Mendelian Randomization Studies: A Metric for Quality Evaluation
Abstract
1. Introduction
2. Methods
2.1. Study Selection and Eligibility Criteria
2.2. Scoring System for Study Quality Assessment
2.2.1. Study Design
2.2.2. Statistical Methods
2.2.3. Interpretation of Results
2.2.4. STROBE Guidelines
2.3. Data Extraction and Statistical Analysis
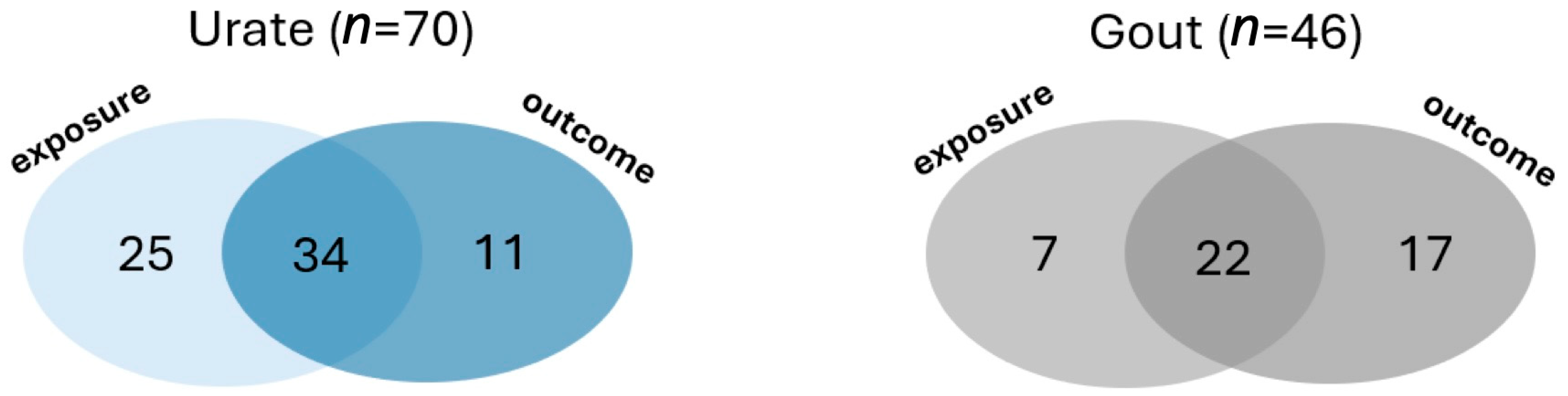
3. Results
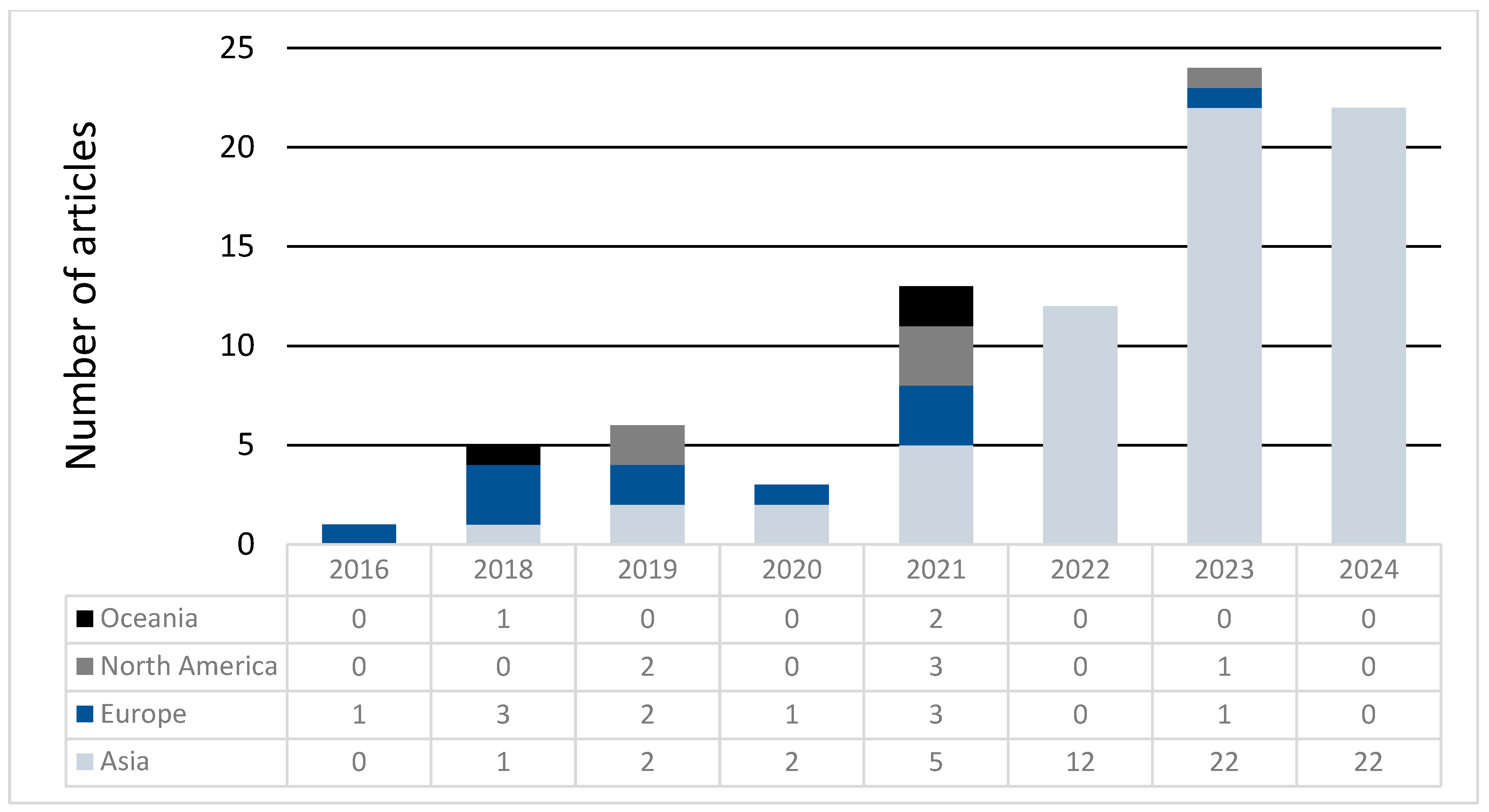
Score Trends per Year and Place of Origin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Muntiu, M.; Joosten, L.A.; Crişan, T.O. Gout basic research: 2023 in review. Gout Urate Cryst. Depos. Dis. 2024, 2, 220–235. [Google Scholar] [CrossRef]
- Drivelegka, P.; Jacobsson, L.T.; Dehlin, M. Gout and gout-related comorbidities: Insight and limitations from population-based registers in Sweden. Gout Urate Cryst. Depos. Dis. 2024, 2, 144–156. [Google Scholar] [CrossRef]
- Andrés, M. Gout and cardiovascular disease: Mechanisms, risk estimations, and the impact of therapies. Gout Urate Cryst. Depos. Dis. 2023, 1, 152–166. [Google Scholar] [CrossRef]
- Robinson, P.C.; Horsburgh, S. Gout: Joints and beyond, epidemiology, clinical features, treatment and co-morbidities. Maturitas 2014, 78, 245–251. [Google Scholar] [CrossRef]
- Robinson, P.C.; Choi, H.K.; Do, R.; Merriman, T.R. Insight into rheumatological cause and effect through the use of Mendelian randomization. Nat. Rev. Rheumatol. 2016, 12, 486–496. [Google Scholar] [CrossRef]
- Richmond, R.C.; Davey Smith, G. Mendelian randomization: Concepts and scope. Cold Spring Harb. Perspect. Med. 2022, 12, a040501. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, D.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Elliott, P.; Chambers, J.C.; Zhang, W.; Clarke, R.; Hopewell, J.C.; Peden, J.F.; Erdmann, J.; Braund, P.; Engert, J.C.; Bennett, D.; et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009, 302, 37–48. [Google Scholar] [CrossRef]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I.; et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 2015, 36, 539–550. [Google Scholar] [CrossRef]
- Jordan, D.M.; Choi, H.K.; Verbanck, M.; Topless, R.; Won, H.H.; Nadkarni, G.; Merriman, T.R.; Do, R. No causal effects of serum urate levels on the risk of chronic kidney disease: A Mendelian randomization study. PLoS Med. 2019, 16, e1002725. [Google Scholar] [CrossRef]
