1. Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common liver primary tumor [
1] and represents up to 20% of all hepatic malignancies [
2]. Surgical resection is the only curative treatment; however, five-year overall survival ranges from 5 to 56%, with only 2–39% of patients being recurrence free by five years [
3]. Unfortunately, this cancer is generally diagnosed at a more advanced stage due to a lack of specific symptoms in the early stages [
4]. Many patients require disease-burden-reducing and symptomatic treatment, such as radiofrequency ablation and endoscopic interventions to reduce biliary obstruction [
5]. However, surgical intervention for advanced ICC cases poses an increased risk of bile leakage and subsequent peritonitis or abscess formation, vascular complications such as portal vein embolisms, and pulmonary complications such as atelectasis, pleural effusions, or bronchobiliary fistulas (BBFs) [
6,
7,
8].
BBFs are defined as an abnormal interconnection between the biliary tract and bronchial trees, most commonly occurring due to obstruction- or infection-induced liver abscess formation or ICC-/hepatocellular carcinoma-induced tumor erosion. More rarely, BBF can form iatrogenically following hepatobiliary interventions or due to postoperative complications, such as infections due to poorly drained bile collections. Generally, presentation with biliptysis, or bile-stained sputum, is pathognomonic for BBF, particularly in the context of the aforementioned relevant medical history [
9]. If BBF is suspected, the fistulous tract can be pre-operatively evaluated with imaging modalities such as hepatobiliary iminodiacetic acid, computed tomography, or magnetic resonance cholangiopancreatography scans [
10,
11].
Existing studies on the treatment of BBF focus on biliary decompression strategies, such as endoscopic retrograde cholangiopancreatography or percutaneous transhepatic biliary drainage, and delayed, definitive fistula corrections, such as thoracotomy and fistula tract excision and repair [
9,
12]. From a systematic perspective, 68 cases of BBF were reported and reviewed between 1980 and 2010, of which very few outline anesthetic considerations despite associations with high morbidity and mortality [
13]. Poor airway management during BBF exacerbations can lead to immediate patient destabilization, post-operative infection risk, or long-term pulmonary damage. Acute management of these cases requires a multidisciplinary approach and a high level of coordination that has yet to be discussed in the current literature, and is necessary to improve patient outcomes during these rare presentations.
2. Patient Information
In brief, this patient was a 61-year-old male with a past medical history of ICC status post left lobe hepatectomy and transarterial chemoembolization of the right hepatic artery. A common bile duct stent was placed in 2018. The patient is currently on maintenance pemigatinib immunotherapy. In January 2024, the patient presented with a persistent cough and yellow, foul-smelling sputum. Magnetic resonance cholangiopancreatography demonstrated a hepatorenal fistula. He was scheduled for endoscopic retrograde cholangiopancreatography (ERCP) in March of 2024 for a stent exchange and drainage of the biliary tree. The patient had no previously reported issues with anesthesia, was appropriately NPO, and only reported allergies to bananas. The patient denied any history not otherwise mentioned.
Vital signs in triage (reference range when abnormal):
Blood Pressure = 104/55
O2 Saturation = 97% on room air
Pulse = 67
Respiratory Rate = 20 breaths per minute
Temperature = 36 °C
BMI = 27 (18.5–24.9); Weight 90.2 kg
Labs from 5 Days Pre-op (reference range when abnormal):
BMP: Na+ 140, K+ 4.0, Cl− 105, HCO3− 28.5 (22–26), BUN 20, Creatinine 1.37 (0.6–1.2),
Glucose 87
CBC: WBC 5.6, Hb/Hct 9.1/25.6% (13.5–17.5/41–53%), Platelets 147
Total Bilirubin = 1.9 (0.2–1.2)
AST/ALT = 25/30
Calcium = 8.8
Phosphorus = 5.2 (2.5–4.5)
3. Procedure, Interventions, and Adverse/Critical Events
A 22-G intravenous line was placed in the patient’s left hand. The patient was consented for monitored anesthesia care (MAC) and general anesthesia with the initial plan of utilizing MAC for the ERCP procedure; however, after discussion with the GI team, general anesthesia was chosen as the safer option given the presentation of a productive yellow cough and known procedural complexity (requiring both a larger scope and longer procedure time). At this point, the patient was deemed optimized for ERCP.
