasitic activity.
a. Liebermann–Burchard Test
In this assay, 1 mg of extract was dissolved in chloroform, followed by an addition of acetic anhydride and concentrated sulfuric acid. After stirring, the presence of triterpenoids was indicated by a purplish-red color [51].
b. Molisch Assay
In this experiment, 1 mg of extract was dissolved in ethanol, followed by the addition of 1% ethanol solution of α-naphthol, then a few drops of concentrated sulfuric acid. The presence of carbohydrates (sugars) was indicated by the appearance of a purplish-red ring at the interface of the solution [52].
c. Ferric Chloride (FeCl3) Test
To identify the presence of phenolic compounds, 1 mg of extract was dissolved in ethanol, followed by the addition of FeCl3 solution. The occurrence of phenolic compounds resulted in the development of blue or violet complexes [51].
d. Dragendorff Test
In this experiment, 10 mg of each sample was added to 1% ethanolic solution of HCl, followed by the addition of the Dragendorff reagent. The generation of an orange precipitate revealed the presence of alkaloids [53].
2.4. Statistical Analysis
For each experiment, Microsoft Excel software (version 2013, Washington, DC, USA) was used to determine the growth inhibition percentages, the values of which were subsequently used to plot the concentration-response curves (percentages of inhibition vs. log10 [sample’s concentration]) for the estimation of the half-maximal inhibitory concentration (IC50) or the median cytotoxic concentration (CC50) using GraphPad Prism 8.0 software. The sample selectivity was estimated by the calculation of the selectivity indices of test samples using the following formula: SI = CC50 Vero cells/IC50 parasites. Each and every experiment was performed in duplicate and repeated two times.
3. Results
3.1. Antiparasitic Tests
In this study, the inhibitory effects of Lippia adoensis extracts were evaluated against Plasmodium falciparum (strains 3D7 and Dd2), Leishmania donovani promastigotes, and trypomastigotes of Trypanosoma brucei brucei. The results of the half-maximal inhibitory concentrations (IC50) of the plant extracts are summarized in Table 1.

Table 1.
Antiprotozoal activity (IC50: μg/mL) and cytotoxicity (CC50: μg/mL) of the hydroethanol extracts of Lippia adoensis twigs and leaves.
3.1.1. Antiplasmodial Effect
The hydroethanolic extracts of leaves and twigs inhibited the growth of P. falciparum 3D7, with IC50 values of 10.008 and 97.467 μg/mL, respectively, vs. chloroquine and artemisinin (IC50 values: 0.859 and 0.003 μg/mL, respectively). Against P. falciparum Dd2, hydroethanolic extracts of leaves and twigs showed inhibitory effects, with IC50 values of 29.48 and 26.96 μg/mL, respectively, vs. chloroquine and artemisinin (IC50 values of 0.0514 and 0.064 μg/mL, respectively).
3.1.2. Antileishmanial Activity
The incubation of L. donovani promastigotes with hydroethanolic extracts from leaves and twigs of Lippia adoensis inhibited the parasite’s population, thus yielding IC50 values of 22.879 and 10.522 μg/mL, respectively, versus amphotericin B (IC50 value: 1.11 μg/mL).
3.1.3. Antitrypanosomal Role
Against T. brucei, the extracts of L. adoensis exhibited IC50 values of 2.308 and 55.06 μg/mL, respectively, versus pentamidine (IC50 value: 0.006 μg/mL).
3.2. Cytotoxicity Test
According to the cytotoxicity test, the hydroethanol extracts from leaves and twigs of Lippia adoensis showed common half-maximal cytotoxic concentration (CC50) values above 100 μg/mL, inferring that these extracts are non-toxic to the human mammalian cells Vero. Thus, the extracts were found to be selective to the parasitic cells with selectivity indices ranging from 1.490 to 99.066.
