Effects of 5% Caffeine Ultrasonophoresis on Gynoid Lipodystrophy—A Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Instruments
2.3.1. Sociodemographic Questionnaire
2.3.2. Short Version of the International Physical Activity Questionnaire (IPAQ)
2.3.3. Semi-Quantitative Food Frequency Questionnaire (FFQ)
2.3.4. Bioimpedance Scale
2.3.5. Measuring Tape
2.3.6. Cellulite Severity Scale CSS
2.3.7. Lipid Profile Meter
2.3.8. Borg Scale
2.3.9. Heart Rate Monitor
2.4. Methods
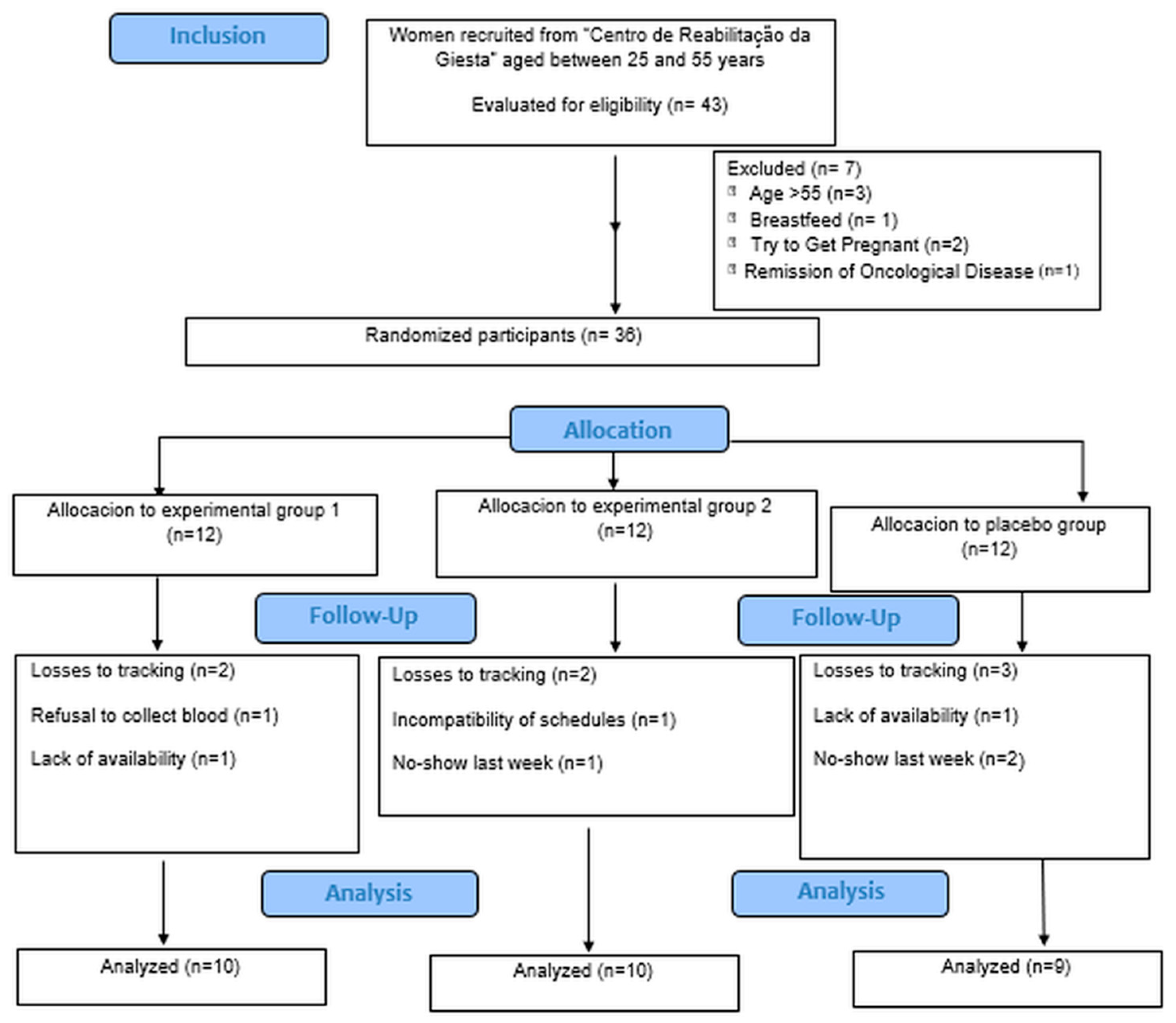
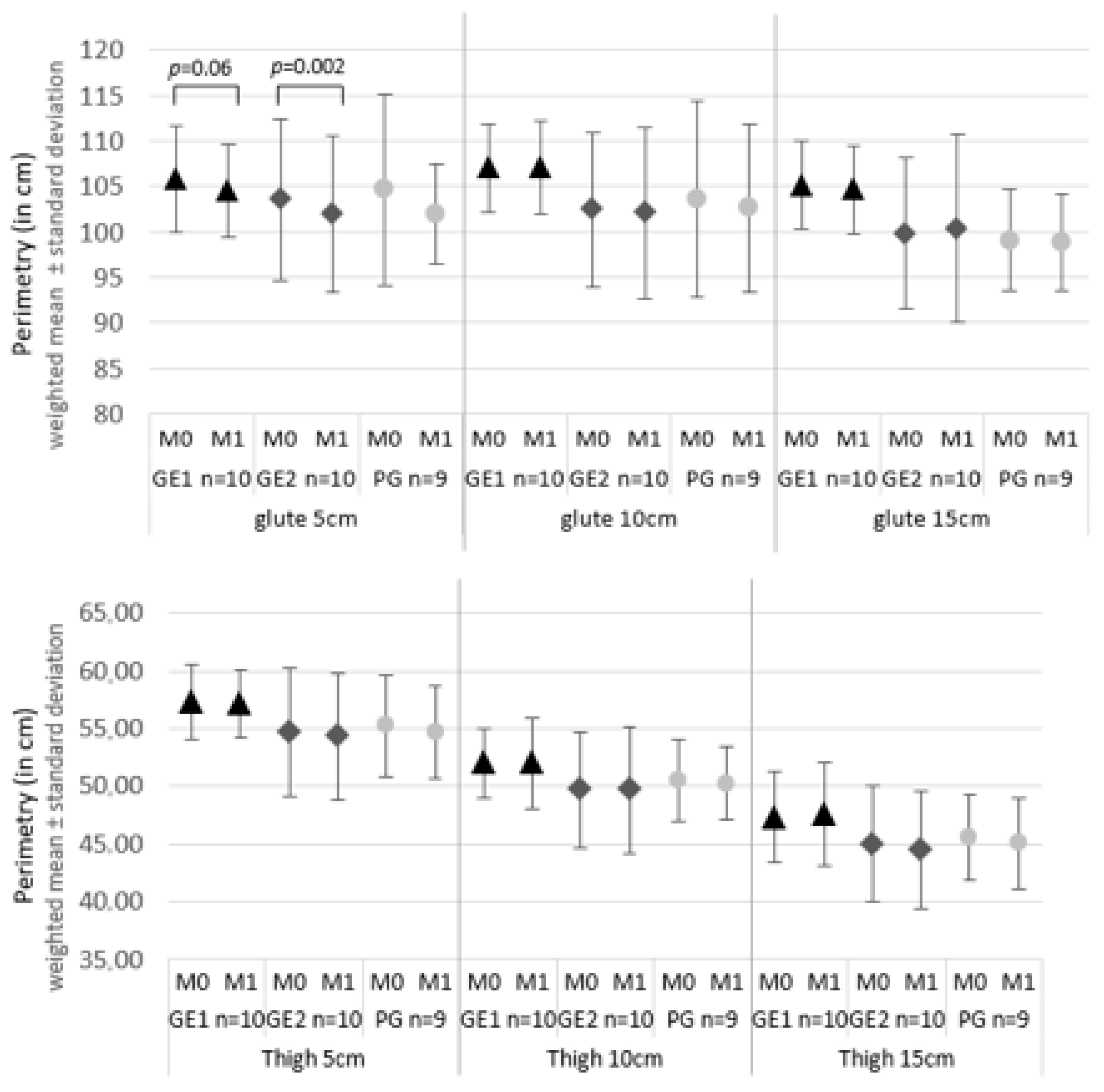
3. Results
3.1. Sample Characterization
3.2. Results of the Effects of the Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACSM | American College of Sports Medicine |
| Bpm | Beats per minute |
| BMI | Body Mass Index |
| Cm | Centimeters |
| C.R.G. | Giesta Rehabilitation Centre |
| CSS | Cellulite Several Scale |
| EP1 | Experimental Group 1 |
| EP2 | Experimental Group 2 |
| FFQ | Semi-Quantitative Food Frequency Questionnaire |
| GL | Gynoid Lipodystrophy |
| HRmax | Maximum Heart Rate |
| ICC | Intraclass Correlation Coefficient |
| IPAQ | International Physical Activity Questionnaire |
| Kg | Kilograms |
| M0 | Before the intervention |
| M1 | After the three interventions |
| MHz | Mega hertz |
| PEF | Physical Exercise Protocol |
| PG | Placebo Group |
| US | Ultrasound Therapy |
| W/cm2 | Watt per centimeter square |
References