The patient was brought into the procedure room, given two puffs of albuterol via an inhaler, and hooked up to an isolyte runner. He was connected to all standard monitors, induced T0 with 1.5 mg/kg propofol, 1 mg/kg lidocaine, 0.6 mg/kg rocuronium, and intubated (T + 3 min) with a 7.0 mm endotracheal tube (ETT) using a McGrath (grade 1 view) atraumatically. His initial ventilation settings were pressure-controlled with a volume guarantee of 500 mL tidal volume, respiratory rate = 10, PEEP = 5 cmH2O, and FiO2 = 60% at a fresh gas flow rate of 2 L per minute. He was maintained on propofol 125 mcg/kg/min (T + 3–10 min), which was then reduced to 75 mcg/kg/min (T + 10 min). Dark yellow/orange liquid was noted in the airway and on the vocal cords during video laryngoscopy. Inline ETT suction was performed on this fluid. The patient was maintained with 0.5 MAC sevoflurane and propofol during the case. High peak pressures were seen as the tube and circuit filled with bilious fluid, requiring suctioning.
The procedure started at T + 30 min. During the procedure, the patient’s vitals were stable, with O2 saturation momentarily dropping to 94%, but correcting to 100% on 100% O2. End-tidal CO2 (ETCO2) increased throughout the case from 40 to 55 mmHg and up to 69 mmHg by the end of the case. Ventilator settings were increased to try to minimize hypercarbia; respiratory rate increased from 10 to 14 to 18 breaths/min. During the procedure, the gastroenterology team was unable to perform the stent exchange, as the intraoperative cholangiogram demonstrated full occlusion of the metal biliary stent without any ability to pass a wire through it. Copious sludge with stones was observed. Extensive scar tissue encasing the metal biliary stent without a visible lumen was reported, and the procedure was aborted (T + 100 min).
At this point, significant bilious fluid was noted to be coming out of the ETT and into the anesthesia circuit. The circuit was changed three times, and inline suction was performed numerous times with only temporary improvement in ETCO2. Help was called, oxygen flows were increased, and the decision to perform a flexible bronchoscopy was made. The patient was extubated and reintubated with an 8.0 mm ETT (T + 110 min) to allow the interventional pulmonology team to perform flexible bronchoscopy. The ETT was noted to be in good position, and large amounts of bilious secretion were noted in both sides of the airway. Therapeutic suctioning was performed.
Discussion between interventional pulmonology (IP), gastroenterology, and anesthesiology teams yielded that the most likely cause was spontaneous fistula formation to the right bronchial tree, given significant bilious output. The decision was made to extubate the 8.0 mm ETT and reintubate with a double-lumen endotracheal tube (DLT) to perform left lung isolated ventilation (T + 125 min). The patient was switched from pressure-control volume guarantee to pressure control with an inspiratory pressure limit of 15 cmH2O, respiratory rate = 22, FiO2 = 100%, and PEEP = 6 cmH2O. Reintubation was successful, and ventilation was appropriate. A radial arterial line and two additional large-bore IVs were placed. The patient was transitioned from sevoflurane to 50 mcg/kg/min propofol and 0.5 mcg/kg/min phenylephrine and remained paralyzed with rocuronium.
