Lipid-Functionalized Electrospun Chitosan Gauze Performs Comparably to Standard of Care in Contaminated Complex Trauma Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospun Chitosan Materials
2.3. Chloride Treatment
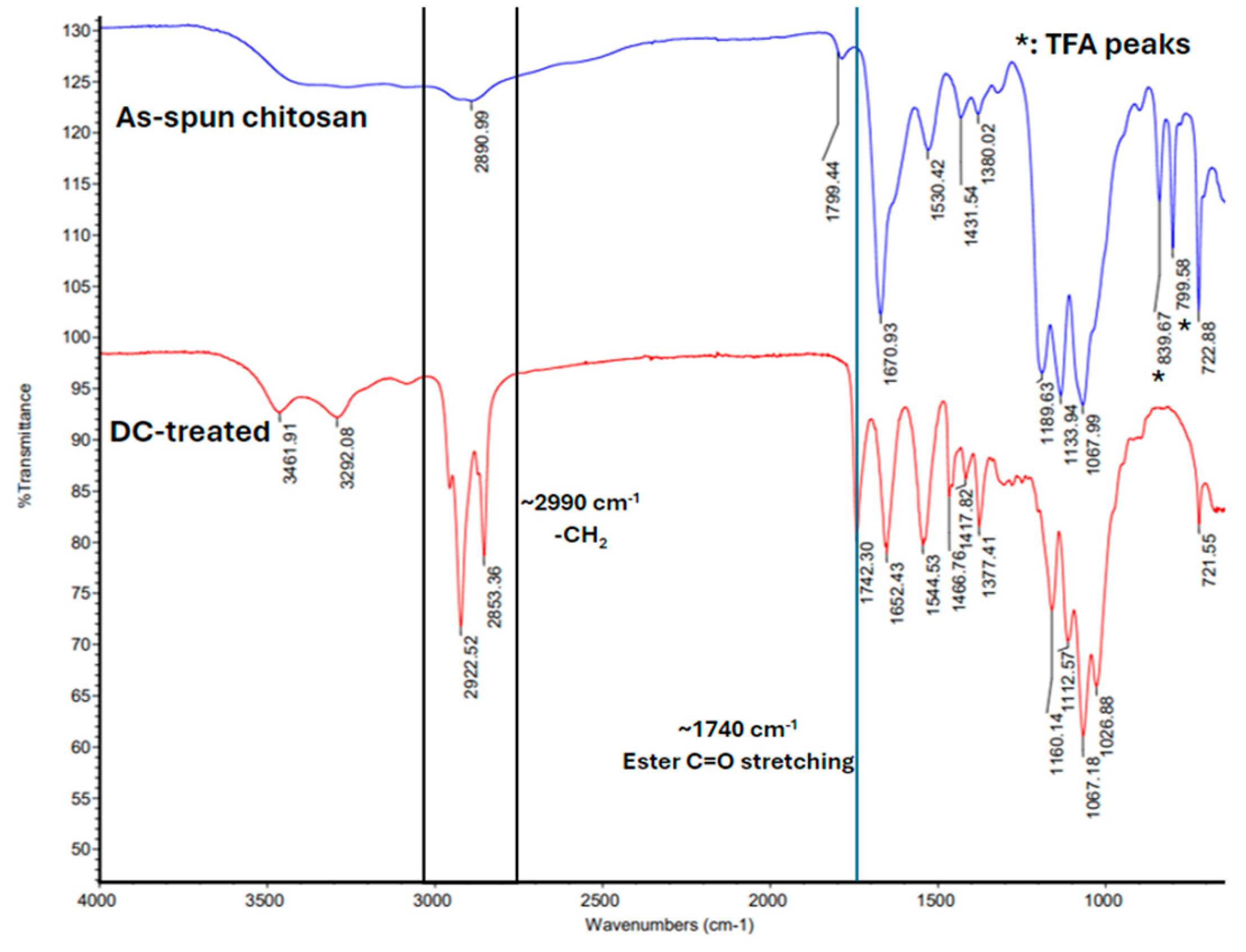
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
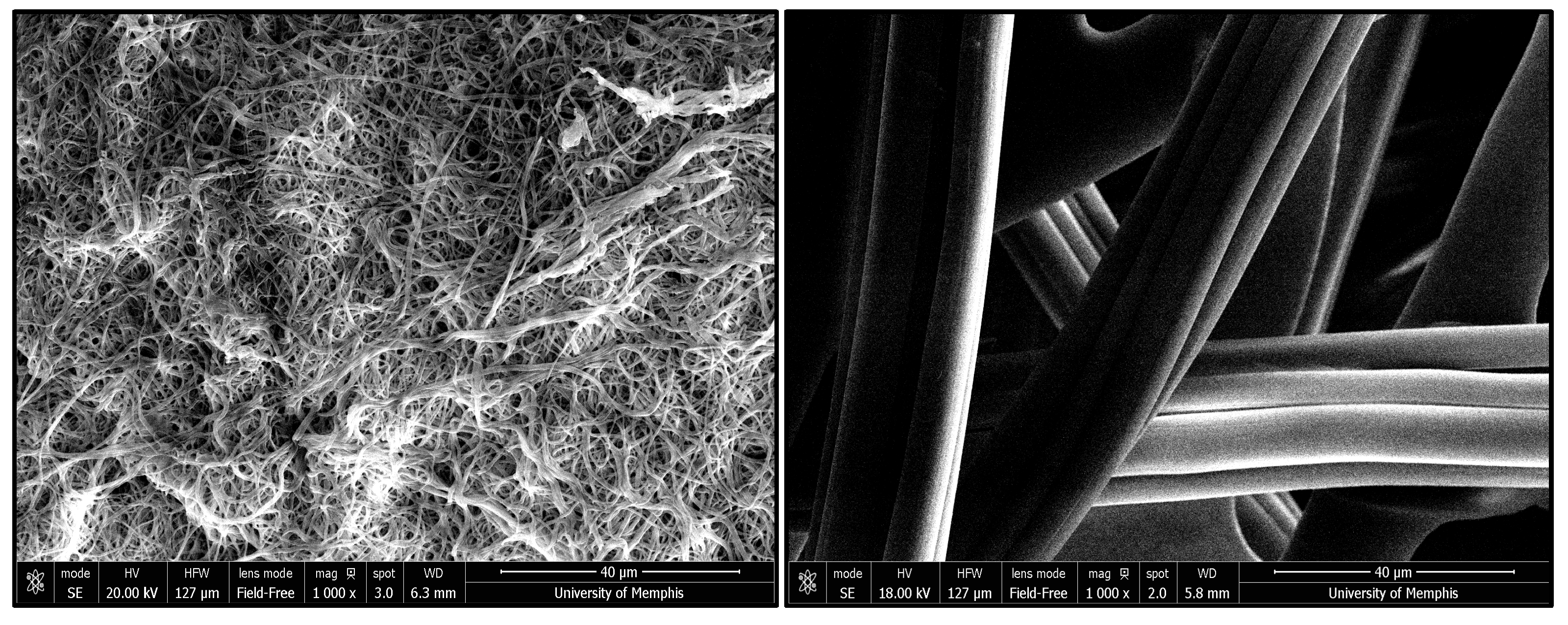
2.5. Scanning Electron Microscopy (SEM)
2.6. Preparation of Commercial Materials and Sterilization
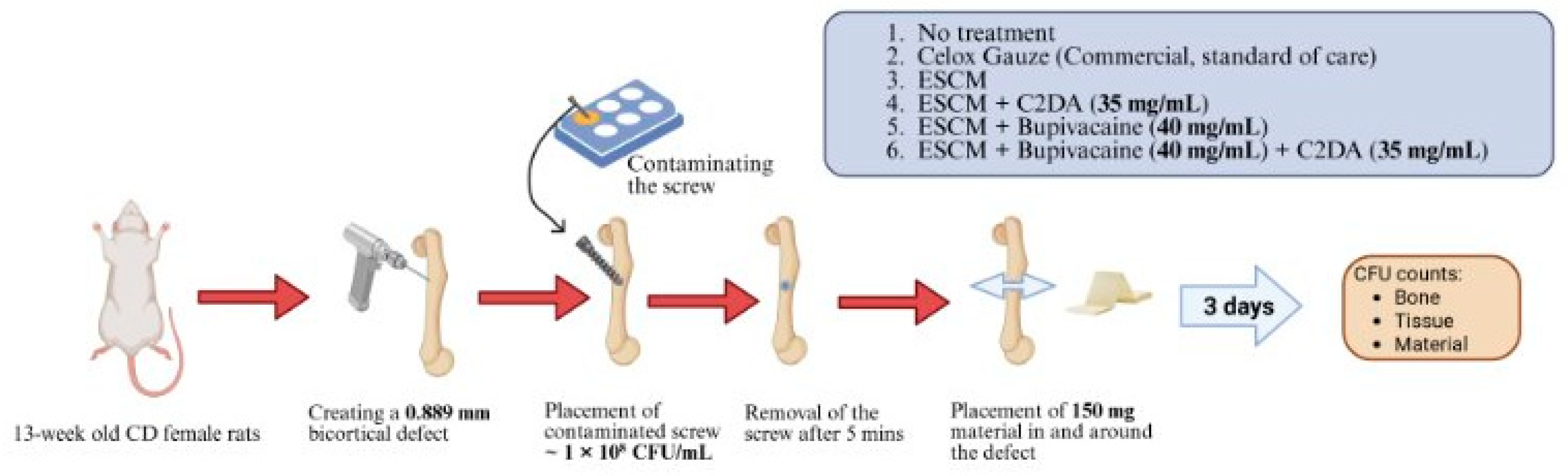
2.7. Surgical Procedures
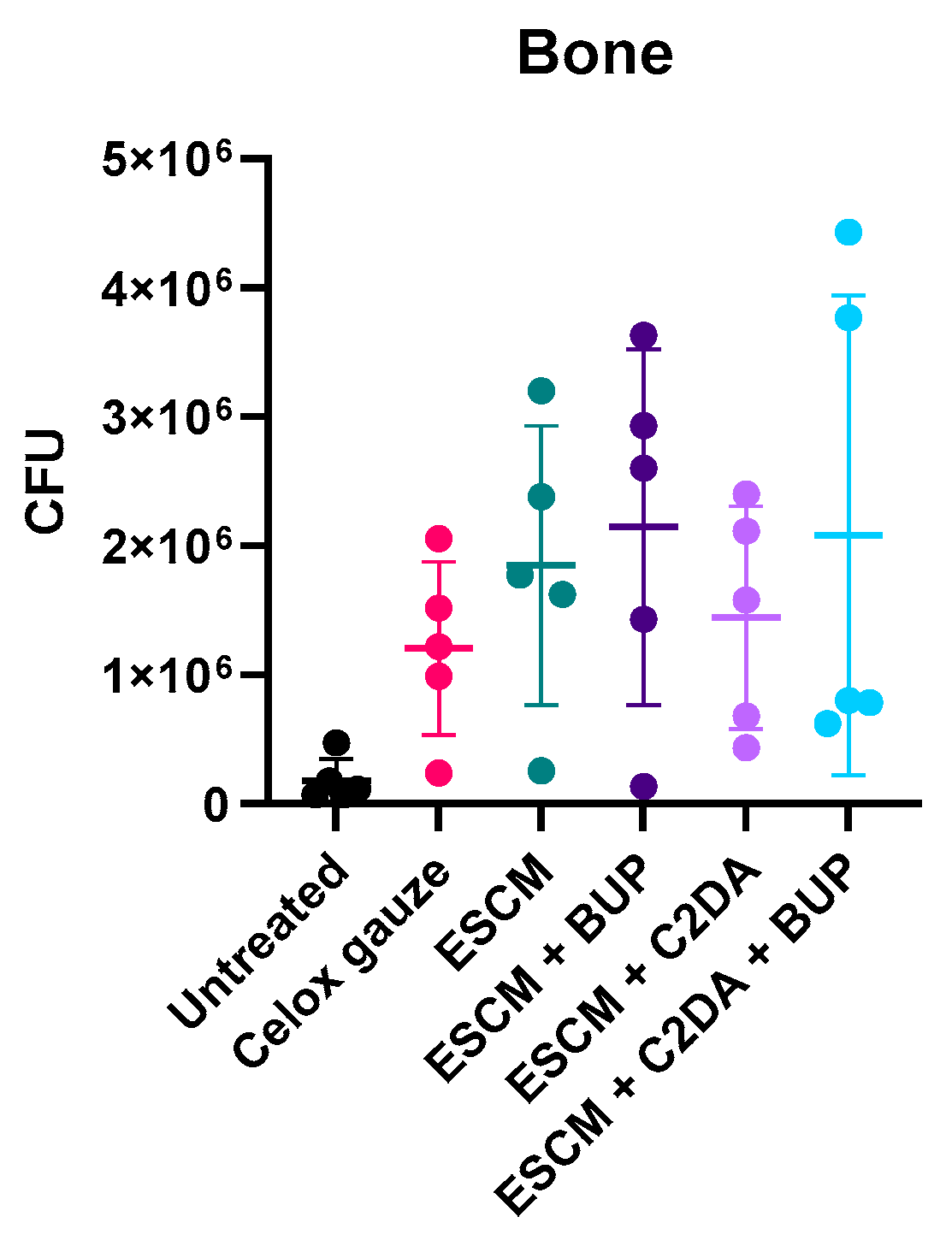
2.8. Colony Forming Units
2.9. Statistical Analysis
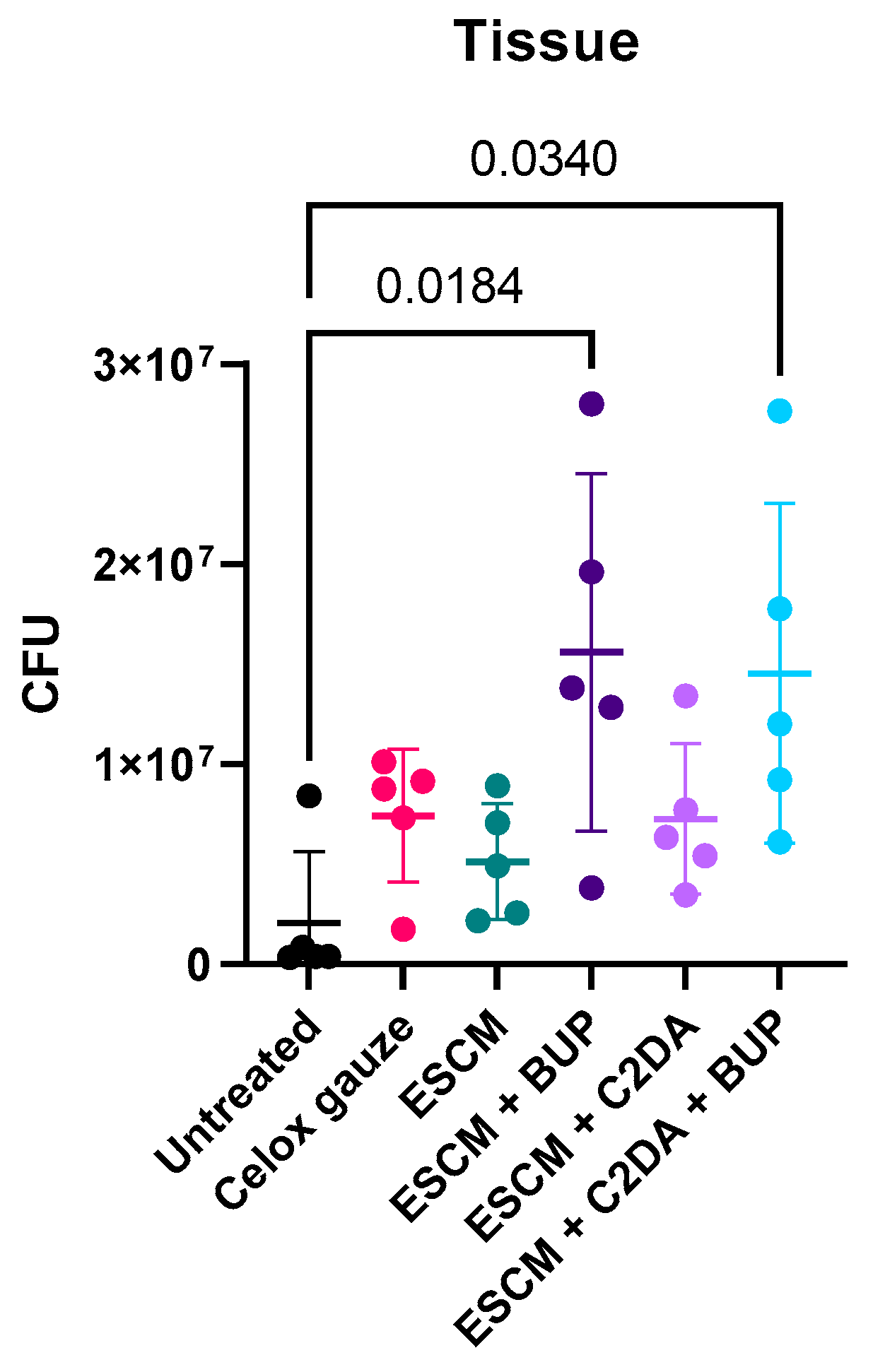
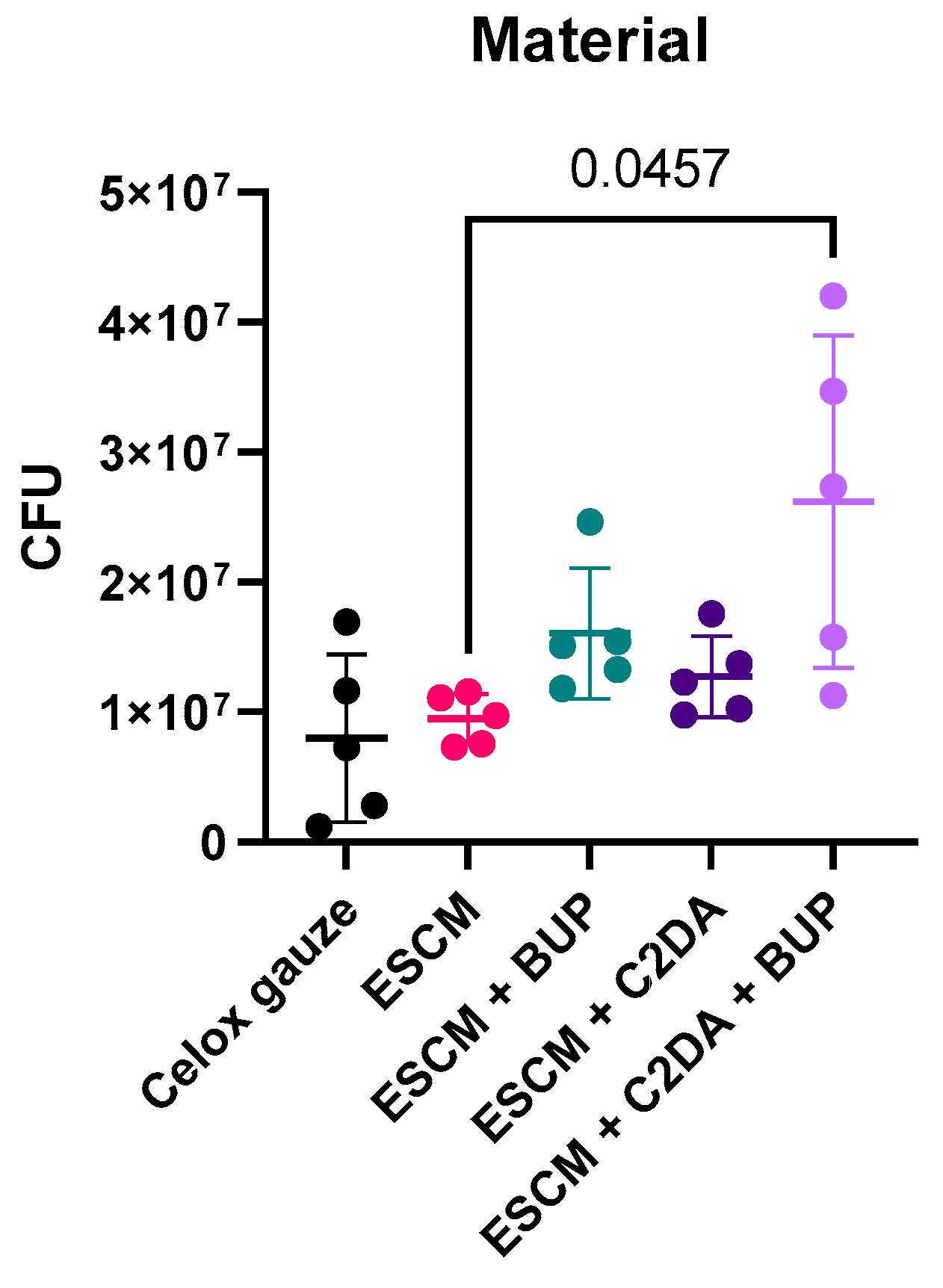
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nana, A.; Nelson, S.B.; McLaren, A.; Chen, A.F. What’s new in musculoskeletal infection: Update on biofilms. J. Bone Jt. Surg. 2016, 98, 1226–1234. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Ward, C.L.; Romano, D.R.; Hurtgen, B.J.; Hardy, S.K.; Woodbury, R.L.; Trevino, A.V.; Rathbone, C.R.; Wenke, J.C. Staphylococcus aureus biofilms decrease osteoblast viability, inhibits osteogenic differentiation, and increases bone resorption in vitro. BMC Musculoskelet. Disord. 