- Bauer, J.; Hoq, N.; Mulcahy, J.; Tofail, S.A.M.; Gulshan, F.; Silien, C.; Podbielska, H.; Akbar, M. Implementation of artificial intelligence and non-contact infrared. EPMA J. 2020, 11, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Dupont, E. An integral topical gel for cellulite reduction: Results from a double-blind, randomized. Clin. Cosmet. Investig. Dermatol. 2014, 7, 73–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almeida, M.D.L.C.; Serrano, C.S.; Sánchez, E.M.M.; Mohedo, E.D.; Moriana, G.C.; Salas, M.R. The efficacy of capacitive radio-frequency diathermy in reducing. J. Cosmet. Laser Ther. 2014, 16, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I. The Pathophysiology of Cellulite: Can the Puzzle Eventually Be Solved? J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Nürnberg, F.M.A. So-Called Cellulite: An Invented Disease. J. Dermatol. Surg. Oncol. 1978, 4, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Atamoros, F.M.P.; Pérez, D.A.; Sigall, D.A.; Romay, A.A.Á.; Gastelum, J.A.B.; Salcedo, J.A.d.l.P.; Salgado, P.E.E.; Palacios, G.J.G.; Guerrero-Gonzalez, G.A.; De la Cerda, R.M.; et al. Evidence-based treatment for gynoid lipodystrophy: A review. J. Cosmet. Dermatol. 2018, 17, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Hexsel, D.; Camozzato, F.O.; Silva, A.F.; Siega, C. Acoustic wave therapy for cellulite, body shaping and fat reduction. J. Cosmet. Laser Ther. 2017, 19, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kania, B.; Goldberg, D.J. Cryolipolysis: A promising nonsurgical technique for localized fat reduction. J. Cosmet. Dermatol. 2023, 22 (Suppl. S3), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.H. Caffeine’s Mechanisms of Action and Its. Ski. Pharmacol. Physiol. 