- Hughes, K.; Flynn, T.; de Zoysa, J.; Dalbeth, N.; Merriman, T.R. Mendelian randomization analysis associates increased serum urate, due to genetic variation in uric acid transporters, with improved renal function. Kidney Int. 2014, 85, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.V.; Pascoe, E.M.; Tiku, A.; Boudville, N.; Brown, F.G.; Cass, A.; Clarke, P.; Dalbeth, N.; Day, R.O.; de Zoysa, J.R.; et al. Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 2020, 382, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.; Galecki, A.T.; Spino, C.; Pop-Busui, R.; Cherney, D.Z.; Lingvay, I.; Parsa, A.; Rossing, P.; Sigal, R.J.; Afkarian, M.; et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N. Engl. J. Med. 2020, 382, 2493–2503. [Google Scholar] [CrossRef]
- McCormick, N.; O’Connor, M.J.; Yokose, C.; Merriman, T.R.; Mount, D.B.; Leong, A.; Choi, H.K. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional Mendelian randomization. Arthritis Rheumatol. 2021, 73, 2096–2104. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Timofeeva, M.; Tzoulaki, I.; Tsilidis, K.K.; Ioannidis, P.; Campbell, H.; Theodoratou, E. Serum uric acid levels and multiple health outcomes: Umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ 2017, 357, j2376. [Google Scholar] [CrossRef]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, D.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipila, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Concato, J.; Brophy, M.; Fiore, L.; Pyarajan, S.; Breeling, J.; Whitbourne, S.; Deen, J.; Shannon, C.; Humphries, D.; et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 2016, 70, 214–223. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Hemani, G.; Bowden, J.; Davey Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 2018, 27, R195–R208. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Use of allele scores as instrumental variables for Mendelian randomization. Int. J. Epidemiol. 2013, 42, 1134–1144. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davies, N.M.; Hemani, G.; Davey Smith, G. Two-sample Mendelian randomization: Avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 2016, 45, 1717–1726. [Google Scholar] [CrossRef]
- Köttgen, A.; Albrecht, E.; Teumer, A.; Vitart, V.; Krumsiek, J.; Hundertmark, C.; Pistis, G.; Ruggerio, D.; O’Seaghdha, C.M.; Haller, T.; et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 2013, 45, 145–154. [Google Scholar] [CrossRef]
- Tin, A.; Marten, J.; Halperin Kuhns, V.L.; Li, Y.; Wüttke, M.; Kirsten, H.; Sieber, K.B.; Qiu, C.; Gorski, M.; Yu, Z.; et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat. Genet. 2019, 51, 1459–1474. [Google Scholar] [CrossRef]