2013, 14, 187. [Google Scholar]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. Apmis 2017, 125, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.K.; Ford, M.A.; Bonnie, R.J. (Eds.) Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use; National Academies Press: Washington, DC, USA, 2017; Available online: https://nap.nationalacademies.org/catalog/24781/pain-management-and-the-opioid-epidemic-balancing-societal-and-individual (accessed on 25 August 2024).
- Mahto, K.U.; Vandana; Priyadarshanee, M.; Samantaray, D.P.; Das, S. Bacterial biofilm and extracellular polymeric substances in the treatment of environmental pollutants: Beyond the protective role in survivability. J. Clean. Prod. 2022, 379, 134759. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar]
- Žiemytė, M.; Carda-Diéguez, M.; Rodríguez-Díaz, J.C.; Ventero, M.P.; Mira, A.; Ferrer, M.D. Real-time monitoring of Pseudomonas aeruginosa biofilm growth dynamics and persister cells’ eradication. Emerg. Microbes Infect. 2021, 10, 2062–2075. [Google Scholar]
- Amato, S.M.; Orman, M.A.; Brynildsen, M.P. Metabolic Control of Persister Formation in Escherichia coli. Mol. Cell 2013, 50, 475–487. [Google Scholar] [CrossRef]
- Davies, D.G.; Marques, C.N.H. A Fatty Acid Messenger Is Responsible for Inducing Dispersion in Microbial Biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef]
- Jennings, J.A.; Courtney, H.S.; Haggard, W.O. Cis-2-decenoic Acid Inhibits S. aureus Growth and Biofilm In Vitro: A Pilot Study. Clin. Orthop. Relat. Res. 2012, 470, 2663–2670. [Google Scholar] [CrossRef]
- Harris, M.A.; Beenken, K.E.; Smeltzer, M.S.; Haggard, W.O.; Jennings, A.J. Phosphatidylcholine coatings deliver local antimicrobials and reduce infection in a murine model: A preliminary study. Clin. Orthop. Relat. Res. 2017, 475, 1847–1853. [Google Scholar] [CrossRef]
- Faustova, M.; Nazarchuk, O.; Dmytriiev, D.; Babina, Y.; Nazarchuk, H.; Dudar, A. The effect of local anesthetics against planktonic forms and film formation of S. aureus strains and its dependence on antiseptics activity. Front. Microbiol. 2023, 14, 1199899. [Google Scholar] [CrossRef] [PubMed]
- Harrison, Z.L.; Bumgardner, J.D.; Fujiwara, T.; Baker, D.L.; Jennings, J.A. In vitro evaluation of loaded chitosan membranes for pain relief and infection prevention. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.R.; Harrison, Z.; Montgomery, E.C.; Bush, J.R.; Abuhussein, E.; Bumgardner, J.D.; Fujiwara, T.; Jennings, J.A. Chitosan Membranes Stabilized with Varying Acyl Lengths Release Cis-2-Decenoic Acid and Bupivacaine at Controlled Rates and Inhibit Pathogenic Biofilm. Front. Biosci. 