1. Introduction
CT-guided percutaneous drainage is a safe, effective, and minimally invasive procedure for the treatment of abdominopelvic abscesses and fluid collections [
1]. With the rapid increase in vascular procedures over the years, the incidence of aortic graft infections (AGIs) has also increased. It is estimated that 1–6% of patients undergoing vascular procedures develop AGIs [
2]. AGI is considered to be a challenging complication, associated with a high perioperative and overall mortality rate of up to 75% and a high reinfection rate ranging from 5% to 40% [
2]. Conservative treatment using percutaneous drainage is often recommended for AGI patients with poor overall health, patients with thoracoabdominal vascular graft infection at high risk of surgery, or patients with severe sepsis [
3].
Patients are often given sedation or general anesthesia to enhance comfort during procedures that require a prolonged prone position, depending on the site and accessibility of the AGI. Commonly used anesthetic drugs in clinical practice include propofol, sufentanyl, fentanyl, and dexmedetomidine. However, most intravenous anesthetics and opioids carry cardiorespiratory risks accompanied by loss of consciousness.
Managing patients with severe systemic comorbidities undergoing radiologic interventional procedures frequently poses a significant challenge for anesthesiologists. It is an anesthesiologist’s responsibility to carefully select an optimal combination of anesthetic drugs that ensures a safe and painless procedure, promotes rapid recovery, and minimizes the risk of complications.
Remimazolam is a novel, short-acting benzodiazepine characterized by a rapid onset of action, quick rapid recovery, and inactive metabolites. It has minimal side effects on the circulatory and respiratory systems. Similarly, esketamine, a novel type of narcotic analgesic, offers potent analgesic efficacy and rapid onset of action with negligible effect on respiratory function [
4]. Yet, remimazolam produces a diminished circulatory and respiratory depressive effect compared to propofol [
5]. On the other hand, esketamine exhibits a beneficial bronchodilatory effect, which is a reason for introducing it to patients with severe COPD [
6]. Therefore, the combination of remimazolam and esketamine may exhibit a synergistic effect, allowing the use of lower doses of each drug. This strategy could reduce the incidence and severity of adverse effects associated with higher individual dosages, ultimately providing a safer sedation profile for patients with severe multiple comorbidities.
Current studies and case series report successful and satisfactory anesthesia and analgesia using a combination of remimazolam and ketamine [
7,
8]. However, these studies predominantly involve patients without severe systemic disease or life-threatening conditions (ASA I, II, or III, respectively) [
9].
To our knowledge, there are no case reports or studies addressing the safety and efficacy of the remimazolam and esketamine combination in patients with multiple system dysfunction classified as ASA V, as in the case presented below.
The choice of anesthetic technique should be based on patient factors and the requirements of the procedure, including anticipated duration and complexity. We used remimazolam here because it is safe for patients with hepatic or renal impairment, as it is eliminated by non-specific tissue esterases. It has a faster onset of sedation and faster recovery compared to midazolam and can be reversed by flumazenil. It also exhibits greater hemodynamic stability and less respiratory depression compared with propofol. In addition, we used esketamine, which produces a dissociative state accompanied by amnesia and intense analgesia with minimal respiratory depression at sedative doses. Esketamine, compared with ketamine, is twice as potent an anesthetic and analgesic, has less severe psychedelic and psychotomimetic side effects, and has similar sympathetic-mediated cardiovascular effects, resulting in comparable increases in BP, HR, and circulating catecholamines. All these factors render this drug combination an optimal anesthetic choice for elderly individuals with significant comorbidities.
2. Case Report
We present the case of a 68-year-old female patient admitted to the hospital with abdominal pain and significantly elevated inflammatory parameters (leukocytes 31.3 × 109/L, C-reactive protein 322.7 mg/L). Two years prior, she had undergone thoraco-abdominal aortic aneurysm repair, which involved thoracophrenolaparotomy, aneurysm resection, graft interpositum implantation, reimplantation of visceral branches of the aorta, and splenectomy.