- Burgess, S.; Woolf, B.; Mason, A.M.; Ala-Korpela, M.; Gill, D. Addressing the credibility crisis in Mendelian randomization. BMC Med. 2024, 22, 374. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef]
- Jiang, T.; Gill, D.; Butterworth, A.S.; Burgess, S. An empirical investigation into the impact of winner’s curse on estimates from Mendelian randomization. Int. J. Epidemiol. 2023, 52, 1209–1219. [Google Scholar] [CrossRef]
- Stender, S.; Gellert-Kristensen, H.; Davey Smith, G. Reclaiming mendelian randomization from the deluge of papers and misleading findings. Lipids Health Dis. 2024, 23, 286. [Google Scholar] [CrossRef] [PubMed]
- Lyngdoh, T.; Vuistiner, P.; Marques-Vidal, P.; Rousson, V.; Waeber, G.; Vollenweider, P.; Bochud, M. Serum uric acid and adiposity: Deciphering causality using a bidirectional Mendelian randomization approach. PLoS ONE 2012, 7, e39321. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.M.; Nordestgaard, B.G.; Benn, M.; Tybjaerg-Hansen, A.; Davey Smith, G.; Lawlor, D.A.; Timpson, N.J. Association of plasma uric acid with ischaemic heart disease and blood pressure: Mendelian randomisation analysis of two large cohorts. BMJ 2013, 347, f4262. [Google Scholar] [CrossRef] [PubMed]
- Oikonen, M.; Wendelin-Saarenhovi, M.; Lyytikainen, L.P.; Siitonen, N.; Loo, B.M.; Jula, A.; Seppälä, I.; Saarikoski, L.; Lehtimäki, T.; Hutri-Kähönen, N.; et al. Associations between serum uric acid and markers of subclinical atherosclerosis in young adults. The cardiovascular risk in Young Finns study. Atherosclerosis 2012, 223, 497–503. [Google Scholar] [CrossRef]
- Yokose, C.; McCormick, N.; Rai, S.K.; Lu, N.; Curhan, G.; Schwarzfuchs, D.; Shai, I.; Choi, H.K. Effects of low-fat, Mediterranean, or low-carbohydrate weight loss diets on serum urate and cardiometabolic risk factors: A secondary analysis of the Dietary Intervention Randomized Controlled Trial (DIRECT). Diabetes Care 2020, 43, 2812–2820. [Google Scholar] [CrossRef]
- Neogi, T.; George, J.; Rekhraj, S.; Struthers, A.D.; Choi, H.; Terkeltaub, R.A. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheumatol. 2012, 64, 327–338. [Google Scholar] [CrossRef]