2024, 29, 108. [Google Scholar] [CrossRef] [PubMed]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Ajalloueian, F.; Zeng, G.; Mendes, A.C.; Chronakis, I.S. Electrospun xanthan gum-chitosan nanofibers as delivery carrier of hydrophobic bioactives. Mater. Lett. 2018, 228, 322–326. [Google Scholar] [CrossRef]
- Wu, C.; Su, H.; Tang, S.; Bumgardner, J.D. The stabilization of electrospun chitosan nanofibers by reversible acylation. Cellulose 2014, 21, 2549–2556. [Google Scholar] [CrossRef]
- Yeasmin, R.; Abuhussein, E.; Perez, F.; Fujiwara, T.; Bumgardner, J.D.; Jennings, J.A. Pyridine catalyzed acylation of electrospun chitosan membranes by C6-C12 acyl chlorides: Effect of reaction time and chain length. Carbohydr. Polym. Technol. Appl. 2024, 7, 100443. [Google Scholar] [CrossRef]
- Wells, C.M.; Coleman, E.C.; Yeasmin, R.; Harrison, Z.L.; Kurakula, M.; Baker, D.L.; Bumgardner, J.D.; Fujiwara, T.; Jennings, J.A. Synthesis and Characterization of 2-Decenoic Acid Modified Chitosan for Infection Prevention and Tissue Engineering. Mar. Drugs 2021, 19, 556. [Google Scholar] [CrossRef]
