Abstract
ATP citrate lyase (ACL) is a highly conserved enzyme across eukaryotes that catalyzes the generation of cytosolic acetyl-CoA from citrate—a pivotal step linking central carbon metabolism to lipid biosynthesis. In the oleaginous yeast Yarrowia lipolytica, ACL is encoded by two genes, ACL1 and ACL2, forming a heteromeric complex that mirrors the multidomain architecture of the single-chain ACL enzymes found in mammals and plants. This conservation of catalytic architecture reflects a shared catalytic strategy across kingdoms, underscoring ACL’s fundamental role in metabolic integration. In Y. lipolytica, ACL is essential for directing mitochondrial citrate toward acetyl-CoA production and subsequent lipid accumulation. Yet, in contrast to well-characterized ACLs in animals and plants, the functional mechanisms and regulation of yeast ACL remain incompletely understood. A deeper understanding of ACL in Y. lipolytica offers not only evolutionary insights but also potential avenues for engineering lipid overproduction in microbial systems.
1. Introduction
ATP citrate lyase (ACL) catalyzes a central reaction in eukaryotic metabolism by converting citrate, exported from the mitochondria, into acetyl-CoA and oxaloacetate (Figure 1). This conversion provides the primary source of cytosolic acetyl-CoA, which fuels multiple anabolic processes, including fatty acid, triacylglycerol (TAG), phospholipid, and sterol biosynthesis, as well as the production of secondary metabolites. In oleaginous yeasts, this reaction is particularly critical, as it establishes the metabolic link between carbohydrate degradation and lipid accumulation, allowing cells to divert excess carbon into storage lipids [1,2,3,4,5]. Beyond its role in lipid metabolism, ACL also contributes to acetyl-CoA supply for protein acetylation and other regulatory processes, highlighting its broader significance in growth, stress adaptation, and metabolic homeostasis.
Figure 1.
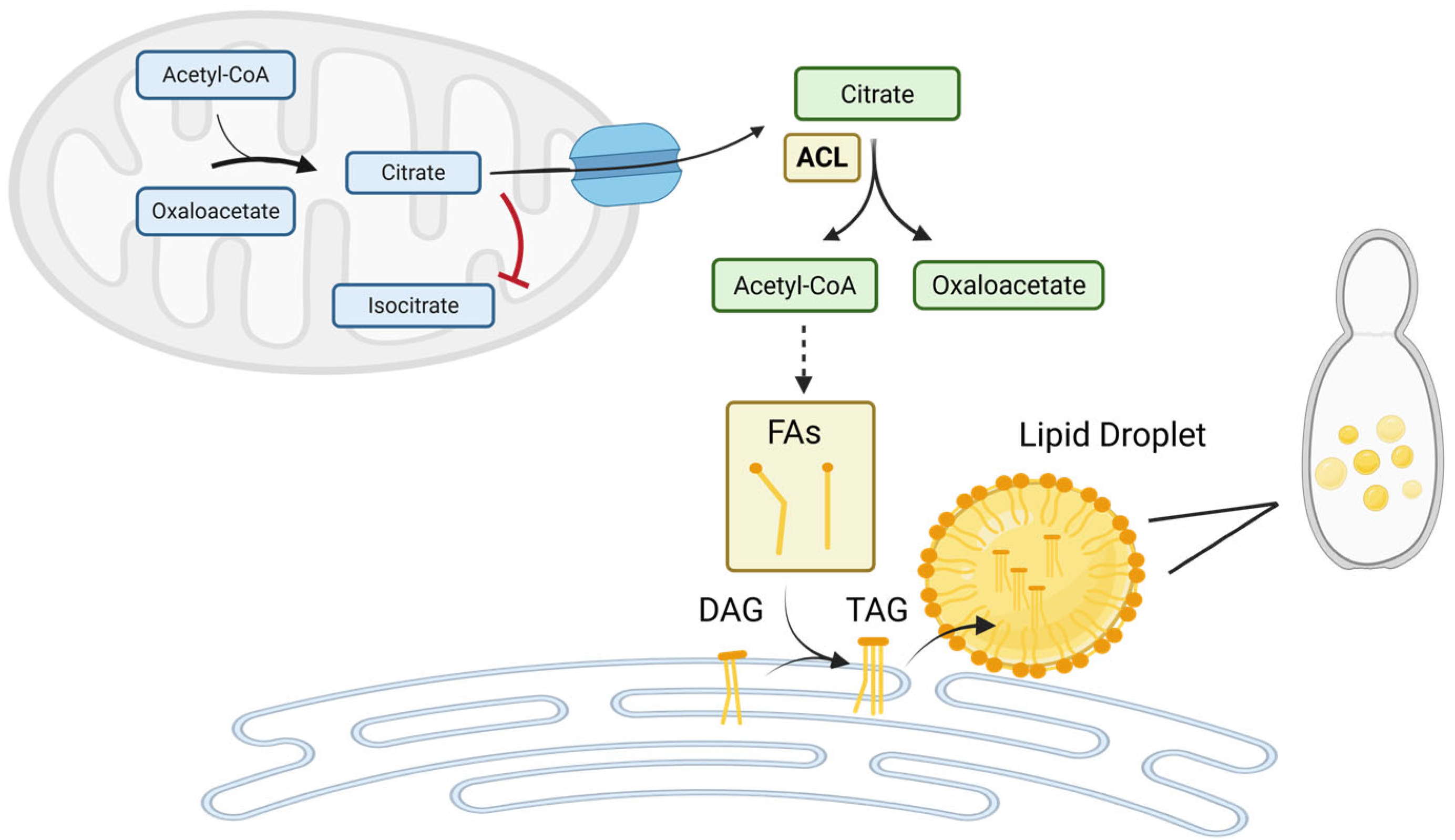
ATP-Citrate Lyase (ACL) Drives Cytosolic Acetyl-CoA Supply and Lipid Biosynthesis in Yarrowia lipolytica. ACL converts cytosolic citrate into acetyl-CoA and oxaloacetate, providing the key precursor for fatty acid (FA) synthesis, triacylglycerol (TAG) formation, and lipid droplet assembly. Under nitrogen-limited conditions, downregulation of mitochondrial isocitrate dehydrogenase (ICDH) which converts citrate to isocitrate leads to citrate accumulation in the mitochondria. Excess citrate is exported to the cytosol, where ACL initiates its conversion into lipid-building blocks. This ACL-dependent pathway is critical for oleaginicity in Yarrowia lipolytica, linking metabolic adaptation to nutrient stress with enhanced lipid storage. (Figure created with BioRender.com).