3.3. Development of Molecular Networking
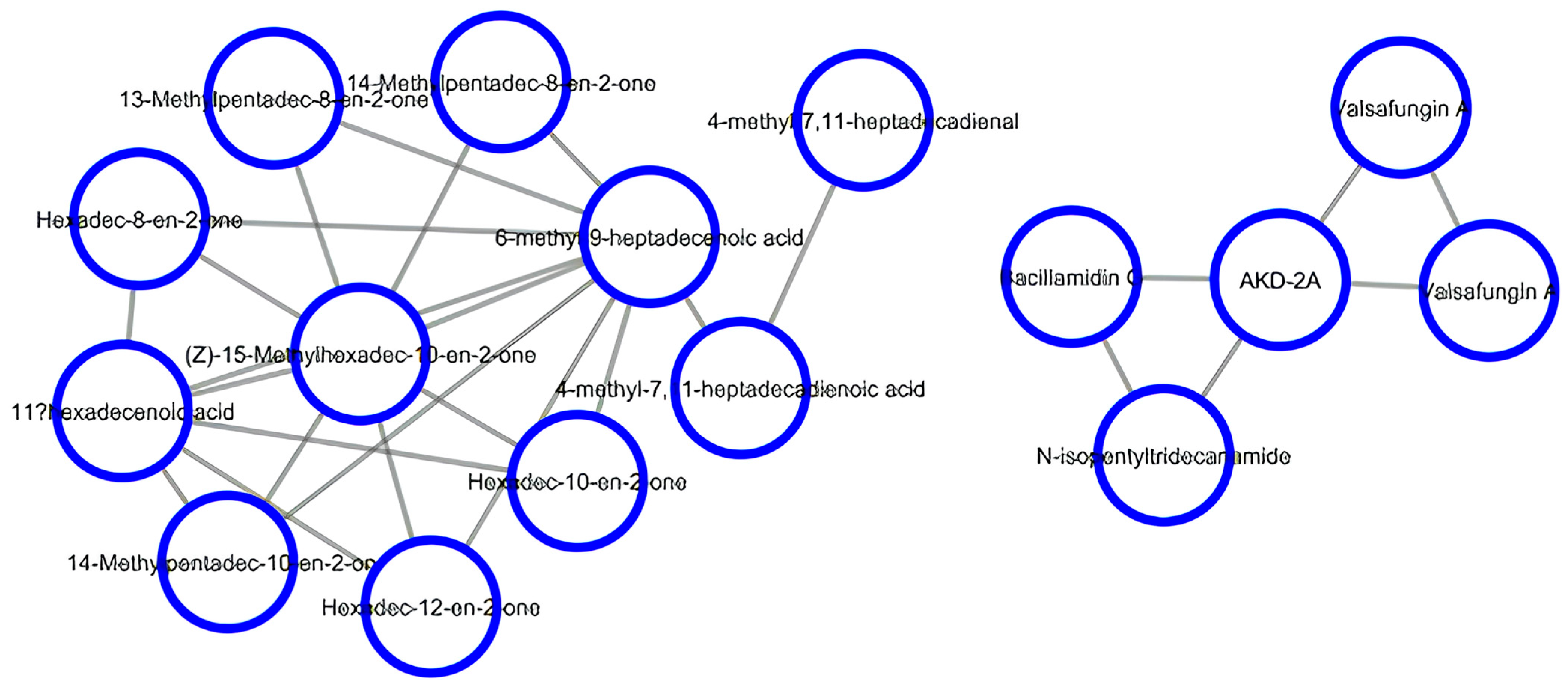
LC-MS feature detection and alignment, followed by a dedicated molecular networking workflow on GNPS, were successively used to predict the chemical constituents present in Lippia adoensis. As a result, two clusters of compounds were obtained. The first cluster was dominated by valsafungin A and bacillamidin, which are well-known antifungal antibiotics [54], in addition to N-isopentyltridecanamide and the codified compound AKD-2A, which were also predicted. The second cluster consisted of ketones (hexadec-10-en-2-one and its isomers hexadec-12-en-2-one and hexadec-8-en-2-one; 14-methylpentadec-8-en-2-one and its isomer 14-methylpentadec-10-en-2-one; 13-methylpentadec-8-en-2-one; (Z)-15-methylhexadec-10-en-2-one), fatty acids (6-methyl-9-heptadecenoic acid; 4-methyl-7,11-heptadecadienoic acid; and 11-hexadecenoic acid), and the aldehyde derivative 4-methyl-7,11-heptadecadienal (Figure 2).
Figure 2.
LC-MS feature-based molecular networking of the leaf extract of Lippia adoensis.
3.4. Phytochemical Analysis
Phytochemical analysis of hydroethanolic extracts from leaves and twigs of Lippia adoensis confirmed the presence of alkaloids, terpenoids, phenolic compounds, and carbohydrates. This information offers important prospects for analyzing the chemical composition of secondary metabolites in hydroethanolic extracts from Lippia adoensis leaves and twigs (Table 2).

Table 2.
Phytochemical screening of hydroethanolic extracts from leaves and twigs of Lippia adoensis.