- White, W.B.; Saag, K.G.; Becker, M.A.; Borer, J.S.; Gorelick, P.B.; Whelton, A.; Hunt, B.; Castillo, M.; Gunawardhana, L. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 2018, 378, 1200–1210. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.Y.; Lee, J.; Yoon, S.; Kim, E.G.; Lee, E.; Kim, N.; Lee, S.; Park, S.-I. Effects of uric acid on ischemic diseases, stratified by lipid levels: A drug-target, nonlinear Mendelian randomization study. Sci. Rep. 2024, 14, 1338. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; He, Y.; Spiliopoulou, A.; Timofeeva, M.; Wei, W.Q.; Gifford, A.; Yang, T.; Varley, T.; Tzoulaki, I.; et al. Genetically determined serum urate levels and cardiovascular and other diseases in UK Biobank cohort: A phenome-wide mendelian randomization study. PLoS Med. 2019, 16, e1002937. [Google Scholar] [CrossRef]
- Rasheed, H.; Hughes, K.; Flynn, T.J.; Merriman, T.R. Mendelian randomization provides no evidence for a causal role of serum urate in increasing serum triglyceride levels. Circ. Cardiovasc. Genet. 2014, 7, 830–837. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Spiliopoulou, A.; Timofeeva, M.; Wei, W.Q.; Gifford, A.; Shen, X.; He, Y.; Varley, T.; McKeigue, P.; et al. MR-PheWAS: Exploring the causal effect of SUA level on multiple disease outcomes by using genetic instruments in UK Biobank. Ann. Rheum. Dis. 2018, 77, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Gaffo, A.L.; Calhoun, D.A.; Rahn, E.J.; Oparil, S.; Li, P.; Dudenbostel, T.; Feig, D.I.; Redden, D.T.; Muntner, P.; Foster, P.J.; et al. Effect of serum urate lowering with allopurinol on blood pressure in young adults: A randomized, controlled, crossover trial. Arthritis Rheumatol. 2021, 73, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Soletsky, B.; Johnson, R.J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA 2008, 300, 924–932. [Google Scholar] [CrossRef]
- Beattie, C.J.; Fulton, R.L.; Higgins, P.; Padmanabhan, S.; McCallum, L.; Walters, M.R.; Dominiczak, A.F.; Touyz, R.M.; Dawson, J. Allopurinol initiation and change in blood pressure in older adults with hypertension. Hypertension 2014, 64, 1102–1107. [Google Scholar] [CrossRef]



| Study design | Rationale | 2: Strong observational evidence 1: Small sample studies or mixed evidence (some studies support the association, while others do not) −1: Minimal information or unclear rationale |
| Comparison direction | 1: Bidirectional 0: Unidirectional | |
| Datasets | 1: Uses the most recent and largest GWAS dataset 0: Does not use latest GWAS dataset | |
| Ancestry comparison | 1: Comparison involves the same ethnicities 0: Ethnicity information is either absent in one or all datasets, or study compares a mixed ancestry database against a single ancestry without appropriate adjustments −1: Comparisons between different ethnicities | |
| Dataset independence | 1: Exposure and outcome datasets are independent −1: Not independent | |