Despite this conserved catalytic role, ACL varies in genetic organization and quaternary structure across eukaryotic lineages. In mammals [6] and certain fungi such as Basidiomycota [5,7,8], ACL is encoded by a single gene that produces a homotetrameric enzyme. By contrast, most fungi [9,10,11,12,13,14,15] and plants [16,17,18,19] possess a two-gene system, referred to as ACL1 and ACL2 in fungi or ACLA and ACLB in plants, which together encode a heteromeric complex. This divergence in genetic architecture reflects an evolutionary split, but the catalytic mechanism remains conserved, underscoring the enzyme’s central importance.
In the oleaginous yeast Yarrowia lipolytica, ACL plays a central role in redirecting carbon flux from central metabolism into lipid biosynthesis [12]. Its activity not only drives TAG accumulation under nutrient-limited conditions but also supports the synthesis of membrane lipids and sterols required for cellular growth and adaptation. Structural predictions of the heterodimeric enzyme suggest a modular organization in which Acl1 provides the catalytic core, while Acl2 contributes regulatory domains that stabilize the active complex and coordinate ATP hydrolysis [20,21,22]. This division of labor differs from the single-chain mammalian ACL, yet functional studies on yeast heterodimeric ACLs remain limited, and the molecular mechanisms that govern their assembly, regulation, and stability are still poorly understood. Deletion of either ACL subunit in Y. lipolytica leads to reduced fatty acid and lipid levels, underscoring the essential role of this enzyme in sustaining oleaginous metabolism [12,20,21,22]. Elucidating these structural and mechanistic features is critical, as they may underlie the metabolic robustness of oleaginous yeasts and could provide new strategies for engineering ACL activity to enhance lipid accumulation. Despite its central importance, research on dimeric ACLs in yeasts like Y. lipolytica is still in its infancy, leaving substantial gaps in our understanding of how this unique architecture shapes both enzyme function and metabolic control.
2. Comparative Overview of ATP-Citrate Lyase in Mammals, Plants, and Fungi
Given the primary focus of this review on yeast ACL, particularly in Y. lipolytica, coverage of ACL enzymes in higher eucaryotes (e.g., humans, rodents, plants) is limited to comparative insights that inform understanding of the fungal system, with only brief references to mammalian roles in disease contexts such as cancer and metabolic disorders. The difference in nomenclature—ACLY for mammals versus ACL for most fungi and plants—reflects these distinct evolutionary organizations of the enzyme, with mammals encoding the full catalytic architecture in one gene product, while fungi and plants divide the domains across two separate proteins.
2.1. ATP-Citrate Lyase in Mammals
In humans, ACLY mediates glucose-dependent histone acetylation during adipocyte differentiation by linking glucose metabolism to epigenetic regulation [23]. Silencing ACLY abolishes serum-induced histone acetylation, reduces global acetylation levels, and significantly impairs lipid accumulation [23]. This is accompanied by decreased expression of the glucose transporter Glut4 and key glycolytic enzymes, as well as a 32% drop in glucose consumption [23]. These findings highlight ACLY’s critical role in coordinating nutrient availability with gene expression during adipogenesis.
Multiple studies have established ACLY as a central regulator of lipid metabolism, inflammation, and cardiometabolic disease. Pharmacological or genetic inhibition of ACLY reduces cholesterol and fatty acid synthesis and decreases plasma lipids in rodents and dogs due to reduced hepatic very low-density lipoprotein (VLDL) production [24]. Infantino et al. further linked ACLY to inflammation, showing that its activity is necessary to produce key inflammatory mediators in macrophages [25]. ACL knockdown disrupts glucose-derived lipogenesis and modulates cell survival under nutrient-deprived conditions [26]. In adipose tissues, ACLY contributes to thermogenic regulation [27] and supports carbohydrate-responsive element-binding protein (ChREBP) activation and fatty acid storage in a sex-specific manner [28]. ACLY inhibition also upregulates low-density lipoprotein (LDL) receptors and attenuates atherosclerosis [29]. Finally, Ference et al. demonstrated that genetic inhibition of ACLY lowers LDL cholesterol and cardiovascular risk similarly to statins, with no increase in cancer incidence, supporting the therapeutic potential of targeting ACL in cardiometabolic disease [30].
ACLY is essential for embryonic development in mice, with complete knockout resulting in embryonic lethality, and is strongly expressed during embryogenesis, particularly in the neural tube [6]. While heterozygous Acly+/− mice exhibit normal metabolic profiles, hepatic deletion in obese mice suppresses lipogenesis by reducing acetyl-CoA and malonyl-CoA levels, downregulating key lipogenic enzymes, and altering transcriptional regulators such as peroxisome proliferator-activated receptor gamma (PPARγ), liver X receptor (LXRα), and sterol regulatory element-binding proteins (SREBPs), leading to improved liver histology and enhanced glucose metabolism [31]. ACLY is also critical in pancreatic β-cells, where it supports cell survival under lipotoxic stress; palmitate exposure initially increases ACLY phosphorylation but eventually reduces its expression and activity, resulting in increased ER stress and apoptosis [32]. These findings underscore ACLY’s central role in metabolic regulation, lipid homeostasis, and cell viability.
Hepatocyte-specific ACLY knockout reduces fatty acid and sterol synthesis while enhancing fatty acid oxidation, as shown both in isolated hepatocytes and in vivo [33]. These mice display lower whole-body respiratory exchange ratio values consistent with reduced carbohydrate oxidation and diminished de novo lipogenesis, accompanied by decreased hepatic steatosis, free fatty acids, and TAG without changes in liver mass or cholesterol content [33]. Unlike ACC inhibition, ACLY deletion also lowers circulating TAG and cholesterol, underscoring its central role in hepatic and systemic metabolism [33].