4. Discussion
A number of modern pharmacological studies have substantiated the ethnopharmacological use of medicinal plants in the treatment of diseases caused by protozoan parasites, thus providing alternative therapies against these maladies [55,56,57]. As a matter of fact, accumulated evidence has shown the antiprotozoal effects of various organs from medicinal plants in the last 20 years [2,58,59,60,61]. In continuation of our search for bioactive compounds of plant origin with antiprotozoal effects, the hydroethanol extracts of Lippia adoensis leaves and twigs were screened for their antiplasmodial, antileishmanial, and antitrypanosomal potential. Notably, to the best of our knowledge, this study represents the first report on the antiparasitic activity of Lippia adoensis against Plasmodium, Leishmania, and Trypanosoma species. The hydroethanolic extract of leaves and twigs revealed antiplasmodial activity against the sensitive strain of P. falciparum (3D7), with IC50 values of 10.008 and 97.467 μg/mL, respectively. Against P. falciparum Dd2, hydroethanolic extracts of leaves and twigs showed IC50 values of 29.48 and 26.96 μg/mL, respectively. In vitro phytochemical analysis of hydroethanolic extracts from Lippia adoensis leaves and twigs revealed the presence of alkaloids, phenolic compounds, terpenoids, and carbohydrates (sugars). The antiprotozoal activity of alkaloids [62,63], terpenoids [64,65], phenolic compounds [66], and sugars [67] has already been reported across the literature. Indeed, growing evidence has also shown that Lippia adoensis contains flavonoids and phenolic compounds [33], monoterpenes (ipsdienone, linalool, cineole, geraniol, caryophyllene), and sesquiterpenes (germacrene D), among others [28,29,30,31,32]. Several authors have shown that these compounds exhibit antiplasmodial activity. For instance, Santos et al. [68] recently described the implication of monoterpenes, such as 1,8-cineole, in the inhibition of P. falciparum in vitro, thus preventing severe malaria in P. berghei-infected mice [68]. In addition, Boyom et al. [69] previously demonstrated that the antiplasmodial activity of the essential oil from Cleistopholis patens was attributed to the presence of β-caryophyllene, germacrene D, and germacrene B, whereas that from Uvariastrum pierreanum was due to the presence of β-bisabolene, α-bisabolol, and α- and β-pinenes [69]. A detailed mechanism of the antiplasmodial action of 1,8-cineole showed the inhibition of hemoglobin degradation, thereby preventing hemozoin formation [68,70,71], even though more experiments are necessary to confirm this. Linalool, the major compound of Lippia adoensis’s essential oil, was also reported to induce leishmanial cell death by nuclear and kinetoplast chromatin destruction, followed by cell lysis, which was observed within 1 h of cell treatment [72]. Rodrigues Goulart et al. [73] previously demonstrated cell cycle arrest by linalool in P. falciparum [73]. Lippia adoensis, which mainly contains monoterpenes, such as linalool, might have exerted antiplasmodial and antileishmanial activities through at least one of these mechanisms, even though additional mechanistic studies are required to validate this claim. Although the antiparasitic mechanisms of the action of flavonoids are unknown, a number of studies have shown that this class of compounds exert antiplasmodial and antitrypanosomatid effects via the inhibition of the type-two fatty acid (FAS II) biosynthesis pathway [74,75,76], inhibition of protein kinase (Pf RIO-2 kinase), or by targeting other functional biomolecules (protein, enzymes, DNA etc.) [77] that are essential for the survival and virulence of the parasites. On the other hand, terpenes are well-known to inhibit the biosynthesis of isoprenoids in P. falciparum [72]. Furthermore, LC-MS feature detection and alignment, with molecular networking workflow on GNPS, allowed for the identification of two clusters, mainly composed of known antifungal compounds, such as valsafungin A and bacillamidin on the one hand, as well as fatty acids, ketone, and aldehyde derivatives on the other hand. Valsafungin A and bacillamidin are well-known to alter drug target and sterol biosynthesis in the membranes of certain microorganisms, such as fungi [51]. It has also been reported that the antiplasmodial activity of aldehyde and ketone derivatives might be attributed to the inhibition of the heme detoxification pathway [78]. Thus, it is not unreasonable to speculate that Lippia adoensis extracts might have exerted antiparasitic activity by at least one of these mechanisms of action. The extracts from L. adoensis were also found to be non-cytotoxic on Vero cells, thus justifying the safe traditional use of this plant in the treatment of several parasitic diseases. However, additional cytotoxicity assays toward other cell lines and in vivo toxicity experiments are needed to confirm this claim.
Overall, L. adoensis extracts, which were predicted to contain a number of secondary metabolites including valsafungin A and bacillamidin, fatty acids, ketone, and aldehyde derivatives; and reported to contain a number of terpenoids, inhibited the growth of three parasites viz. P. falciparum, T. brucei brucei, and Leishmania donovani. As per our knowledge, this is the first report on the antiparasitic activity of L. adoensis, thus justifying the use of this plant in the traditional treatment of fever and malaria symptoms in ethnomedicine. However, additional studies, including in-depth toxicity tests, pharmacokinetics, and in vivo antiparasitic experiments, are warranted to support the successful utilization of this plant in treating malaria and other parasitic diseases.