- Cobb, L.H.; Park, J.; Swanson, E.A.; Beard, M.C.; McCabe, E.M.; Rourke, A.S.; Seo, K.S.; Olivier, A.K.; Priddy, L.B. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS ONE 2019, 14, e0220421. [Google Scholar] [CrossRef]
- Lamret, F.; Colin, M.; Mongaret, C.; Gangloff, S.C.; Reffuveille, F. Antibiotic tolerance of Staphylococcus aureus biofilm in periprosthetic joint infections and antibiofilm strategies. Antibiotics 2020, 9, 547. [Google Scholar] [CrossRef]
- Heffernan, J.M.; McLaren, A.C.; Glass, C.M.; Overstreet, D.J. Extended release of bupivacaine from temperature-responsive hydrogels provides multi-day analgesia for postoperative pain. Pain Med. 2023, 24, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lencova, S.; Svarcova, V.; Stiborova, H.; Demnerova, K.; Jencova, V.; Hozdova, K.; Zdenkova, K. Bacterial biofilms on polyamide nanofibers: Factors influencing biofilm formation and evaluation. ACS Appl. Mater. Interfaces 2020, 13, 2277–2288. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; Kingshott, P.; McArthur, S.L. Electrospun polystyrene fiber diameter influencing bacterial attachment, proliferation, and growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Tochitsky, I.; Jo, S.; Andrews, N.; Kotoda, M.; Doyle, B.; Shim, J.; Talbot, S.; Roberson, D.; Lee, J.; Haste, L.; et al. Inhibition of inflammatory pain and cough by a novel charged sodium channel blocker. Br. J. Pharmacol. 2021, 178, 3905–3923. [Google Scholar] [CrossRef]