| Replication | 3: Replication study included −1: no replication | |
| Statistical methods | SNP selection | 1: SNPs were associated with exposure at genome-wide significance (p < 5 × 10−8) or F-statistic > 10 and 1: SNPs were pruned for LD with R2 < 0.1. |
| Mediator analysis | 1 If a mediator variable analysis was conducted | |
| Confounder analysis | 1 If testing for confounders was performed | |
| Presented SNPs | 2: SNPs significantly associated with the exposure were clearly listed, including their effect alleles, effect sizes, and p-values. 1: SNPs associated with the exposure were listed but without complete information on effect alleles, effect sizes, and p-values. −1: SNPs were not listed | |
| p-value correction | 2: Applied −1: When correction required was <10 tests but not applied −3: When correction required was ≥10 tests but not applied 0: Not required | |
| Was the study power considered? | 2: Yes −1: No | |
| Interpretation of results | 2: Results concluded appropriately according to statistical evidence −2: Results not concluded appropriately according to statistical evidence | |
| STROBE guidelines presented? | 1: Yes 0: No | |
| Exposure | Outcome | N Articles | Articles That Found Association | Articles That Found No Association | ||
|---|---|---|---|---|---|---|
| Mean Score | Articles | Mean Score | Articles | |||
| Urate → trait | ||||||
| Urate | Coronary heart disease | 8 | 9.3 | 75, 176, 200 | 2.3 | 12, 100, 129, 131, 136 |
| Urate | Hypertension | 6 | 2.1 | 46, 78 | 10.5 | 65, 97, 129, 131 |
| Urate | BMI | 4 | - | - | 1.9 | 5, 31, 60, 65 |
| Urate | Heart failure | 4 | 9.1 | 62, 78 | 2.1 | 1, 26 |
| Urate | CKD | 3 | - | - | 10.7 | 97, 41, 137 |
| Urate | Gut microbiota | 4 | - | - | 6.25 | 2, 30, 43, 199 |
| Urate | Myocardial infarction | 3 | 14 | 75 | 10 | 129, 131 |
| Urate | Fasting insulin | 3 | - | - | 12.3 | 65, 91, 99 |
| Gout → trait | ||||||
| Gout | Coronary heart disease | 2 | 5 | 78, 200 | - | - |
| Trait → Urate | ||||||
| BMI | Urate | 7 | 9 | 5, 15, 31, 60, 65, 93, 139 | - | - |
| Coffee | Urate | 4 | 7 | 9, 38 | 8 | 73, 106 |
| Gut Microbiota | Urate | 4 | - | - | 6.25 | 2, 30, 43, 199 |
| Fasting Insulin | Urate | 3 | 12.3 | 65, 91, 99 | - | - |
| Waist/Hip ratio | Urate | 3 | 9 | 31 | 9 | 139, 65 |
| HDLc | Urate | 3 | 11.3 | 65, 93, 102 | - | - |
| TG | Urate | 3 | 11.3 | 65, 93, 102 | - | - |
| T2DM | Urate | 2 | 9 | 99 | 11 | 65 |
| Trait → Gout | ||||||
| Tea intake | Gout | 4 | 10 | 26, 215 | 3 | 16, 211 |