5. Limitations and Perspectives
The present study summarizes a preliminary screening of the antiprotozoal action of Lippia adoensis extracts. Crude extracts from L. adoensis inhibited the growth of P. falciparum (3D7 and Dd2) (IC50s; (3D7): 10.00 and 97.46 μg/mL; (Dd2): 29.48 and 26.96 μg/mL), L. donovani (IC50s: 22.87–10.52 μg/mL), and T. brucei brucei (IC50s: 2.30–55.06 μg/mL). The extracts were found to be non-cytotoxic to Vero cells, thus yielding median cytotoxic concentrations (CC50s) above 100 μg/mL. In vitro phytochemical analysis of the crude extracts revealed the presence of alkaloids, terpenoids, phenolic compounds, and carbohydrates. This novel contribution substantiates the use of Lippia adoensis in the traditional treatment of fever and malaria conditions. As it is essential that plant extracts be thoroughly studied to identify the active principles, a phytochemical analysis of Lippia adoensis extracts was performed using standard protocols. However, the major limitations of this study include the use of the latest generation of separation techniques, such as HPLC, UPLC, etc., and spectroscopic analyses (NMR, IR, etc.), among others, as a complement for a detailed chemical study to identify the antiprotozoal compounds of Lippia adoensis. Nonetheless, to achieve a comprehensive identification of active compounds of Lippia adoensis, bioactivity-guided fractionation and isolation of antiprotozoal compounds, as well as their characterization should be considered among the main perspectives to this work. Furthermore, other studies, including in-depth toxicity tests, pharmacokinetics, and in vivo antiparasitic experiments should be investigated to support the successful utilization of this plant in treating malaria and other parasitic diseases.
6. Conclusions
This study sought to investigate the antiparasitic potential of the hydroethanol extracts from Lippia adoensis leaves and twigs. The obtained results provide adequate evidence lending support, to some extent, to the use of Lippia adoensis preparations in traditional practice to treat fever and certain parasitic infections in humans. In vitro phytochemical screening of L. adoensis extracts revealed the presence of alkaloids, phenolic compounds, carbohydrates, and terpenoids, which might be responsible for the observed antiparasitic effects. Further LC-MS feature detection tandem molecular networking on GNPS predicted valsafungin A and bacillamidin, quite a few fatty acids, ketone, and aldehyde derivatives as the main plant constituents responsible for the observed antiparasitic effects. The low IC50 values recorded for the plant extracts against the four test parasites might be attributable to the synergy or combined effects elicited by multi-component extracts, so achieved by concerted actions of the ligands to produce suitable perturbations of cellular targets. Isolation, purification, and structure elucidation of major constituents from Lippia adoensis leaves and twigs are warranted to support the discovery of novel antiplasmodial, antileishmanial, and antitrypanosomal compounds. In addition, more studies on toxicity and antiprotozoal effects in vivo, in-depth mechanistic studies, and pharmacokinetics of L. adoensis are warranted to prospect this plant as a potential source for effective antiprotozoal pharmacophores.
Author Contributions
Conceptualization, B.P.K., E.A.M.K. and F.F.B.; methodology, E.A.M.K., M.B.T.T., D.D. and C.A.N.N.; software, E.A.M.K., M.B.T.T., D.D. and C.A.N.N.; validation, B.P.K., E.A.M.K. and F.F.B.; formal analysis, E.A.M.K., M.B.T.T., D.D. and C.A.N.N.; investigation, E.A.M.K., M.B.T.T., D.D. and C.A.N.N.; resources, B.P.K., E.A.M.K. and F.F.B.; data curation, E.A.M.K., M.B.T.T., D.D. and C.A.N.N.; writing—original draft preparation, E.A.M.K., M.B.T.T., D.D. and C.A.N.N.; writing—review and editing, B.P.K. and F.F.B.; visualization, B.P.K. and F.F.B.; supervision, B.P.K. and F.F.B.; project administration, B.P.K. and F.F.B.; funding acquisition, B.P.K. and F.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation received funds from the Yaounde–Bielefeld Bilateral Graduate School for Natural Products with Anti-parasite and Antibacterial Activity (YaBiNaPA) (grant number 57316173). The APC was funded by the authors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding authors upon reasonable request. The data are not publicly available due to the sensitive nature of the research supporting the data.
Acknowledgments
The authors acknowledge the Cameroon National Herbarium (Yaounde, Cameroon) for their assistance with plant identification. The authors are also thankful to the Biodefense and Emerging Infections Research Resources Repository (Bei Resources) for providing Leishmania, Trypanosoma, and Plasmodium parasites. Thanks to the “Centre Pasteur” of Cameroon and for providing the mammalian African Green monkey Vero ATCC CRL 1586.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- The World Health Organization (WHO). Neglected Tropical Diseases. 2023. Available online: https://www.who.int/news-room/questions-and-answers/item/neglected-tropical-diseases (accessed on 5 April 2024).