- Wang, Y.-C.; He, F.; Feng, F.; Liu, X.-W.; Dong, G.-Y.; Qin, H.-Y.; Hu, X.-B.; Zheng, M.-H.; Liang, L.; Feng, L.; et al. Notch signaling determines the m1 versus m2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010, 70, 4840–4849. [Google Scholar] [CrossRef]
- Monteiro, J.M.; Fernandes, P.B.; Vaz, F.; Pereira, A.R.; Tavares, A.C.; Ferreira, M.T.; Pereira, P.M.; Veiga, H.; Kuru, E.; VanNieuwenhze, M.S.; et al. Cell shape dynamics during the staphylococcal cell cycle. Nat. Commun. 2015, 6, 8055. [Google Scholar] [CrossRef]
- Kim, T.; Kwon, S.; Lee, J.; Lee, J.S.; Kang, S. A metallic anti-biofouling surface with a hierarchical topography containing nanostructures on curved micro-riblets. Microsystems Nanoeng. 2022, 8, 1–14. [Google Scholar] [CrossRef]
- Gracia, E.; Laclériga, A.; Monzón, M.; Leiva, J.; Oteiza, C.; Amorena, B. Application of a rat osteomyelitis model to comparein vivoandin vitrothe antibiotic efficacy against bacteria with high capacity to form biofilms. J. Surg. Res. 1998, 79, 146–153. [Google Scholar] [CrossRef]