Upon admission, the patient was started on dual empiric parenteral antibiotic therapy with ceftriaxone and metronidazole, in addition to continuous analgesic therapy and parenteral hydration. An urgent CT aortography revealed inflammatory collection in the area of the aneurysmal sac surrounding the graft interpositum, specifically in the left subphrenic space, along the suprarenal part of the abdominal aorta, and between the stomach and the left kidney.
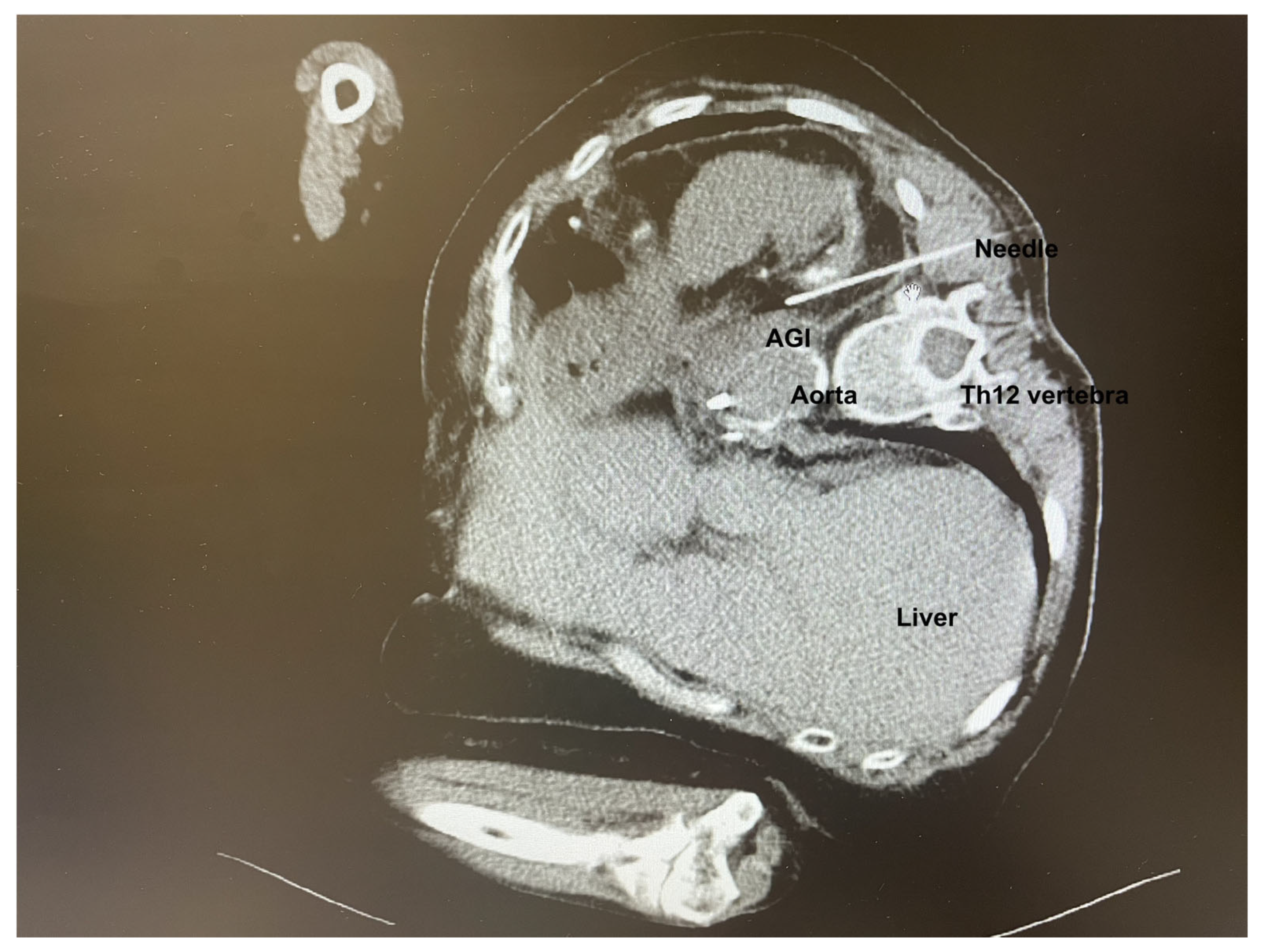
Following confirmation of an aortic graft infection (AGI), antibiotic therapy was adjusted to meropenem and linezolid. A multidisciplinary team comprising of a vascular surgeon and an interventional radiologist decided to proceed with CT-guided percutaneous drainage of the AGI (
Figure 1). Due to the extremely challenging localization of the AGI, general anesthesia in the prone position was preferred to enhance the safety of the procedure, to minimize patient discomfort, and prevent unintended movement during the procedure.