- Tchatat Tali, M.B.; Pone Kamdem, B.; Tchouankeu, J.C.; Boyom, F.F. Current developments on the antimalarial, antileishmanial, and antitrypanosomal potential and mechanisms of action of Terminalia spp. S. Afr. J. Bot. 2023, 156, 309–333. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Wu, B.; Deng, Y.; Li, M.; Yang, Q.; Huang, L.; Cao, Y.; Liu, Y. Drinking water and sanitation conditions are associated with the risk of malaria among children under five years old in sub-Saharan Africa: A logistic regression model analysis of national survey data. J. Adv. Res. 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tigabu, A.; Taye, S.; Aynalem, M.; Adane, K. Prevalence and associated factors of intestinal parasitic infections among patients attending Shahura Health Center, Northwest Ethiopia. BMC Res. Notes 2019, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (WHO). World Malaria Report 2023. 2024. Available online: https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/world-malaria-report-2023-spreadview.pdf (accessed on 21 March 2025).
- Hustedt, J.; Prasetyo, D.B.; Fiorenzano, J.M.; von Fricken, M.E.; Hertz, J.C. Phlebotomine sand flies (Diptera: Psychodidae) and sand fly-borne pathogens in the Greater Mekong Subregion: A systematic review. Parasit. Vectors 2022, 15, 355. [Google Scholar] [CrossRef]
- The World Health Organization (WHO). Health Topics/Leishmaniasis. 2024. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 27 May 2024).
- Hannan, T.B.; Hossain, Z.; Roy, U.; Rahman, S.M.M.; Rahman, M.S.; Sabah, S.; Rahat, M.A.; Chowdhury, R.; Hossain, F.; Mondal, D.; et al. Successful treatment of recurrent visceral leishmaniasis relapse in an immunocompetent adult female with functional hypopituitarism in Bangladesh. PLoS Negl. Trop. Dis. 2024, 18, e0012134. [Google Scholar] [CrossRef]
- Olías-Molero, A.I.; de la Fuente, C.; Cuquerella, M.; Torrado, J.J.; Alunda, J.M. Antileishmanial Drug Discovery and Development: Time to Reset the Model? Microorganisms 2021, 9, 2500. [Google Scholar] [CrossRef]
- The World Health Organization (WHO). Key Facts. Trypanosomiasis, African. 2024. Available online: https://www.afro.who.int/health-topics/trypanosomiasis-african#:~:text=Since%20the%20number%20of%20new,public%20health%20problem%20by%202020.&text=Sleeping%20sickness%20threatens%20millions%20of,countries%20in%20sub%2DSaharan%20Africa (accessed on 20 April 2024).
- Castro, J.A.; de Mecca, M.M.; Bartel, L.C. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef]
- Kwofie, K.D.; Tung, N.H.; Suzuki-Ohashi, M.; Amoa-Bosompem, M.; Adegle, R.; Sakyiamah, M.M.; Ayertey, F.; Owusu, K.B.; Tuffour, I.; Atchoglo, P.; et al. Antitrypanosomal activities and mechanisms of action of novel tetracyclic iridoids from Morinda lucida Benth. Antimicrob. Agents Chemother. 2016, 60, 3283–3290. [Google Scholar] [CrossRef]
- Lozano-Cruz, O.A.; Jiménez, J.V.; Olivas-Martinez, A.; Ortiz-Brizuela, E.; Cárdenas-Fragos, J.L.; Azamar-Llamas, D.; Rodríguez-Rodríguez, S.; Oseguera-Moguel, J.C.; Dorantes-García, J.; Barrón-Magdaleno, C.; et al. Adverse effects associated with the use of antimalarials during the COVID-19 pandemic in a tertiary care center in Mexico City. Front. Pharmacol. 2021, 12, 668678. [Google Scholar] [CrossRef]
- Scariot, D.B.; Staneviciute, A.; Zhu, J.; Li, X.; Scott, E.A.; Engman, D.M. Leishmaniasis and Chagas disease: Is there hope in nanotechnology to fight neglected tropical diseases? Front. Cell Infect. Microbiol. 2022, 12, 1000972. [Google Scholar] [CrossRef]
- Ceravolo, I.P.; Aguiar, A.C.; Adebayo, J.O.; Krettli, A.U. Studies on activities and chemical characterization of medicinal plants in search for new antimalarials: A ten-year review on ethnopharmacology. Front. Pharmacol. 2021, 12, 734263. [Google Scholar] [CrossRef] [PubMed]