2013, 26, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Pires-de-Campos, M.S.M.; Leonardi, G.R.; Chorilli, M.; Spadari-Bratfisch, R.C.; Polacow, M.L.O.; Grassi-Kassisse, D.M. The effect of topical caffeine on the morphology of swine hypodermis as measured by ultrasound. J. Cosmet. Dermatol. 2008, 7, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Kiens, B. Regulation and limitations to fatty acid oxidation. J. Physiol. 2012, 590, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Gary, L. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer Health: Philadelphia, PA, USA, 2022. [Google Scholar]
- Miwa, H.; Kino, M.; Han, L.-K.; Takaoka, K.; Tsujita, T.; Furuhata, H.; Sugiyama, M. Effect of ultrasound application on fat mobilization. Pathophysiology 2002, 9, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Thyfault, J.P.; Johnson, E.A.; Kraus, R.M.; Choi, M.D.; Hickner, R.C. Fat metabolism and acute resistance exercise in trained men. J. Appl. Physiol. 2007, 102, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Otero-Díaz, B.; Rodríguez-Flores, M.; Sánchez-Muñoz, V.; Monraz-Preciado, F.; Ordoñez-Ortega, S.; Becerril-Elias, V.; Baay-Guzmán, G.; Obando-Monge, R.; García-García, E.; Palacios-González, B.; et al. Exercise induces white adipose tissue browning across the weight spectrum in humans. Front. Physiol. 2018, 9, 1781. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Torres, D.; Oliveira, A.; Severo, M.; Alarcão, V.; Guiomar, S.; Mota, J.; Teixeira, P.; Ramos, E.; Rodrigues, S.; et al. Inquérito Alimentar Nacional e de Atividade Física, IAN-AF-2015-2016: Relatório metodológico. 2017. Available online: www.ian-af.up.pt (accessed on 6 June 2025).