The risk of general anesthesia was extremely high due to the patient’s extensive comorbidities and previous surgical procedures. The patient had severe COPD characterized by a centrilobular and panlobular emphysema of the lungs, with bronchiectasis resulting from her long history of smoking. Additionally, she had post-COVID-19 syndrome, which made her dependent on a home oxygenator (oxygen flow of 5 L/min), for at least 16–18 h a day. Other comorbidities included arterial hypertension, diabetes type 2, chronic renal impairment with creatinine clearance of 29 mL/min, and hyperlipoproteinemia. She also had ischemic cardiomyopathy previously treated with myocardial revascularization followed by percutaneous coronary intervention.
During the preprocedural anesthesiological assessment, the patient’s condition was serious, appearing exhausted due to abdominal pain that had progressively worsened over the previous two days. Her functional capacity prior to hospitalization was very limited; she could only walk a few steps at home, mostly due to severe COPD and dyspnea. On examination, the patient exhibited tachyarrhythmia (heart rate 105 bpm) but was hemodynamically stable (blood pressure 135/80 mmHg). She was dyspneic, with an oxygen saturation (SpO2) of 85% without supplemental oxygen, and with auscultation signs of prolonged expiration and wheezing. Regarding her comorbidities, previous interventions, and physical condition, she was at high risk for developing pulmonary and cardiac perioperative complications, a risk that was clearly explained to her. She was classified as ASA score V, with a metabolic equivalent of task (MET) score below four and a Revised Cardiac Risk Index (LEE score) of four.
Upon arrival in the preoperative area, the patient remained hemodynamically stable (BP 140/80 mmHg) with tachycardia (HR 115 bpm), and tachydyspnea (SpO
2 88%, RR 32/min). Her abdomen was tense and distended, and she experienced moderate to severe abdominal pain despite tramadol/paracetamol analgesia.
Table 1 presents vital parameters from the preoperative exam to postoperative recovery.
Considering her worsening clinical condition over time due to abdominal pain, the attending anesthesiologist determined that the general anesthesia posed an unacceptably high risk of prolonged respiratory complications and potential for failed weaning from ventilatory support. In agreement with the interventional radiologist, it was recommended that the procedure be performed under sedation with the maintenance of spontaneous breathing, despite the requirement for the prone position. The patient was informed of the decision and the updated anesthesia plan. She understood the risks and agreed to proceed with the procedure.
However, upon being placed in the prone position, the patient’s dyspnea worsened significantly despite oxygen supplementation of 10 L/min, accompanied by a severe drop in oxygen saturation (SpO2 of 69%). She was promptly repositioned to an upright position and administered an inhalation of ipratropium bromide/salbutamol (0.5 mg/2.5 mg). Following this intervention, the symptoms of dyspnea decreased and her symptoms subsided.
To achieve an optimal position for the procedure and to minimize the risk of respiratory depression and airway compromise, the patient was placed in the right lateral decubitus position, which she tolerated better. After standard monitoring was established (HR, NIBP, BIS, SpO2, and oxygen supplementation via facial mask), sedation was initiated with a bolus dose of 5 mg of remimazolam and 20 mg of esketamine. This was followed by a continuous infusion of remimazolam at 0.2 mg/kg/h and esketamine at 0.4 mg/kg/h to maintain the patient’s Modified Observer Assessment of Alertness/Sedation (MOAA/S) score below three.