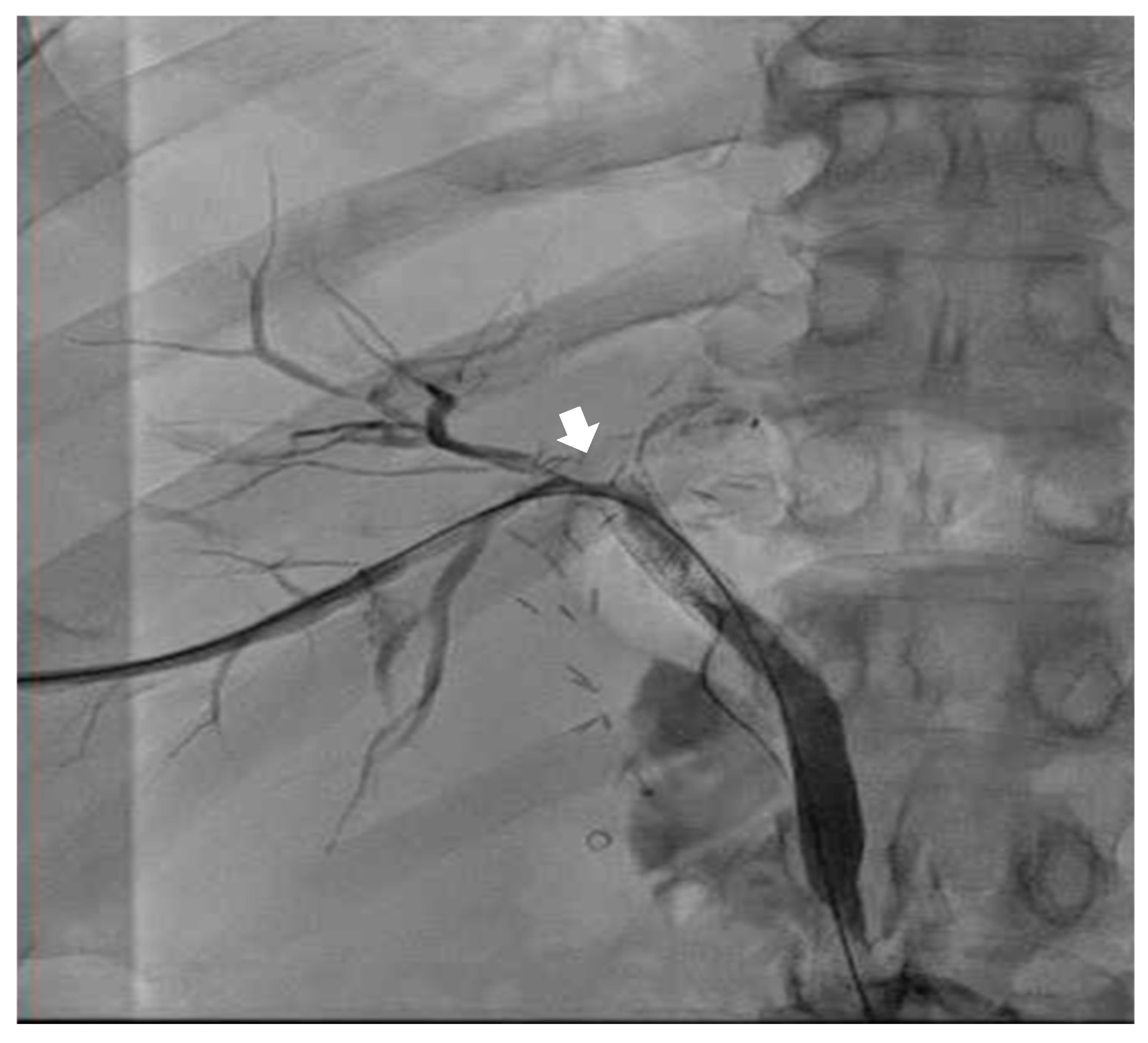
Emergent biliary drain placement with interventional radiology (IR) was performed. The patient remained stable on single-lung ventilation. The IR team noted a biliary pleural fistula (T + 180 min) with connection to the right airway (see
Figure 1). During the procedure, more bilious and bloody secretions were suctioned out of the right lung by DLT. Mean arterial pressure was greater than 65 mmHg during the entirety of the procedure. Pressure support of 15 cmH
2O provided adequate ventilation with O
2 saturation > 99% during the entirety of the procedure with an average tidal volume of 250 mL. At the end of the procedure, the patient was admitted to the medical intensive care unit (MICU) with the DLT on single-lung ventilation with phenylephrine and propofol on the intravenous line. The external biliary drain had significant bloody and bilious drainage to gravity.