2.2. ATP-Citrate Lyase in Plants
Fatland et al. [16] conclusively demonstrated that ACLA-1 and ACLB-2 encode two distinct and essential subunits of cytosolic ACL in Arabidopsis, contributing to the cytosolic acetyl-CoA pool. The nearly identical spatial and temporal expression patterns of ACLA and ACLB mRNAs throughout development suggest tightly coordinated regulation of both subunits. Even moderate reductions in ACL activity lead to a severe, pleiotropic growth phenotype characterized by stunted development, cellular abnormalities, altered plastid morphology, and metabolic imbalances [17]. The severity of these defects correlates with the extent of ACL activity loss. Additionally, reduced expression of the ACLA subunit leads to decreased accumulation of the ACLB subunit, suggesting coordinated regulation of both components of the ACL complex [17].
In Arabidopsis, ACL is essential for providing cytosolic acetyl-CoA required for the acetylation of plant cell wall polysaccharides. Expression of ACL genes is upregulated in secondary wall-forming cells and is induced by transcriptional activators associated with secondary wall biosynthesis [34]. RNAi-mediated downregulation of ACL expression led to a significant reduction in xylan acetylation and moderate decreases in the acetylation of glucomannan, xyloglucan, and pectin. These findings support a key role for ACL-derived acetyl-CoA in supplying the acetyl groups necessary for the modification of structural polysaccharides during secondary wall formation.
Three ACL genes were identified in the citrus genome: CitACLα1, CitACLα2, and CitACLβ1. CitACLα1 and CitACLα2 encode putative alpha subunits sharing 82.5% amino acid identity, while CitACLβ1 encodes a beta subunit [18]. Expression profiling demonstrated strong tissue-specific regulation, with CitACLα1 and CitACLβ1 most highly expressed in fully opened flowers, whereas CitACLα2 showed elevated expression in stems and fibrous roots. Notably, CitACLβ1 expression in flowers and stems was nearly thirty-fold higher than in fruit juice sacs, suggesting distinct metabolic roles of the ACL subunits across citrus tissues [18].
In pepper, CaACLA forms a functional complex with CaACLB to utilize cytoplasmic citrate, thereby supplying acetyl-CoA for essential metabolites and sustaining normal growth [35]. Silencing of CaACLA leads to impaired growth, reduced acetyl-CoA–derived metabolites, and diminished tolerance to heat stress, underscoring its dual role in development and stress adaptation. Interestingly, overexpression of CaACLA in tomato disrupts cellular energy metabolism and compromises heat tolerance, pointing to species-specific regulatory mechanisms. Under heat stress in pepper, CaACLA is rapidly degraded through both ubiquitin–proteasome and autophagy pathways, a process that preserves cytoplasmic citrate for antioxidant defenses and membrane protection. Together, these findings suggest that fine-tuned regulation of CaACLA allows pepper to balance growth with environmental resilience, highlighting ACL as a central metabolic node in plant stress physiology [35].
2.3. ATP-Citrate Lyase in Filamentous Fungi
ACL was identified in multiple species of both higher and lower fungi, suggesting that the presence of this enzyme is a common feature among filamentous fungi, particularly within the Ascomycota and Zygomycota phyla [15,36]. Its activity has been reported in genera such as Fusarium, Penicillium, Aspergillus, Mucor, and Mortierella, where it is strongly associated with oleaginicity and the ability to accumulate large amounts of storage lipids [36]. Enzyme assays confirmed ACL activity in Endomyces exitialis, Candida nanodes, and Mortierella alpina-peyron, all of which accumulated over 20% lipid by dry weight, consistent with their classification as oleaginous species [37]. In contrast, no activity was detected in marine fungi or Sapra parasitica, which showed limited lipid accumulation, reinforcing the concept that ACL is indispensable for high lipid storage. Strikingly, fungi lacking ACL rarely exceeded 10% lipid content, while those with detectable activity were capable of accumulating much higher amounts, although their polyunsaturated fatty acid (PUFA) profiles varied. For example, M. alpina-peyron reached the highest lipid content but produced relatively little PUFA, whereas S. parasitica and Thraustochytrium aureum synthesized significant amounts of long-chain PUFA but stored little total lipid, reflecting the trade-off between bulk storage lipids and specialized fatty acids [37]. These observations suggest that the most promising candidates for industrial lipid or PUFA production are those fungi that combine oleaginicity, indicated by ACL activity, with the ability to tailor lipid composition toward valuable fatty acid species.
Comparative studies of Mucor circinelloides strains with differing lipid-producing capacities reveal a strong correlation between ACL activity and fatty acid biosynthesis [38]. In high lipid-producing strains, ACL activity is markedly higher than in low lipid-producing strains, suggesting an increased supply of cytosolic acetyl-CoA for fatty acid synthesis. This enhanced acetyl-CoA availability likely underpins the greater lipid accumulation observed in these strains, highlighting ACL as a key determinant of oleaginicity in filamentous fungi. These findings support the broader concept that variations in ACL activity among strains or species can directly influence lipid biosynthetic capacity [38]. Consequently, modulation of ACL activity represents a potential strategy to optimize lipid production in industrially relevant oleaginous fungi.
In Cunninghamella species, ACL plays a central role in coordinating lipogenesis under nitrogen-limited conditions. Activation of lipid biosynthesis is marked by increased activities of ACL, along with malic enzyme and fatty acid synthase, highlighting the enzyme’s contribution to providing cytosolic acetyl-CoA for fatty acid production [15]. Lipid accumulation ceases when ACL activity declines, indicating that reduced acetyl-CoA supply limits further fatty acid synthesis. Restoration of ACL activity following ammonium supplementation confirmed that its downregulation under nitrogen depletion is reversible and directly linked to nutrient status. Additional experiments mixing cell-free extracts from early and late cultures suggested that inhibitory factors may modestly suppress ACL activity, but nutrient limitation remains the primary determinant of its regulation and, consequently, lipid accumulation [15].
In Sordaria macrospora, ACL activity fluctuates during development, peaking early in mycelial growth and declining as the culture progresses. The temporal pattern of ACL expression closely aligns with the transition from vegetative growth to sexual development, suggesting a regulatory role in fruiting body formation. This developmental link is further supported by observations that peak ACL activity shifts in accordance with delayed mycelial density and subsequent onset of reproductive structures. These findings point to ACL as a crucial factor that integrates metabolic status with developmental timing [13]. In Gibberella pulicaris, ACL2 is constitutively expressed in uninduced mycelium and is upregulated in response to rishitin, a potato phytoalexin [9]. This induction is likely part of a membrane-repair response, as ACL supplies acetyl-CoA required for phospholipid biosynthesis and membrane restoration [9].