- Charlton, R.L.; Rossi-Bergmann, B.; Denny, P.W.; Steel, P.G. Repurposing as a strategy for the discovery of new anti-leishmanials: The-state-of-the-art. Parasitology 2018, 145, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An overview on target-based drug design against kinetoplastid protozoan infections: Human African Trypanosomiasis, Chagas Disease and Leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M. The International Natural Product Sciences Taskforce, Supuran CT, 2021. Natural products in drug discovery: Advances and opportunities. Nat. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Laftouhi, A.; Eloutassi, N.; Ech-Chihbi, E.; Rais, Z.; Abdellaoui, A.; Taleb, A.; Beniken, M.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M.; et al. The impact of environmental stress on the secondary metabolites and the chemical compositions of the essential oils from some medicinal plants used as food supplements. Sustainability 2023, 15, 7842. [Google Scholar] [CrossRef]
- Butler, M.S. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2005, 22, 162–195. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Armson, A.; Lymbery, A.J.; Zahedi, A.; Ash, A. Medicinal plants as a source of antiparasitics: An overview of experimental studies. Pathog. Glob. Health 2023, 117, 535–553. [Google Scholar] [CrossRef]
- Godeto, Y.G.; Bachheti, R.K.; Bachheti, A.; Saini, S.; Wabaidur, S.M.; Mohammed, A.A.A.; Širić, I.; Kumar, P.; Fayssal, S.A.; Rai, N. Sustainable use of extracts of some plants growing in Ethiopia for the formulation of herbal shampoo and its antimicrobial evaluation. Sustainability 2023, 15, 3189. [Google Scholar] [CrossRef]
- Shiferaw, M.; Yusuf, Z.; Desta, M. Physicochemical properties and biological activities of Koseret (Lippia adoensis Hochst. Var. Koseret) seed and leaf oil extracts. Recent Pat. Biotechnol. 2023, 17, 142–150. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology 1–5; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Wansi, J.D.; Sewald, N.; Nahar, L.; Martin, C.; Sarker, S.D. Bioactive essential oils from the Cameroonian rain forest: A review—Part II. Trends Phytochem. Res. 2019, 3, 3–52. [Google Scholar]
- Maroyi, A. Lippia javanica (Burm.f.) Spreng.: Traditional and commercial uses and phytochemical and pharmacological significance in the African and Indian Subcontinent. Evid. Based Complement. Alternat. Med. 2017, 2017, 6746071. [Google Scholar] [CrossRef] [PubMed]
- Boye, A.T.; Ekanem, P.E.; Hailu, T.B.; Hordofa, I.D.; Asfaw, M.S. Histopathological evaluation of ethanolic leaf extract of Lippia adoensis on liver, kidney, and biochemical parameters in Swiss albino mice. Hepatic Med. Evid. Res. 2022, 14, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Stella, D.; Elakovich, S.D.; Oguntimein, B.O. The essential oil of Lippia adoensis leaves and flowers. J. Nat. Prod. 1987, 50, 503–506. [Google Scholar]
- Abegaz, B.; Asfaw, N.; Lwande, W. Constituents of the essential oils from Wild and cultivated Lippia adoensis Hochst. ex Walp. J. Essent. Oil Res. 1993, 5, 487–491. [Google Scholar] [CrossRef]
- Kasali, A.A.; Ekundayo, O.; Winterhalter, P.; Koenig, W.A.; Eshilokun, A.O. Chemical constituents of the essential oil of Lippia adoensis Hochst. ex Walp. Flavour Fragr. J. 2004, 19, 210–212. [Google Scholar] [CrossRef]
- Adelani, B.S.; Olusegun, O.S.; Olulakin, A.G.; Adeolu, A.M. Chemical composition and bioactivity of Lippia adoensis Hochst ex. Walp (Verneneaceae) leaf essential oil against Callosobruchus maculatus Fabricius (Coleoptera: Chrysomelidae). J. Northeast Agric. Univ. 2016, 23, 8–14. [Google Scholar] [CrossRef]
- Fikadu, Y.; Yaya, E.E.; Chandravanshi, B.S. Chemical composition and antioxidant activities of the essential oils of Lippia adoensis hochst ex. walp and Ocimum sanctum linn. Bull. Chem. Soc. Ethiop. 2022, 36, 95–108. [Google Scholar] [CrossRef]
- Dessalegn, E.; Bultosa, G.; Haki, G.D.; Rupasinghe, H.P.V. Effect of extraction solvents on total phenolic contents and in vitro antioxidant activity of the leaves of Lippia adoensis var. Koseret Sebsebe. Food Sci. Qual. Manag. 2020, 94, 29–37. [Google Scholar]
- Watrous, J.; Roach, P.; Alexandrov, T.; Heath, B.S.; Yang, J.Y.; Kersten, R.D.; van der Voort, M.; Pogliano, K.; Gross, H.; Raaijmakers, J.M.; et al. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. USA 2012, 109, E1743–E1752. [Google Scholar] [CrossRef]
- Quinn, R.A.; Nothias, L.F.; Vining, O.; Meehan, M.; Esquenazi, E.; Dorrestein, P.C. Molecular networking as a drug discovery, drug metabolism, and precision medicine strategy. Trends Pharmacol. Sci. 2017, 38, 143–154. [Google Scholar] [CrossRef]