- Monzón, M.; García-Álvare, F.; Laclériga, A.; Gracia, E.; Leiva, J.; Oteiza, C.; Amorena, B. A simple infection model using pre-colonized implants to reproduce rat chronic Staphylococcus aureus osteomyelitis and study antibiotic treatment. J. Orthop. Res. 2001, 19, 820–826. [Google Scholar] [CrossRef]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Stemberger, A.; Haas, N.P.; Raschke, M. A new model of implant-related osteomyelitis in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 67, 593–602. [Google Scholar]
- Korniienko, V.; Husak, Y.; Diedkova, K.; Varava, Y.; Grebnevs, V.; Pogorielova, O.; Bērtiņš, M.; Korniienko, V.; Zandersone, B.; Ramanaviciene, A.; et al. Antibacterial potential and biocompatibility of chitosan/polycaprolactone nanofibrous membranes incorporated with silver nanoparticles. Polymers 2024, 16, 1729. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Validi, M.; Gholipour, A.; Makvandi, P.; Sharifi, E. Chitosan nanofiber biocomposites for potential wound healing applications: Antioxidant activity with synergic antibacterial effect. Bioeng. Transl. Med. 2022, 7, e10254. [Google Scholar] [CrossRef] [PubMed]
| Treatment Groups | Material Mass (mg) | Sample Size (n) |
|---|---|---|
| Celox | 150 | 5 |
| ESCMs | 150 | |
| ESCMs + C2DA | 150 | |