| BMI | Gout | 3 | 9.67 | 31, 65, 93 | - | - |
| Coffee | Gout | 2 | 5.5 | 73, 142 | - | - |
| Blood pressure | Gout | 2 | 13 | 65, 198 | - | - |
| Gut microbiota | Gout | 2 | - | - | 9.5 | 30, 43 |
| Dataset | Ancestry | Year | Urate Sample Size | Gout Sample Size (Cases/Controls) | Freq (%) | PMID |
|---|---|---|---|---|---|---|
| Köttgen | European | 2013 | 110,347 | 2115/67,259 | 44 (51.16%) | 23263486 |
| Tin | European | 2019 | 288,649 | 13,179/750,634 | 20 (23.26%) | 31578528 |
| Japan Biobank | East Asian | 2019 | 109,029 | 3053/4554 | 6 (6.98%) | 32238385 |
| UK Biobank | European | NA | 6542/456,391 | 12 (13.95%) | ||
| Sakaue | European + East Asian | 2021 | 343,836 | - | 2 (2.33%) | 34594039 |
| FinnGen | European | - | 3576/147,221 | 8 (9.3%) | ||
| UK Biobank | African | 2021 | 6206 | - | 1 (1.16%) | |
| Taiwan Biobank | East Asian | 2008 | 3483 | - | 1 (1.16%) | 18370851 |
| Nakatochi | East Asian | 2019 | 121,745 | - | 1 (1.16%) | 30993211 |
| Kolz | European | 2009 | 28,141 | - | 1 (1.16%) | 19503597 |
| Huffman | European | 2015 | 42,569 | 2 (2.33%) | 25811787 | |
| White | European | 2016 | 166,486 | - | 2 (2.33%) | 26781229 |
| Leon-Mimila | Hispanic | 2013 | 1073 adults, 1080 children | - | 1 (1.16%) | 23950976 |
| Dönertaş | European | 2021 | - | 488,295 | 1 (1.16%) | 33959723 |
| Zhou | European + East Asian | 2022 | - | 30,549/1,039,290 | 1 (1.16%) | 36777996 |
| Continent | Mean | IQR |
|---|---|---|
| Asia | 8.9 ± 0.5 | 5 |
| Europe | 9.8 ± 0.7 | 4 |
| North America | 9.5 ± 2 | 3 |
| Oceania | 10 ± 0.6 | 2 |
| Total | 9.1 ± 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Gout, Hyperuricemia and Crystal Associated Disease Network. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Chavez, F.; Merriman, T.R. Mendelian Randomization Studies: A Metric for Quality Evaluation. Gout Urate Cryst. Depos. Dis. 2025, 3, 8. https://doi.org/10.3390/gucdd3020008
Rosas-Chavez F, Merriman TR. Mendelian Randomization Studies: A Metric for Quality Evaluation. Gout, Urate, and Crystal Deposition Disease. 2025; 3(2):8. https://doi.org/10.3390/gucdd3020008
Chicago/Turabian StyleRosas-Chavez, Fiorella, and Tony R. Merriman. 2025. "Mendelian Randomization Studies: A Metric for Quality Evaluation" Gout, Urate, and Crystal Deposition Disease 3, no. 2: 8. https://doi.org/10.3390/gucdd3020008
APA StyleRosas-Chavez, F., & Merriman, T. R. (2025). Mendelian Randomization Studies: A Metric for Quality Evaluation. Gout, Urate, and Crystal Deposition Disease, 3(2), 8. https://doi.org/10.3390/gucdd3020008