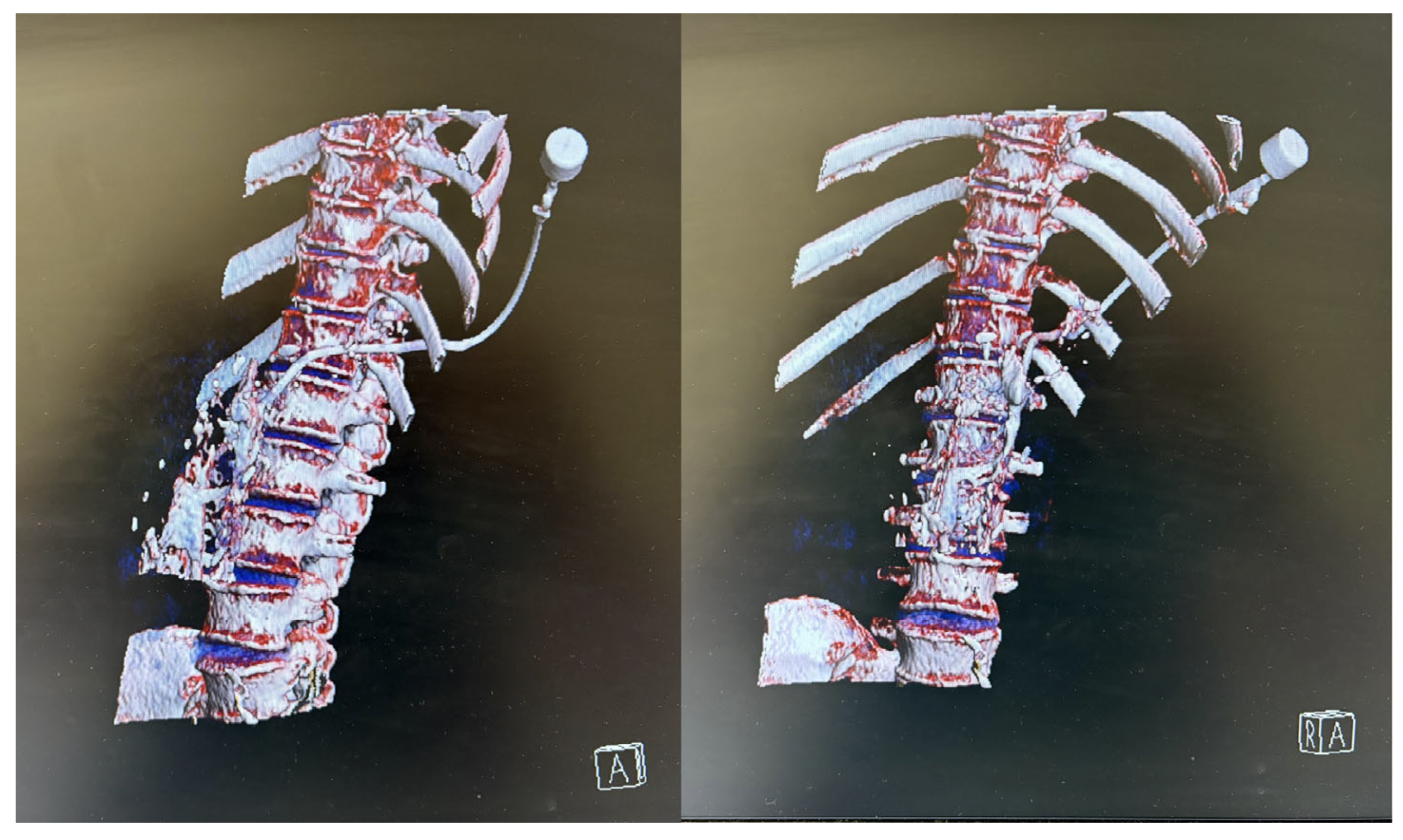
Throughout the procedure, the patient remained sedated, comfortable, and free of unwanted movements, with oxygen saturation between 92 and 96%. The interventional radiologist infiltrated the skin with 2% lidocaine and successfully performed the puncture through the left paravertebral space of the 12th thoracic vertebra. Upon puncturing the AGI, purulent material was aspirated and an 8.5 F drain with an internal locking mechanism was placed (
Figure 2). The entire procedure, including preparation, lasted 105 min, with the sedation for the CT-guided percutaneous drainage lasting 65 min. The process was uneventful, with stable vital signs throughout the whole procedure. No adverse events such as hypotension, bradycardia, or respiratory depression were observed.