ACL from Aspergillus niger is a cytosolic, constitutively expressed enzyme encoded by the aclA and aclB genes that plays a major role in acetyl-CoA production during growth on carbohydrates [39]. It has a relatively low citrate affinity and is inhibited by nucleoside phosphates and palmitoyl-CoA [39]. Deletion of acl genes impaired growth on carbon sources metabolized via 2-oxoglutarate, but growth was restored by acetate [10]. In contrast, growth was normal on acetate or ethanol, although ethanol-dependent growth repair was blocked by glucose due to catabolite repression [10]. The acl genes were expressed during growth on glucose but were repressed when acetate or ethanol was the sole carbon source, indicating feedback repression by acetyl-CoA [10]. The deletion strains were also able to grow on fatty acids metabolized via β-oxidation, suggesting that acetyl-CoA generated through peroxisomal fatty acid catabolism can partially bypass the requirement for ACL [10]. Similar results were obtained by Chen et al. [11], who also demonstrated that both subunits are required to form a functional ACL.
3. ATP: Citrate Lyase in Yeasts
3.1. Regulation by Growth Conditions
Early research established ACL as a key determinant of lipid accumulation in yeasts [1,2,40,41]. Its consistent presence in lipid-rich species, and absence in non-oleaginous yeasts, provided one of the first direct genetic and biochemical links between ACL and oleaginicity [1]. In extracts from both nitrogen- and carbon-limited cells of Candida 107, an oleaginous yeast capable of accumulating high levels of lipids, ACL activity was consistently present but remained relatively low compared to other oleaginous species [40]. This observation highlights that although ACL is essential for channeling citrate-derived acetyl-CoA into lipid biosynthesis, the enzyme does not always operate at high activity levels. By contrast, the enzyme could not be detected in more than ten independently prepared extracts of Candida utilis, a non-oleaginous species, grown under either carbon- or nitrogen-limiting conditions, regardless of whether exogenous AMP was included in the assay [40].
ACL is thought to serve as the primary source of acetyl-CoA for lipid synthesis, enabling the diversion of carbon from central metabolism toward fatty acid and TAG production. Work in Lipomyces starkeyi further strengthened this connection, showing that ACL activity closely tracked lipid synthesis rates under nitrogen-limited conditions, a classic trigger for oleaginous metabolism [1]. These findings suggested that ACL is not only required for lipid accumulation but may also represent a rate-limiting step in the pathway. During transitions from carbon to nitrogen limitation in L. starkeyi, no significant changes in the specific activity of ACL were observed. This finding was confirmed in independent transition experiments, indicating that the enzyme activity remains stable under these nutrient shifts [42].
Regulatory studies confirmed that ACL is subject to tight metabolic control, consistent with its central role in coordinating carbon flux. In L. starkeyi, ACL activity is reversibly inhibited by oleoyl-CoA and other long-chain acyl-CoA esters at micromolar concentrations, highlighting a feedback mechanism that prevents uncontrolled lipid accumulation [2]. Comparable findings in Rhodotorula gracilis extended this regulatory picture, where ACL activity was inhibited in a dose-dependent manner by ADP and AMP, but not other monophosphate nucleotides [5]. In addition, glucose 6-phosphate acted as a strong inhibitor, whereas structurally related sugar phosphates were ineffective, pointing to a high degree of substrate specificity in regulation. Long-chain acyl-CoA esters such as oleoyl-, palmitoyl-, and myristoyl-CoA produced potent concentration-dependent inhibition, particularly when the enzyme was preincubated with the effector, suggesting conformational sensitivity. Moreover, inhibition by sulfhydryl-reactive compounds such as pCMB implicated cysteine residues as critical for catalysis [5]. Together, these studies demonstrate that yeast ACL functions not merely as a passive supplier of acetyl-CoA, but as a highly regulated metabolic control point that integrates energy status, carbon supply, and lipid feedback signals to balance growth and storage lipid production.
In Starmerella bombicola, deletion and overexpression of the ACL1 subunit revealed its central role in regulating growth, glycolipid, and citric acid metabolism. Loss of ACL1 reduced the yeast’s specific growth rate and glycolipid synthesis while increasing extracellular and intracellular citric acid accumulation, although the final cell density and intracellular acetyl-CoA levels were largely unaffected [14]. Overexpression of ACL1 enhanced intracellular acetyl-CoA, reduced extracellular citric acid, and slightly increased malic acid, but unexpectedly decreased sophorolipid production, indicating a complex influence on secondary metabolite partitioning. Both deletion and overexpression altered the lactonic-to-acidic sophorolipid ratio under conditions without initial citrate supplementation, highlighting the sensitivity of glycolipid profiles to ACL1 expression [14]. These findings suggest that ACL1 is a key metabolic node in S. bombicola, balancing growth, acetyl-CoA availability, citric acid accumulation, and sophorolipid biosynthesis.
3.2. Molecular and Biochemical Characterization
Biochemical characterization in Rhodotorula toruloides confirms ACL’s central role in oleaginous yeast lipid accumulation. The partially purified ~480 kDa enzyme showed optimal activity at pH 8.2 and followed Michaelis-Menten kinetics, with Km values of 0.19 mM for ATP and 0.15 mM for citrate [8]. Activity was specific for ATP and inhibited by ADP, suggesting classic product inhibition. Notably, ammonium ions enhanced activity under low citrate concentrations, likely by increasing substrate affinity. Inhibitory metabolites included glucose-6-phosphate and fatty acyl-CoAs (palmitoyl-CoA, oleoyl-CoA), with glucose-6-phosphate’s inhibition being dependent on citrate concentration [8]. The observed regulatory patterns closely paralleled those in L. starkeyi [2,3], supporting a conserved role for ACL in integrating carbon flux toward lipogenesis in oleaginous yeasts.