4. Follow-Up and Outcomes
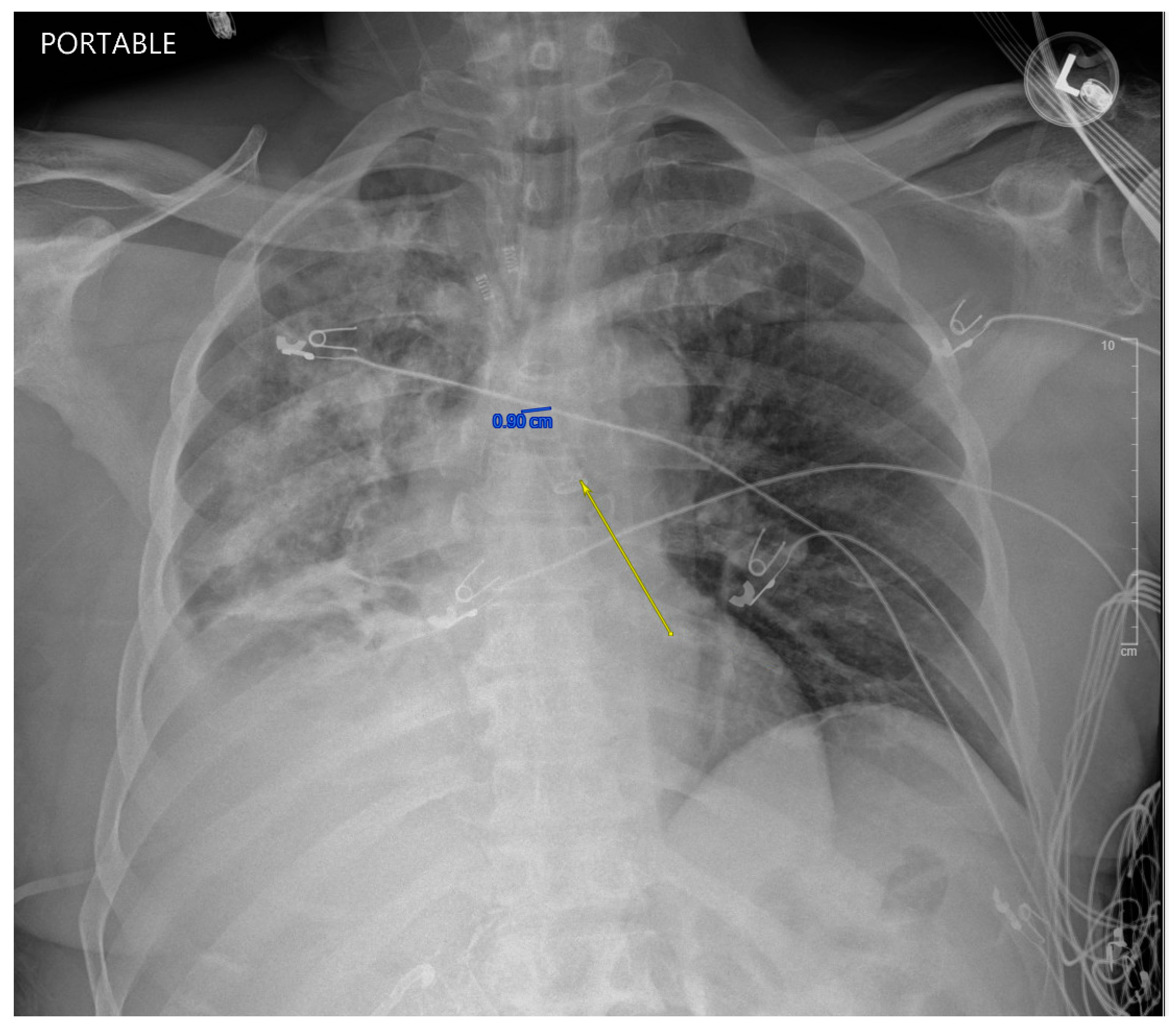
While in the MICU, the patient was weaned off pressors, and IP conducted another bronchoscopy, which showed thick, bloody secretions in the right lower lobe airway. There were no obvious secretions in the left airway. Therapeutic suctioning was performed. The patient remained intubated during post-operative day one and was started on a five-day course of Zosyn given the acute infiltrations seen on chest X-ray in the right lung (see
Figure 2). Bile cultures were negative. The patient was extubated on post-operative day two with the difficult airway team present. The patient was alert and oriented to person, place, and time with appropriate biliary drain function (200 cc/day) and ambulating with assistance (ICU mobility level 8). In April 2024, the patient underwent a staging cholangiogram with internalization of the biliary drain (see
Figure 3). Finally, the patient underwent a successful embolization of the biliary-pleural fistula in March 2024.