- Fox Ramos, A.E.; Evanno, L.; Poupon, E.; Champy, P.; Beniddir, M.A. Natural products targeting strategies involving molecular networking: Different manners, one goal. Nat. Prod. Rep. 2019, 36, 960–980. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Buli, G.A.; Duga, A.G.; Dessalegn, E. Antimicrobial activity of Lippia adoensis var. koseret against human pathogenic bacteria and fungi. Adv. J. Clin. Exp. Med. 2015, 3, 118–123. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Bagavan, A.; Rahuman, A.A.; Zahir, A.A.; Kamaraj, C.; Elango, G.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Marimuthu, S.; et al. Evaluation of antiplasmodial activity of medicinal plants from North Indian Buchpora and South Indian Eastern Ghats. Malar. J. 2015, 14, 65. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fuorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Khanjani, J.S.; Farazmand, A.; Amin, M.; Doroodgar, A.; Shirzadi, M.; Razavi, M. Methanolic extract’s activity of Artemisia absinthium, Vitex agnuscastus and Phytolaca americana against Leishmania major in vitro and in vivo. Int. Arch. Health Sci. 2015, 2, 69–74. [Google Scholar]
- Siqueira-Neto, J.L.; Song, O.R.; Oh, H.; Sohn, J.H.; Yang, G.; Nam, J.; Jang, J.; Cechetto, J.; Lee, C.B.; Moon, S.; et al. Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl. Trop. Dis. 2010, 4, e675. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for Human African Trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rosenthal, P.J. Comparison of efficacies of cysteine protease inhibitors against five strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 2001, 45, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jarmusch, A.K.; Vargas, F.; Aksenov, A.A.; Gauglitz, J.M.; Weldon, K.; Petras, D.; da Silva, R.; Quinn, R.; Melnik, A.; et al. Mass spectrometry searches using MASST. Nat. Biotechnol. 2020, 38, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Demchak, B.; Ideker, T. Cytoscape tools for the web age: D3.js and Cytoscape.js exporters. F1000Research 2014, 3, 143. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Evans, W.C.; Evans, D. General methods associated with the phytochemical investigation of herbal products. In Trease and Evans’ Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 1989; pp. 135–147. [Google Scholar]
- Odebiyi, O.O.; Sofowora, E.A. Antimicrobial alkaloids from a Nigerian chewing stick (Fagara zanthoxyloides). Planta Medica 1979, 36, 204–207. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods; Springer: Dordrecht, The Netherlands, 1984; Epub ahead of print. [Google Scholar] [CrossRef]
- Ishijima, H.; Uchida, R.; Ohtawa, M.; Kondo, A.; Nagai, K.; Shima, K.; Nonaka, K.; Masuma, R.; Iwamoto, S.; Onodera, H.; et al. Simplifungin and valsafungins, antifungal antibiotics of fungal origin. J. Org. Chem. 2016, 81, 7373–7383. [Google Scholar] [CrossRef]
- Phillipson, J.D.; Wright, C.W. Antiprotozoal agents from plant sources. Planta Medica 1991, 57, 53–59. [Google Scholar] [CrossRef]
- Chan-Bacab, M.J.; Peña-Rodríguez, L.M. Plant natural products with leishmanicidal activity. Nat. Prod. Rep. 2001, 18, 674–688. [Google Scholar]
- Al-Musayeib, N.M.; Mothana, R.A.; Matheeussen, A.; Cos, P.; Maes, L. In vitro antiplasmodial, antileishmanial and antitrypanosomal activities of selected medicinal plants used in the traditional Arabian Peninsular region. BMC Complement. Altern. Med. 2012, 12, 49. [Google Scholar] [CrossRef]
- Boniface, P.K.; Pal, A. Susbtantiation of the ethnopharmacological use of Conyza sumatrensis in the treatment of malaria through in vivo evaluation in Plasmodium berghei K173 infected mice. J. Ethnopharmacol. 2013, 145, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Boniface, P.K.; Singh, M.; Maurya, A.K.; Pal, A. Acute and sub-chronic toxicity of HPLC fingerprinted extract of Conyza sumatrensis in rodents. J. Ethnopharmacol. 2013, 149, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, E.; Kwenti, T.E.; Kheyri, P.; Nezaratizade, S.; Lindsay, D.S.; Khamesipour, F. Antiplasmodial, antimalarial activities and toxicity of African medicinal plants: A systematic review of literature. Malar. J. 2021, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Amang, À.; Ngnoung, G.A.; Sidjui, L.S.; Leutcha, P.B.; Nganso Ditchou, Y.O.; Tchokouaha, L.R.Y.; Herbette, G.; Baghdikian, B.; Kowa, T.K.; Soh, D.; et al. Antileishmanial and antiplasmodial activities of secondary metabolites from the root of Antrocaryon klaineanum Pierre (Anacardiaceae). Molecules 2023, 28, 2730. [Google Scholar] [CrossRef]