The ACL from Phaffia rhodozyma was biochemically characterized after purification and shown to retain the canonical features of this enzyme. Kinetic analyses revealed Michaelis–Menten behavior with Km values of 70.2 mM for citrate, 0.064 mM for CoA, and 1.6 mM for ATP, highlighting a particularly high affinity for CoA and ATP but a relatively high substrate requirement for citrate [8]. The corresponding Vmax values were 2.02, 0.35, and 0.45 µmol mg−1 min−1, confirming efficient turnover under substrate-saturated conditions. The enzyme displayed an optimal activity at 21 °C and pH 8.5, consistent with adaptation to moderately alkaline conditions and lower temperature optima. These biochemical parameters underscore the conserved catalytic features of ACL while providing valuable insights into the enzymatic properties of this basidiomycetous yeast, linking its activity to central carbon metabolism and lipid biosynthesis [8].
3.3. Yarrowia lipolytica ACL
3.3.1. Modular Organization and Functional Domains of Y. lipolytica ACL
Y. lipolytica and human ACLs share a conserved five-domain architecture and a C-terminal citrate synthase (CS)-like domain, organized into three functional catalytic sites [43]. Site I carries out the initial phosphorylation of a histidine residue (His760 in humans [44] and His291 in Y. lipolytica) and subsequent activation of citrate to citryl-phosphate. Site II is part of the Acl2 subunit and includes an ATP-grasp fold, which facilitates ATP hydrolysis. Site III, located in the CS-like domain, catalyzes the cleavage of citryl-CoA to yield acetyl-CoA and oxaloacetate and mediates tetramerization in the human enzyme [45,46,47]. While human ACL is a single polypeptide forming a homotetramer [46], Y. lipolytica ACL functions as a heteromeric complex composed of two subunits (Acl1 and Acl2) that together reconstitute this catalytic architecture.
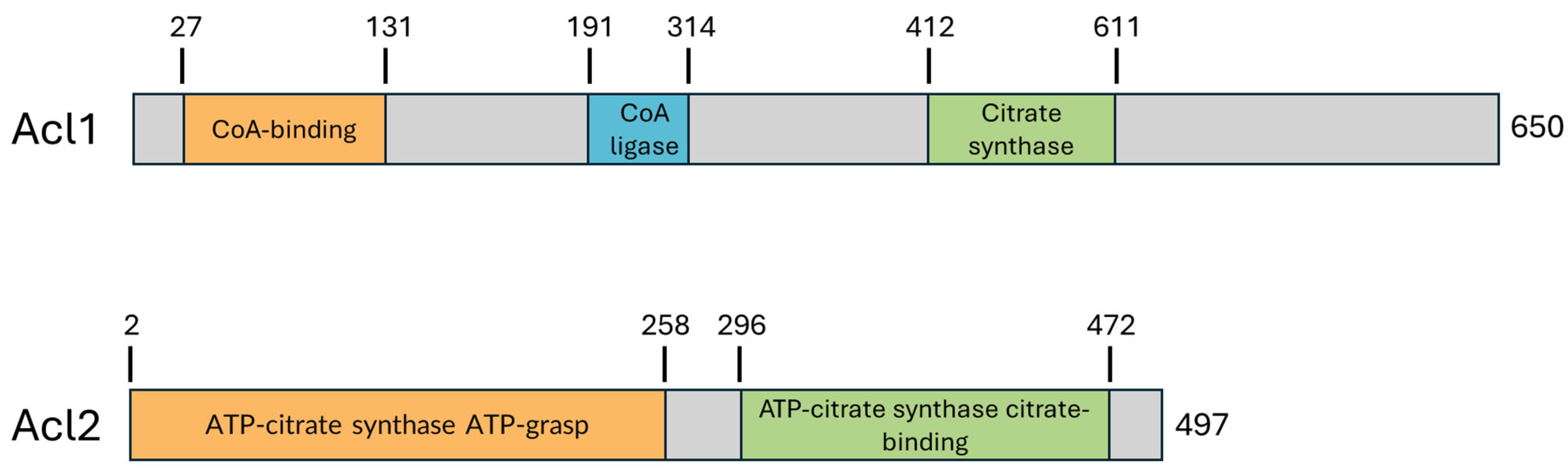
In Y. lipolytica, ACL functions as a heterodimer composed of the catalytic subunit Acl1 (UniProt ID: Q6C3H5) and regulatory subunit Acl2 (UniProt ID: Q6C7Y1), encoded by the genes ACL1 (1953 bp) and ACL2 (1494 bp), respectively (Figure 2). The Acl1 subunit consists of 650 amino acids (71.4 kDa) and contains three key domains arranged from N- to C-terminus: a CoA-binding domain (residues 27–131), which anchors CoA as a substrate; a CoA ligase domain (191–314), which mediates the transfer of the phosphoryl group to citrate; and a citrate synthase–like domain at the C-terminus (412–611), which catalyzes the cleavage of citryl-CoA into acetyl-CoA and oxaloacetate. The Acl2 subunit, comprising 497 amino acids (54.4 kDa), features two functional regions: an ATP-grasp domain (72–228), which facilitates ATP hydrolysis, and a citrate-binding domain (296–497), which positions citrate for catalysis. Together, Acl1 and Acl2 reconstitute the three catalytic sites of ACL, functioning in a manner analogous to the single-chain mammalian enzyme and reflecting a conserved modular organization across species. A multiple sequence alignment of the Yarrowia, fungal, plant, and mammalian ACL sequences is provided in the Supplementary Material.
Figure 2.
Schematic representation of domain organization in Yarrowia lipolytica Acl1 and Acl2. The catalytic subunit Acl1 contains an N-terminal acyl-CoA synthetase domain, a central CoA-ligase/AMP-binding domain, and a C-terminal citrate-binding domain, consistent with its role in acetyl-CoA production. Acl2, which functions as a non-catalytic regulatory subunit, includes an ATP-citrate synthase ATP-grasp domain and a citrate-binding domain. Domain assignments were based on UniProt and Pfam annotations.
3.3.2. Regulation of ACL in Y. lipolytica
ACL activity in Y. lipolytica is highly dependent on environmental factors. High dissolved oxygen concentrations increase its activity [48], while growth in a double-limited medium (i.e., limited in nitrogen and magnesium) significantly lowers it compared to a nitrogen-limited one [49]. Interestingly, although glucose as a substrate yields higher biomass and lipid production than glycerol, the preferred carbon source of Y. lipolytica, ACL activity is paradoxically lower [49]. Furthermore, mRNA expression levels for ACL1 and ACL2 were found to be unaffected by the presence or absence of magnesium [49].