| ESCMs + Bup | 150 | |
| ESCMs + C2DA + Bup | 150 | |
| No treatment | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuhussein, E.; Tucker, L.J.; Tubbs, A.R.; Priddy, L.B.; Jennings, J.A. Lipid-Functionalized Electrospun Chitosan Gauze Performs Comparably to Standard of Care in Contaminated Complex Trauma Model. Lipidology 2025, 2, 7. https://doi.org/10.3390/lipidology2020007
Abuhussein E, Tucker LJ, Tubbs AR, Priddy LB, Jennings JA. Lipid-Functionalized Electrospun Chitosan Gauze Performs Comparably to Standard of Care in Contaminated Complex Trauma Model. Lipidology. 2025; 2(2):7. https://doi.org/10.3390/lipidology2020007
Chicago/Turabian StyleAbuhussein, Ezzuddin, Luke J. Tucker, Andie R. Tubbs, Lauren B. Priddy, and Jessica Amber Jennings. 2025. "Lipid-Functionalized Electrospun Chitosan Gauze Performs Comparably to Standard of Care in Contaminated Complex Trauma Model" Lipidology 2, no. 2: 7. https://doi.org/10.3390/lipidology2020007
APA StyleAbuhussein, E., Tucker, L. J., Tubbs, A. R., Priddy, L. B., & Jennings, J. A. (2025). Lipid-Functionalized Electrospun Chitosan Gauze Performs Comparably to Standard of Care in Contaminated Complex Trauma Model. Lipidology, 2(2), 7. https://doi.org/10.3390/lipidology2020007