- Lopes, C.; Aro, A.; Azevedo, A.; Ramos, E.; Barros, H. Intake and adipose tissue composition of fatty acids and risk of myocardial infarction in a male Portuguese community sample. J. Am. Diet. Assoc. 2007, 107, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Gogia, P.P.; Braatz, J.H. Validity and reliability of leg length measurements. J. Orthop. Sports Phys. Ther. 1986, 8, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: A meta-analysis. J. Sports Sci. 2002, 20, 873–899. [Google Scholar] [CrossRef] [PubMed]
- Lintsi, M.K. Comparison of hand-to-hand bioimpedance and anthropometry equations versus dual-energy X-ray absorptiometry for the assessment of body fat percentage in 17–18-year-old conscripts. Clin. Physiol. Funct. Imaging 2004, 24, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Predicted Maximal Heart Revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Tassinary, J.A.; Rogeri, L.N.; Schmitt, B.; Marder, F.; Sinigaglia, G.; Herbele, J.; Hilgemann, M.; Stülp, S. Efeito do ultrassom terapêutico na liberação, permeação e retenção de ácido kójico em sistema de difusão vertical. Acta Biomed. Bras. 2011, 9, 8–16. [Google Scholar] [CrossRef]
- Chorilli, M.; Tamascia, P.; Rossim, C.; Salgado, H. Ensaios biológicos para avaliação de segurança de produtos cosméticos. Rev. Cienc. Farm. Basica E Apl. 2009, 30, 19–30. [Google Scholar]
- Leung, M.C.; Ng, G.Y.; Yip, K.K. Effect of ultrasound on acute inflammation of transected medial. Arch. Phys. Med. Rehabil. 2004, 85, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Leitão-Correia, F.; Sousa, M.J.; Leão, C. Dietary Restriction and Nutrient Balance in Aging. Oxidative Med. Cell. Longev. 2015, 2016, 4010357. [Google Scholar] [CrossRef] [PubMed]
- Presotto, L.; Rogeri, L.N.; Sinigaglia, G.; Bitencourt, S.; Tassinary, J.A.F. Aesthetic nonthermal ultrasound and electric current combination therapy for body sculpting. J. Cosmetol. 2018, 2, 000109. [Google Scholar]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Glisezinski, I.D. Mobilisation des lipides du tissu adipeux au cours de l’exercice physique. Sci. Sports 2007, 22, 280–285. [Google Scholar] [CrossRef]
- Ahmadian, M.; Wang, Y.; Sul, H.S. Lipolysis in adipocytes. Int. J. Biochem. Cell Biol. 2010, 42, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Stich, V.; de Glisezinski, I.; Berlan, M.; Bulow, J.; Galitzky, J.; Harant, I.; Suljkovicova, H.; Lafontan, M.; Rivière, D.; Crampes, F. Adipose Tissue Lipolysis Is Increased during a Repeated Bout of Aerobic Exercise. J. Appl. Physiol. 2000, 88, 1277–12833. [Google Scholar] [CrossRef] [PubMed]