Overexpression and deletion studies of ACL1 and ACL2 in Yarrowia lipolytica highlight the essential role of ACL in fatty acid synthesis, independent of lipid accumulation capacity. While overexpressing both genes in a mutant strain engineered for enhanced fatty acid production led to only modest increases in fatty acid levels, deletion of ACL1 significantly impaired growth and reduced fatty acid content by up to 80%, even under non-lipogenic conditions [12]. The ACL1 deletion mutant also exhibited altered fatty acid profiles, with increased unsaturation, and reprogrammed carbon flux, including slower glucose consumption and elevated secretion of citrate and mannitol. These results demonstrate that ACL is a critical source of cytosolic acetyl-CoA for fatty acid synthesis and a key regulator of central carbon metabolism in Y. lipolytica.
Inactivation of ACL2 in Y. lipolytica significantly impairs growth on minimal medium with glucose as the sole carbon source, though it does not abolish it entirely [50]. Compared to the control strain, the mutant displays a pronounced lag phase and delayed entry into stationary phase, reaching it considerably later. The mutant also shows a reduced specific growth rate and increased doubling time relative to the control. These findings indicate that while ACL2 is not strictly essential for growth on glucose, it plays a significant role in supporting efficient carbon utilization and cell proliferation under minimal conditions.
Native ACL in Y. lipolytica has a relatively high Km for citrate (~3.6 mM), indicating low affinity and suggesting that high intracellular citrate levels are required for effective acetyl-CoA generation [51]. Comparative studies have shown that replacing the endogenous enzyme with heterologous ACLs possessing lower Km values (e.g., from Mus musculus) enhances acetyl-CoA availability and reduces citrate secretion, highlighting the importance of enzyme affinity in regulating cytosolic citrate flux [51]. Conversely, reducing ACL activity leads to increased citrate accumulation and altered metabolic profiles, underscoring ACL’s key function at the intersection of central carbon metabolism.
In Y. lipolytica, Acl1 and Acl2 are expressed during early growth but their levels decline at later stages, suggesting reduced stability or turnover. Loss of either subunit impairs growth, while overexpression has little effect on growth but markedly alters lipid metabolism [20]. These changes include reduced storage lipid accumulation in deletion strains and enhanced lipid accumulation in overexpression strains, along with shifts in phospholipid content. Fatty acid composition is also remodeled, highlighting the key role of ACL in shaping lipid balance in Y. lipolytica [20]. Complementation experiments showed that reintroduction of ACL1 into the acl1Δ mutant restored and even enhanced lipid accumulation, particularly in the form of TAGs, to levels comparable to or exceeding those of the wild type. Conversely, loss of ACL1 caused major reductions in both fatty acids and TAG. Preliminary results indicated that ACL2 complementation in the acl2Δ mutant produced a similar restoration of lipid and TAG levels, supporting a model in which ACL1 serves as the primary driver of lipid metabolism, especially TAG synthesis, with ACL2 contributing a supporting role [21].
4. Conclusions
ACL is a central metabolic enzyme that converts mitochondrial citrate into cytosolic acetyl-CoA, fueling lipid biosynthesis in Y. lipolytica and other eukaryotes. Its activity directs carbon flux toward fatty acid and TAG, particularly under nutrient-limited conditions. In yeasts and plants, ACL functions as a heteromeric complex, whereas in mammals, ACLY forms a single polypeptide homotetramer, reflecting evolutionary differences while preserving its core metabolic role. Structural analyses of Y. lipolytica ACL have illuminated its architecture and catalytic sites, but key aspects of its mechanism, regulation, and integration with cellular metabolism remain unclear. Future studies across species are needed to define structure–function relationships and how ACL/ACLY orchestrates acetyl-CoA production and lipid accumulation under diverse physiological contexts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/lipidology2040020/s1, Figure S1: Multiple sequence alignment of Yarrowia lipolytica Acl1 (A) and Acl2 (B) with representative bacterial, plant, fungal, and human ATP-citrate lyases; Table S1: Proteins used for ACL sequence alignment.
Author Contributions
S.F.: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing—original draft, Writing—review and editing. A.O.: Formal analysis, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by USDA/NIFA through Evans-Allen Grant 7000736.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACL | ATP-citrate lyase |
| ICDH | Isocitrate dehydrogenase |
| ChREBP | Carbohydrate-Responsive Element-Binding Protein |
| CS | Citrate synthase |
| ER | Endoplasmic reticulum |
| LDL | Low-density lipoprotein |
| LXR | Liver X receptor |
| PPAR | Peroxisome proliferator-activated receptor |
| SREB | Sterol regulatory element-binding protein |
| TAG | Triacylglycerol |
| VLDL | Very low-density lipoprotein |
References
- Boulton, C.; Ratledge, C. Correlation of lipid accumulation in yeasts with possession of ATP: Citrate lyase. J. Gen. Microbiol. 1981, 127, 169–176. [Google Scholar] [CrossRef]
- Boulton, C.; Ratledge, C. ATP:Citrate lyase-The regulatory enzyme for lipid biosynthesis in Lipomyces starkeyi? J. Gen. Microbiol. 1981, 127, 423–426. [Google Scholar]