Following the procedure, the patient regained full consciousness within minutes, was hemodynamically stable, and demonstrated satisfactory respiratory parameters. Her abdomen appeared flattened, was soft and elastic, and painless on palpation. She no longer reported abdominal pain. After a standard period of 30 min observation in the recovery room, the patient was discharged to a high dependency unit on the surgical ward in a stable condition, with no need for ICU admission.
3. Discussion
Globally, the aging population is leading to an increase in patients with multiple comorbidities, resulting in a growing prevalence of individuals classified as ASA IV or higher in clinical practice [
10,
11]. Despite this trend, there remains a lack of randomized controlled trials to guide the safe selection of anesthetic drugs that are safe for periprocedural sedation in this vulnerable population.
To optimize anesthesia quality and reduce the total dose of general anesthetics, co-induction techniques have become increasingly popular. This approach can also be adapted for periprocedural sedation, where it is critical to ensure the patient’s safety by selecting the optimal drug combination and monitoring strategy. In our case, we employed a combination of remimazolam and esketamine to achieve more stable sedation. This decision was primarily guided by the pharmacological profiles of the drugs and the patient’s complex comorbidities, aligning with the goal of minimizing risks while providing effective sedation.
COPD is a progressive inflammatory disease characterized by persistent and irreversible airflow limitation primarily due to small airway obstruction. The condition makes patients highly susceptible to dyspnea. Preoperative evaluation of COPD in elective patients is crucial and should include comprehensive history, spirometry, and arterial blood gas analysis [
12]. However, in emergency situations, as was the case with our patient, such thorough preparations may not be feasible. Despite limited preoperative assessment, her dyspnea and dependence on a home oxygenator indicated severe COPD with compromised pulmonary function. Patients with advanced COPD experience significant risks during general anesthesia, endotracheal intubation, and intermittent positive pressure ventilation. These factors increase the incidence of postoperative pulmonary complications, including hypoxemia, laryngospasm, bronchospasm, barotrauma injuries, and compromised hemodynamic stability. The selection and management of anesthesia in COPD patients should aim to minimize COPD-triggering factors, reduce the perioperative hypoxemia, minimize the perioperative cardiopulmonary disease complications, and improve patient prognosis. Suboptimal sedation depth can lead to poor patient cooperation and increased patient distress, resulting in circulatory compromises and possible acute COPD exacerbations. Therefore, selecting appropriate sedative drugs is crucial for maintaining appropriate sedation depth and ensuring safe and effective sedation. Commonly used sedatives, such as propofol, midazolam, and dexmedetomidine, each have limitations in patients with severe or multiple comorbidities.
Here, we provide an elaboration on our choice of anesthetic protocol based on remi-mazolam and esketamine. Propofol is an excellent intravenous anesthetic due to its rapid onset of action, short elimination half-life, rapid awakening, and low incidence of postoperative nausea and vomiting. Nevertheless, its dose-dependent circulatory and respiratory depressant effects can lead to hypotension, hypoventilation, or even apnea [
13]. Given the patient’s advanced age, severe COPD combined with low cardiac performance, we considered that propofol could markedly increase the risk of respiratory and circulatory depression, potentially leading to adverse outcome, such as apnea and the need for vasopressors.
Midazolam, often used for anxiolytic and hypnotic properties due to its rapid onset of action, may lead to the loss of airway reflexes, increasing the risk of respiratory depression or aspiration, and induce delirium to some extent in elderly patients [
14]. Our primary concern was that midazolam could complicate respiratory management in this already vulnerable patient.
Dexmedetomidine, an α-adrenoceptor agonist with dose-dependent α2-adrenoceptor selectivity, has anesthetic, analgesic, and antisympathetic properties [
15]. It exerts sedative and weak analgesic effects, with minimal impact on the respiratory function, making it, in theory, ideal for our patient. However, dexmedetomidine can cause cardiovascular depression, leading to bradycardia and hypotension, which led us to exclude it from our protocol. Additionally, the relatively slow onset of action (10–15 min), a long elimination half-life, and accumulation effect were inconsistent with our need for rapid and highly controlled sedation.