5. Discussion
This case report presents a unique complication to a routine ERCP. Given the acuteness of the presentation, the decision to perform flexible bronchoscopy, therapeutic suctioning, and exchange of the ETT for a DLT to perform one-lung ventilation was adequate for this patient to maintain saturation, protect his left lung, and allow the IR team to successfully drain and embolize the BBF. With the increasing incidence of cholangiocarcinoma cases, BBF should be considered when a chronic productive cough is uncovered in a thorough history and physical exam. Workup with appropriate imaging can confirm this diagnosis. In the absence of preoperative suspicion, many BBFs are detected only after bilious contamination of the airway is evident perioperatively. In our case, a double-lumen endotracheal tube (DLT) was utilized after initial endotracheal intubation and bronchoscopy revealed persistent bile in the right mainstem bronchus. Lung isolation was critical to preserve the contralateral (left) lung and allow therapeutic suction and stabilization. In contrast, bronchial blockers (BB) placed through single-lumen endotracheal tubes (SLTs) have been described in planned cases of BBF or when DLT placement is contraindicated due to airway anatomy or limited resources. While a BB offers selective lung isolation, they can be technically more challenging, especially in an emergent setting with active contamination and poor bronchoscopic visualization [
14]. Additionally, bronchial blockers do not provide robust suctioning capability as a DLT, which was a decisive advantage in our case, given the continuous bile influx [
15].
In patients with a known BBF, preoperative planning for one-lung ventilation has been shown to be successful [
16]. However, no case reports or trials have described intraoperative management of a spontaneous/spontaneously worsening BBF. Furthermore, no gradation of the severity of BBFs has been published to guide proceduralists and anesthesiologists for case management, traditionally based on symptomatology. Ultimately, the treatment of BBFs is either endoscopic drainage or embolization [
13]. Patients with known BBFs can be optimized with biliary drains or embolization preoperatively to avoid the risk of chemical pneumonitis or aspiration. Even with a drain, the anesthesia provider may want to still elect to intubate with a DLT to preserve the right lung, given the pressure from endoscopic insufflation (as seen with this case report) is often greater than the PEEP provided and thus, bilious fluid could enter the lung regardless; although a drain should act as an escape valve for excess bilious fluid to egress.
We propose the following strategy for management of unexpected perioperative BBF exacerbations, which can be managed by the anesthesia team:
Call for help for additional anesthesia teams and interventional pulmonology (if available), alert procedural/surgical teams, and stop the procedure.
Airway management: Increase FiO2 to 100% and fresh gas flow to 10 L, perform in-line suctioning and airway maintenance to allow for ventilation and oxygenation.
Perform flexible bronchoscopy to confirm BBF, ideally with an interventional pulmonology team.
Isolate the contaminated lung and stabilize the patient on one-lung ventilation.
Coordinate with interventional radiology to place a biliary drain and with the ICU for post-operative care.
Further research into the gradation of BBF severity is warranted, and pre-operative discussions between proceduralists and anesthesiologists if a patient presents with a BBF are fundamental for ensuring patient safety. The anesthesiologists in the case immediately recognized the urgency associated with the BBF and threat to airway security, appropriately called interventional pulmonology to help confirm the diagnosis, secured the airway with the clamped DLT, appropriately resuscitated the patient, and coordinated with interventional radiology for the biliary drain placement. The role of the anesthesiologist in coordinating patient safety cannot be understated in this case report and is highlighted by a thorough understanding of anatomy, physiology, and care coordination. Of note, anatomical variability may alter the risks associated with a BBF, as well as interventional strategies—for example, external biliary drain placement may not be possible.
In the present case, the patient ultimately returned to embolize the fistula two weeks later, and a remote follow-up confirmed the patient was performing very well without any productive bilious cough.