- Boulton, C.; Ratledge, C. Partial purification and some properties of ATP:citrate lyase from the oleaginous yeast Lipomyces starkeyi. J. Gen. Microbiol. 1983, 129, 2863–2869. [Google Scholar]
- Evans, C.; Ratledge, C. Possible regulation roles of ATP: Citrate lyase, malic enzyme, and AMP deaminase in lipid accumulation by Rhodosporidium toruloides CBS 14. Can. J. Microbiol. 1985, 31, 1000–1004. [Google Scholar] [CrossRef]
- Shashi, K.; Bachhawat, A.K.; Joseph, R. ATP: Citrate lyase of Rhodotorula gracilis: Purification and properties. BBA-Gen. Subj. 1990, 1033, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Beigneux, A.P.; Kosinski, C.; Gavino, B.; Horton, J.D.; Skarnes, W.C.; Young, S.G. ATP-citrate lyase deficiency in the mouse. J. Biol. Chem. 2004, 279, 9557–9564. [Google Scholar] [CrossRef]
- Reķēna, A.; Pals, K.; Gavrilović, S.; Lahtvee, P.-J. The role of ATP citrate lyase, phosphoketolase, and malic enzyme in oleaginous Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2025, 109, 77. [Google Scholar] [CrossRef]
- Chávez-Cabrera, C.; Marsch, R.; Bartolo-Aguilar, Y.; Flores-Bustamante, Z.R.; Hidalgo-Lara, M.E.; Martínez-Cárdenas, A.; Cancino-Díaz, J.C.; Sánchez, S.; Flores-Cotera, L.B. Molecular cloning and characterization of the ATP citrate lyase from carotenogenic yeast Phaffia rhodozyma. FEMS Yeast Res. 2015, 15, fov054. [Google Scholar] [CrossRef][Green Version]
- Nowrousian, M.; Kück, U.; Loser, K.; Weltring, K.-M. The fungal acl1 and acl2 genes encode two polypeptides with homology to the N-and C-terminal parts of the animal ATP citrate lyase polypeptide. Curr. Genet. 2000, 37, 189–193. [Google Scholar] [CrossRef]
- Hynes, M.J.; Murray, S.L. ATP-citrate lyase is required for production of cytosolic acetyl coenzyme A and development in Aspergillus nidulans. Eukaryot. Cell 2010, 9, 1039–1048. [Google Scholar] [CrossRef]
- Chen, H.; He, X.; Geng, H.; Liu, H. Physiological characterization of ATP-citrate lyase in Aspergillus niger. J. Ind. Microbiol. Biotechnol. 2014, 41, 721–731. [Google Scholar] [CrossRef]
- Dulermo, T.; Lazar, Z.; Dulermo, R.; Rakicka, M.; Haddouche, R.; Nicaud, J.-M. Analysis of ATP-citrate lyase and malic enzyme mutants of Yarrowia lipolytica points out the importance of mannitol metabolism in fatty acid synthesis. Biochim. Biophys. Acta 2015, 1851, 1107–1117. [Google Scholar] [CrossRef]
- Nowrousian, M.; Masloff, S.; Pöggeler, S.; Kück, U. Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP-citrate lyase activity. Mol. Cell. Biol. 1999, 19, 450–460. [Google Scholar] [CrossRef]
- Jezierska, S.; Claus, S.; Van Bogaert, I.N.A. Identification and importance of mitochondrial citrate carriers and ATP citrate lyase for glycolipid production in Starmerella bombicola. Appl. Microbiol. Biotechnol. 2020, 104, 6235–6248. [Google Scholar] [CrossRef]
- Hamid, A.A.; Mokhtar, N.F.; Taha, E.M.; Omar, O.; Yusoff, W.M.W. The role of ATP citrate lyase, malic enzyme and fatty acid synthase in the regulation of lipid accumulation in Cunninghamella sp. 2A1. Ann. Microbiol. 2011, 61, 463–468. [Google Scholar] [CrossRef]
- Fatland, B.L.; Ke, J.; Anderson, M.D.; Mentzen, W.I.; Cui, L.W.; Allred, C.C.; Johnston, J.L.; Nikolau, B.J.; Wurtele, E.S. Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol. 2002, 130, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Fatland, B.L.; Nikolau, B.J.; Wurtele, E.S. Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis. Plant Cell 2005, 17, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Shi, C.-Y.; Liu, X.; Jin, L.-F.; Liu, Y.-Z.; Peng, S.-A. Genome-wide identification of citrus ATP-citrate lyase genes and their transcript analysis in fruits reveals their possible role in citrate utilization. Mol. Genet. Genom. 2015, 290, 29–38. [Google Scholar] [CrossRef]
- Liu, F.; Ma, Z.; Cai, S.; Dai, L.; Gao, J.; Zhou, B. ATP-citrate lyase B (ACLB) negatively affects cell death and resistance to Verticillium wilt. BMC Plant Biol. 2022, 22, 443. [Google Scholar] [CrossRef]
- Anche, V.; Fakas, S. ATP-citrate lyase regulates lipid biosynthesis in Yarrowia lipolytica. J. Biol. Chem. 2023, 299, 349. [Google Scholar] [CrossRef]
- Jackson, K.; Fakas, S.; Odunsi, A. Role of ATP-citrate lyase subunits in Yarrowia lipolytica. J. Biol. Chem. 2025, 301, 109431. [Google Scholar] [CrossRef]
- Odunsi, A.; Fakas, S. Functional Characterization of ATP-citrate lyase mutants in Yarrowia lipolytica. J. Biol. Chem. 2025, 301, 109435. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; CB, T. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 1994, 22, 1076–1080. [Google Scholar] [CrossRef]
- Pearce, N.J.; Yates, J.W.; Berkhout, T.A.; Jackson, B.; Tew, D.; Boyd, H.; Camilleri, P.; Sweeny, P.; Gribble, A.D.; Shaw, A. The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Biochem. J. 1998, 334, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Iacobazzi, V.; Palmieri, F.; Menga, A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem. Biophys. Res. Commun. 1992, 440, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.E.; Hatzivassiliou, G.; Zhao, F.; Andreadis, C.; Thompson, C.B. ATP citrate lyase is an important component of cell growth and transformation. Oncogene 2005, 24, 6314–6322. [Google Scholar] [CrossRef] [PubMed]
- Korobkina, E.D.; Calejman, C.M.; Haley, J.A.; Kelly, M.E.; Li, H.; Gaughan, M.; Chen, Q.; Pepper, H.L.; Ahmad, H.; Boucher, A.; et al. Brown fat ATP-citrate lyase links carbohydrate availability to thermogenesis and guards against metabolic stress. Nat. Metabol. 2024, 6, 2187–2202. [Google Scholar] [CrossRef]