- Osorio, E.J.; Robledo, S.M.; Bastida, J. Alkaloids with antiprotozoal activity. Alkaloids Chem. Biol. 2008, 66, 113–190. [Google Scholar]
- Tempone, A.G.; Pieper, P.; Borborema, S.E.T.; Thevenard, F.; Lago, J.H.G.; Croft, S.L.; Anderson, E.A. Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria. Nat. Prod. Rep. 2021, 38, 2214–2235. [Google Scholar] [CrossRef]
- Durão, R.; Ramalhete, C.; Madureira, A.M.; Mendes, E.; Duarte, N. Plant terpenoids as hit compounds against trypanosomiasis. Pharmaceuticals 2022, 15, 340. [Google Scholar] [CrossRef]
- Rodrigues, A.C.J.; Carloto, A.C.M.; Gonçalves, M.D.; Concato, V.M.; Detoni, M.B.; Dos Santos, Y.M.; Cruz, E.M.S.; Madureira, M.B.; Nunes, A.P.; Pires, M.F.M.K.; et al. Exploring the leishmanicidal potential of terpenoids: A comprehensive review on mechanisms of cell death. Front. Cell Infect. Microbiol. 2023, 13, 1260448. [Google Scholar] [CrossRef]
- Mamede, L.; Ledoux, A.; Jansen, O.; Frédérich, M. Natural phenolic compounds and derivatives as potential antimalarial agents. Planta Medica 2020, 86, 585–618. [Google Scholar] [CrossRef]
- Dziduch, K.; Greniuk, D.; Wujec, M. The current directions of searching for antiparasitic drugs. Molecules 2022, 27, 1534. [Google Scholar] [CrossRef]
- Santos, E.C.D.; Silva, L.S.; Pinheiro, A.S.; Teixeira, D.E.; Peruchetti, D.B.; Silva-Aguiar, R.P.; Wendt, C.H.C.; Miranda, K.R.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H.; et al. The monoterpene 1,8-cineole prevents cerebral edema in a murine model of severe malaria. PLoS ONE 2022, 17, e0268347. [Google Scholar] [CrossRef] [PubMed]
- Boyom, F.F.; Ngouana, V.; Kemgne, E.A.; Zollo, P.H.; Menut, C.; Bessiere, J.M.; Gut, J.; Rosenthal, P.J. Antiplasmodial volatile extracts from Cleistopholis patens Engler & Diels and Uvariastrum pierreanum Engl. (Engl. & Diels) (Annonaceae) growing in Cameroon. Parasitol. Res. 2011, 108, 1211–1217. [Google Scholar] [PubMed]
- Pagola, S.; Stephens, P.W.; Bohle, D.S.; Kosar, A.D.; Madsen, S.K. The structure of malaria pigment beta-haematin. Nature 2000, 404, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.J. Haemozoin formation. Mol. Biochem. Parasitol. 2008, 157, 127–136. [Google Scholar] [CrossRef]
- Kamatou, P.P.; Viljoen, A.M. Linalool—A review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar] [CrossRef]
- Rodrigues Goulart, H.; Kimura, E.A.; Peres, V.J.; Couto, A.S.; Aquino Duarte, F.A.; Katzin, A.M. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 2502–2509. [Google Scholar] [CrossRef]
- van Schaijk, B.C.; Kumar, T.R.; Vos, M.W.; Richman, A.; van Gemert, G.J.; Li, T.; Eappen, A.G.; Williamson, K.C.; Morahan, B.J.; Fishbaugher, M.; et al. Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot. Cell 2014, 13, 550–559. [Google Scholar] [CrossRef]
- Parreira de Aquino, G.; Mendes Gomes, M.A.; Köpke Salinas, R.; Laranjeira-Silva, M.F. Lipid and fatty acid metabolism in trypanosomatids. Microb. Cell 2021, 8, 262–275. [Google Scholar] [CrossRef]
- Arya, R.; Dhembla, C.; Makde, R.D.; Sundd, M.; Kundu, S. An overview of the fatty acid biosynthesis in the protozoan parasite Leishmania and its relevance as a drug target against leishmaniasis. Mol. Biochem. Parasitol. 2021, 246, 111416. [Google Scholar] [CrossRef]
- Almeida Rezende, B.; Pereira, A.C.; Cortes, S.F.; Lemos, V.S. Vascular effects of flavonoids. Curr. Med. Chem. 2016, 23, 87–102. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, S.; Kumar, R.; Kumar, A.; Verma, R.; Darokar, M.P.; Rout, P.; Pal, A. Inhibition of heme detoxification pathway in malaria parasite by 3-hydroxy-11-keto-β-boswellic acid isolated from Boswellia serrata. Biomed. Pharmacother. 2021, 144, 112302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Oman Medical Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).