| GE1 (n = 10) | GE2 (n = 10) | PG (n = 9) | Difference Groups | ||||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | Valuer p* | |
| Age (years) | 35.20 | 12.54 | 39.40 | 10.34 | 32.78 | 11.82 | 0.46 |
| Height (cm) | 163.30 | 6.40 | 165.60 | 5.19 | 168.78 | 7.14 | 0.18 |
| Weight (kg) | 69.09 | 6.81 | 66.64 | 11.50 | 63.77 | 6.70 | 0.42 |
| BMI (kg/cm2) | 25.91 | 2.11 | 24.24 | 3.70 | 22.52 | 3.40 | 0.08 |
| IPAQ | |||||||
| MET min/week | 1.60 | 0.70 | 2.20 | 0.79 | 1.89 | 0.78 | 0.23 |
| QFA | |||||||
| Calories (kcal) | 1791.87 | 855.67 | 2339.23 | 742.26 | 1790.04 | 656.31 | 0.22 |
| Protein | 78.05 | 42.15 | 119.61 | 52.04 | 87.40 | 26.91 | 0.09 |
| Carbohydrates | 190.05 | 85.69 | 310.59 | 123.91 | 180.57 | 86.16 | 0.01 a |
| Fat | |||||||
| Total | 79.63 | 44.54 | 122.49 | 115.22 | 82.61 | 29.28 | 0.38 |
| Satured | 20.30 | 11.04 | 31.46 | 15.69 | 21.51 | 9.11 | 0.11 |
| Monosaturated | 37.04 | 21.68 | 58.84 | 70.87 | 40.32 | 14.87 | 0.51 |
| Polysaturated | 16.32 | 10.58 | 23.18 | 23.83 | 14.19 | 4.77 | 0.43 |
| Cholesterol | 252.61 | 136.21 | 364.79 | 96.97 | 294.52 | 123.61 | 0.13 |
| Fibers | 23.14 | 12.01 | 33.52 | 23.08 | 20.02 | 8.02 | 0.17 |
| Sugar | 77.08 | 27.30 | 145.63 | 72.68 | 92.77 | 52.07 | 0.02 b |
| Alcohol | 4.34 | 3.21 | 1.21 | 2.14 | 2.82 | 3.41 | 0.08 |
| Caffeine | 65.47 | 41.94 | 58.62 | 43.57 | 35.48 | 31.67 | 0.25 |
| Post-hoc analysis | |||||||
| (a) GC > GE: p = 0.03; GC > GP: p = 0.02 (b) GC > GE: p = 0.02 | |||||||
| GE1 (n = 10) | GE2 (n = 10) | PG (n = 9) | Difference Groups | ||||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | 9SD | Value p | |
| Lipid profile Triglycerides (mg/dL) | |||||||
| M0 | 220.60 | 93.36 | 161.20 | 47.89 | 235.00 | 80.02 | 0.10 |
| M1 | 176.00 | 62.90 | 188.20 | 49.71 | 188.78 | 63.02 | 0.86 |
| Value p | 0.23 | 0.08 | 0.01 * | ||||
| Cholesterol (mg/dL) | |||||||
| M0 | 204.20 | 49.33 | 221.00 | 62.51 | 204.56 | 44.54 | 0.73 |
| M1 | 173.10 | 55.85 | 199.70 | 45.58 | 189.78 | 32.19 | 0.44 |
| Value p | <0.001 | 0.04 | 0.04 | ||||
| Bioimpedance Adipose tissue % | |||||||
| M0 | 31.47 | 3.56 | 28.41 | 8.62 | 28.22 | 3.58 | 0.40 |
| M1 | 31.07 | 4.09 | 28.40 | 8.62 | 27.76 | 3.99 | 0.45 |
| Value p | 0.20 | 0.98 | 0.31 | ||||
| Muscle tissue (kg) | |||||||
| M0 | 44.67 | 3.22 | 44.39 | 3.30 | 44.24 | 4.17 | 0.97 |
| M1 | 44.84 | 3.24 | 44.35 | 3.01 | 45.00 | 4.40 | 0.92 |