- Fernandez, S.; Viola, J.M.; Torres, A.; Wallace, M.; Trefely, S.; Zhao, S.; Affronti, H.C.; Gengatharan, J.M.; Guertin, D.A.; Snyder, N.W.; et al. Adipocyte ACLY facilitates dietary carbohydrate handling to maintain metabolic homeostasis in females. Cell Rep. 2019, 27, 2772–2784.e6. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Newton, R.S.; Day, E.A.; Ford, R.J.; Lhotak, S.; Austin, R.C.; Birch, C.M.; Smith, B.K.; Filippov, S.; Groot, P.H.E.; et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat. Comm. 2016, 7, 13457. [Google Scholar] [CrossRef]
- Ference, B.A.; Ray, K.K.; Catapano, A.L.; Ference, T.B.; Burgess, S.; Neff, D.R.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; Di Angelantonio, E. Mendelian randomization study of ACLY and cardiovascular disease. N. Engl. J. Med. 2019, 380, 1033–1042. [Google Scholar]
- Wang, Q.; Jiang, L.; Wang, J.; Li, S.; Yu, Y.; You, J.; Zeng, R.; Gao, X.; Rui, L.; Li, W. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology 2009, 49, 1166–1175. [Google Scholar] [CrossRef]
- Chu, K.Y.; Lin, Y.; Hendel, A.; Kulpa, J.E.; Brownsey, R.W.; Johnson, J.D. ATP-citrate lyase reduction mediates palmitate-induced apoptosis in pancreatic beta cells. J. Biol. Chem. 2010, 285, 32606–32615. [Google Scholar] [CrossRef]
- Morrow, M.R.; Batchuluun, B.; Wu, J.; Ahmadi, E.; Leroux, J.M.; Mohammadi-Shemirani, P.; Desjardins, E.M.; Wang, Z.; Tsakiridis, E.E.; Lavoie, D.C. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metab. 2022, 34, 919–936.e8. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Richardson, E.A.; Phillips, D.R.; Azadi, P.; Lu, G.; Ye, Z.-H. Cytosolic acetyl-CoA generated by ATP-citrate lyase is essential for acetylation of cell wall polysaccharides. Plant Cell Physiol. 2020, 61, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Yang, J.; Li, X.; Li, H.; Huang, G.; Chen, T.; Lu, M. Rapid degradation of ACLA, a subunit of ATP citrate lyase, via autophagy and 26S proteasome pathways to promote pepper growth-to-tolerance transition under heat stress. Plant J. 2025, 122, e70212. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.P.; Hamid, A.A.; Midgley, M.; Ratledge, C. Widespread occurrence of ATP:citrate lyase and carnitine acetyltransferase in filamentous fungi. World J. Microbiol. Biotechnol. 1998, 14, 145–147. [Google Scholar] [CrossRef]
- Kendrick, A.; Ratledge, C. Lipids of selected molds grown for production of n-3 and n-6 polyunsaturated fatty acids. Lipids 1992, 27, 15–20. [Google Scholar] [CrossRef]
- Tang, X.; Chen, H.; Chen, Y.Q.; Chen, W.; Garre, V.; Song, Y.; Ratledge, C. Comparison of biochemical activities between high and low lipid-producing strains of Mucor circinelloides: An explanation for the high oleaginicity of strain wj11. PLoS ONE 2015, 10, e0128396. [Google Scholar] [CrossRef]
- Pfitzner, A.; Kubicek, C.; Röhr, M. Presence and regulation of ATP: Citrate lyase from the citric acid producing fungus Aspergillus niger. Arch. Microbiol. 1987, 147, 88–91. [Google Scholar] [CrossRef]
- Botham, P.; Ratledge, C. A biochemical explanation for lipid accumulation in Candida 107 and other oleaginous micro-organisms. J. Gen. Microbiol. 1979, 114, 361–375. [Google Scholar] [CrossRef]
- Boulton, C.; Ratledge, C. Cryptococcus terricolus, an oleaginous yeast re-appraised. Appl. Microbiol. Biotechnol. 1984, 20, 72–73. [Google Scholar] [CrossRef]
- Boulton, C.; Ratledge, C. Use of transition studies in continuous cultures of Lipomyces starkeyi, an oleaginous yeast, to investigate the physiology of lipid accumulation. J. Gen. Microbiol. 1983, 129, 2871–2876. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Singh, N.; Medina, A.; Usón, I.; Fraser, M.E. Identification of the active site residues in ATP-citrate lyase’s carboxy-terminal portion. Protein Sci. 2019, 28, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Williams, H.J.; Boyer, J.G.; Graham, T.L.; Zhao, H.; Lehr, R.; Qi, H.; Schwartz, B.; Raushel, F.M.; Meek, T.D. On the catalytic mechanism of human ATP citrate lyase. Biochemistry 2012, 51, 5198–5211. [Google Scholar] [CrossRef]
- Verstraete, K.; Verschueren, K.H.G.; Dansercoer, A.; Savvides, S.N. Acetyl-CoA is produced by the citrate synthase homology module of ATP-citrate lyase. Nat. Struct. Mol. Biol. 2021, 28, 636–638. [Google Scholar] [CrossRef]
- Verschueren, K.H.G.; Blanchet, C.; Felix, J.; Dansercoer, A.; De Vos, D.; Bloch, Y.; Van Beeumen, J.; Svergun, D.; Gutsche, I.; Savvides, S.N.; et al. Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature 2019, 568, 571–575. [Google Scholar] [CrossRef]
- Wei, X.; Schultz, K.; Pepper, H.L.; Megill, E.; Vogt, A.; Snyder, N.W.; Marmorstein, R. Allosteric role of the citrate synthase homology domain of ATP citrate lyase. Nat. Commun. 2023, 14, 2247. [Google Scholar] [CrossRef]
- Bellou, S.; Makri, A.; Triantaphyllidou, I.-E.; Papanikolaou, S.; Aggelis, G. Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology 2014, 160, 807–817. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.E.; Mizerakis, P.; Aggelis, G. High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J. Biotechnol. 2016, 234, 116–126. [Google Scholar] [CrossRef]
- Yuzbasheva, E.Y.; Yuzbashev, T.V.; Vinogradova, E.B.; Kosikhina, I.M.; Taratynova, M.O.; Dementev, D.A.; Solovyev, A.I.; Egorova, D.A.; Sineoky, S.P. Inactivation of Yarrowia lipolytica YlACL2 gene coding subunit of ATP Citrate Lyase using CRISPR/Cas9 System. Appl. Biochem. Microbiol. 2020, 56, 885–892. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Chen, H.; Chen, Y.Q.; Chen, W.; Song, Y.; Ratledge, C. Enhanced lipid accumulation in the yeast Yarrowia lipolytica by over-expression of ATP:citrate lyase from Mus musculus. J. Biotechnol. 2014, 192 Pt A, 78–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).