Remimazolam is a novel ester-based benzodiazepine that is quickly metabolized into inactive metabolites by tissue carboxylesterases. It acts on the GABA (γ-aminobutyric acid)-A receptor benzodiazepine binding site and potentiates the effects of GABA. Remi-mazolam offers several advantages over other intravenous anesthetics, including a high clearance rate, a short and low context-sensitive half-life, rapid onset and recovery time, and low liability for cardiorespiratory depression [
5]. Approved by the FDA for clinical use in 2020, remimazolam has been studied in clinical trials as a sedative for various procedural sedations, such as gastrointestinal endoscopy [
16,
17], bronchoscopy [
18], hys-teroscopy [
19], sedation during cardiac catheterization [
20], and for dental procedures [
21]. Nevertheless, careful titration and respiratory monitoring are warranted, due to apnea incidence when used as a single agent in continuous infusion for moderate to deep sedation [
22].
Esketamine is a relatively novel anesthetic drug with sedative and analgesic properties. It is the pure S-enantiomer of ketamine, acting as an NMDA antagonist. Esketamine is characterized by rapid onset of action, strong anesthetic potency, reduced intraoperative drug requirements, shorter postoperative awakening times, and higher controllability compared to other anesthetic drugs. Additionally, its cardiostimulatory and bronchodilatory properties can counteract the cardiopulmonary depressive effects of many sedatives [
23,
24].
The combination of remimazolam and esketamine is becoming more widely used in clinical practice. Compared to propofol, this combination has the same advantages of a rapid awakening time with fewer side effects on patients’ circulatory and respiratory functions. Moreover, it accelerates the onset of unconsciousness compared to remimazolam alone or propofol, without prolonging sedation time, while reducing the total induction dose of remimazolam [
7,
25]. These findings make this combination an attractive and effective anesthetic option for procedural sedation, especially in patients with multiple comorbidities. Therefore, based on our case, conducting a cohort study would help to strengthen and validate our findings.
Remimazolam and esketamine are medications with rapid onset and short duration of action, which is preferred for sedation/analgesia during CT guided procedures. It was crucial to avoid respiratory depression and hypotension, which are side effects of most anesthetic drugs, in our patient considering her condition. As our patient was in the prone position, thereby restricting access to the airway, there was a need for modified airway management during anesthesia. Therefore, we used remimazolam, a new short-acting benzodiazepine, which can be safely used in patients with hepatic or renal impairment, without fear of prolonged duration of action, and can be reversed with flumazenil. The onset effect is similar to midazolam, but the recovery time is shorter because of remimazolam’s unique metabolism (metabolized by non-specific tissue esterases). The main benefits of remimazolam are higher hemodynamic stability and lower respiratory depression occurrence compared to propofol and rapid recovery compared with midazolam (three times as quickly as midazolam) [
5,
7]. Patients with severe comorbidities, including poor cardiac reserve, or who are at risk for respiratory depression, may be good candidates for remimazolam sedation, especially during procedures that involve low stimulation intensity. We also used esketamine, which produces a dissociative state accompanied by amnesia and intense analgesia with minimal respiratory depression at sedation doses. Esketamine, compared with ketamine, is twice as potent anesthetic and analgesic, has less severe psychedelic and psychotomimetic side effects, and has similar sympathetic-mediated cardiovascular effects, resulting in comparable increases in BP, HR, and circulating catecholamines [
6].
The limitation of the study is that we were unable to extrapolate data from the bispectral index (BIS) due to electrode malfunction and signal disturbances. Nevertheless, we believe that our findings had no significant impact on the course of sedation itself. Interestingly, previous reports suggest that ketamine can increase the BIS values while paradoxically deepening the level of hypnosis. BIS reflects cortical activity rather than the level of consciousness, and ketamine-induced changes in EEG pattern can increase BIS levels independent of the depth of anesthesia [
26]. Additionally, the appropriate BIS index ranges for remimazolam anesthesia remain to be clarified [
27]. In our case, we repeatedly assessed the MOAA/S score to evaluate the patient’s awareness throughout the procedure. We asked the patient, fully awake after the procedure, about her experience and the quality of her sedation. She reported being comfortable throughout the procedure and expressed satisfaction with its course.