| Value p | 0.71 | 0.92 | 0.17 | ||||
| Weight (kg) | |||||||
| M0 | 69.09 | 6.81 | 66.64 | 11.50 | 63.77 | 6.70 | 0.42 |
| M1 | 66.63 | 8.78 | 64.56 | 9.75 | 64.07 | 8.23 | 0.80 |
| Value p | 0.11 | 0.09 | 0.83 | ||||
| BMI (kg/m2) | |||||||
| M0 | 25.91 | 2.11 | 24.24 | 3.70 | 22.52 | 3.40 | 0.08 |
| M1 | 25.01 | 3.26 | 23.51 | 3.21 | 22.58 | 3.49 | 0.29 |
| Value p | 0.11 | 0.10 | 0.91 | ||||
| GE1 (n = 10) | GE2 (n = 10) | PG (n = 9) | Difference Groups | ||||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | Value p | |
| CSS—total score | |||||||
| M0 | 2.50 | 0.50 | 2.00 | 1.00 | 2.00 | 0.50 | 0.060 |
| M1 | 2.00 | 0.63 | 2.00 | 0.50 | 2.00 | 0.50 | 0.188 |
| Value p | 0.25 | 0.500 | ≈1.000 | ||||
| Sub categories Number of depressions | |||||||
| M0 | 2.00 | 1.00 | 2.00 | 0.63 | 2.00 | 0.50 | 0.645 |
| M1 | 1.50 | 1.00 | 2.00 | 0.50 | 1.00 | 0.50 | 0.590 |
| Value p | 0.500 | ≈1.000 | 0.500 | ||||
| Depth | |||||||
| M0 | 2.00 | 0.63 | 2.00 | 0.63 | 1.00 | 0.25 | 0.101 |
| M1 | 2.00 | 0.63 | 2.00 | 0.50 | 1.00 | 0.25 | 0.148 |
| Value p | 0.375 | ≈1.000 | 0.500 | ||||
| Appearance | |||||||
| M0 | 2.00 | 1.00 | 1.50 | 1.00 | 1.00 | 0.50 | 0.671 |
| M1 | 1.50 | 0.63 | 1.50 | 0.63 | 1.00 | 0.50 | 0.809 |
| Value p | 0.25 | ≈1.000 | ≈1.000 | ||||
| Nurnberger | |||||||
| M0 | 2.00 | 0.63 | 2.00 | 0.50 | 2.00 | 0.50 | 0.128 |
| M1 | 2.00 | 1.00 | 1.50 | 0.63 | 1.00 | 0.75 | 0.191 |
| Value p | 0.500 | ≈1.000 | 0.250 | ||||
| Sagging | |||||||
| M0 | 2.00 | 1.00 | 1.50 | 1.00 | 2.00 | 0.50 | 0.836 |
| M1 | 2.00 | 1.00 | 1.50 | 0.63 | 1.00 | 0.50 | 0.357 |
| Value p | ≈1.000 | ≈1.000 | 0.500 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, D.S.; Noites, A. Effects of 5% Caffeine Ultrasonophoresis on Gynoid Lipodystrophy—A Randomized Controlled Study. Lipidology 2025, 2, 13. https://doi.org/10.3390/lipidology2030013
Cabral DS, Noites A. Effects of 5% Caffeine Ultrasonophoresis on Gynoid Lipodystrophy—A Randomized Controlled Study. Lipidology. 2025; 2(3):13. https://doi.org/10.3390/lipidology2030013
Chicago/Turabian StyleCabral, Diana Santos, and Andreia Noites. 2025. "Effects of 5% Caffeine Ultrasonophoresis on Gynoid Lipodystrophy—A Randomized Controlled Study" Lipidology 2, no. 3: 13. https://doi.org/10.3390/lipidology2030013
APA StyleCabral, D. S., & Noites, A. (2025). Effects of 5% Caffeine Ultrasonophoresis on Gynoid Lipodystrophy—A Randomized Controlled Study. Lipidology, 2(3), 13. https://doi.org/10.3390/lipidology2030013







