Comparison of Pathophysiological Mechanisms Among Crystal-Induced Arthropathies
Abstract
1. Introduction
2. Physical–Chemical Properties and Detection Methods of Crystals
2.1. Compensated Polarized Light Microscopy (CPLM)
2.2. Microscopy with Staining
2.3. Ultrasounds (US)
2.4. Dual Energy Computed Tomography (DECT)
2.5. Raman Spectroscopy
3. Crystal Formation
4. Induction of Inflammation
4.1. Cells Mainly Involved in Crystal-Induced Inflammatory Processes
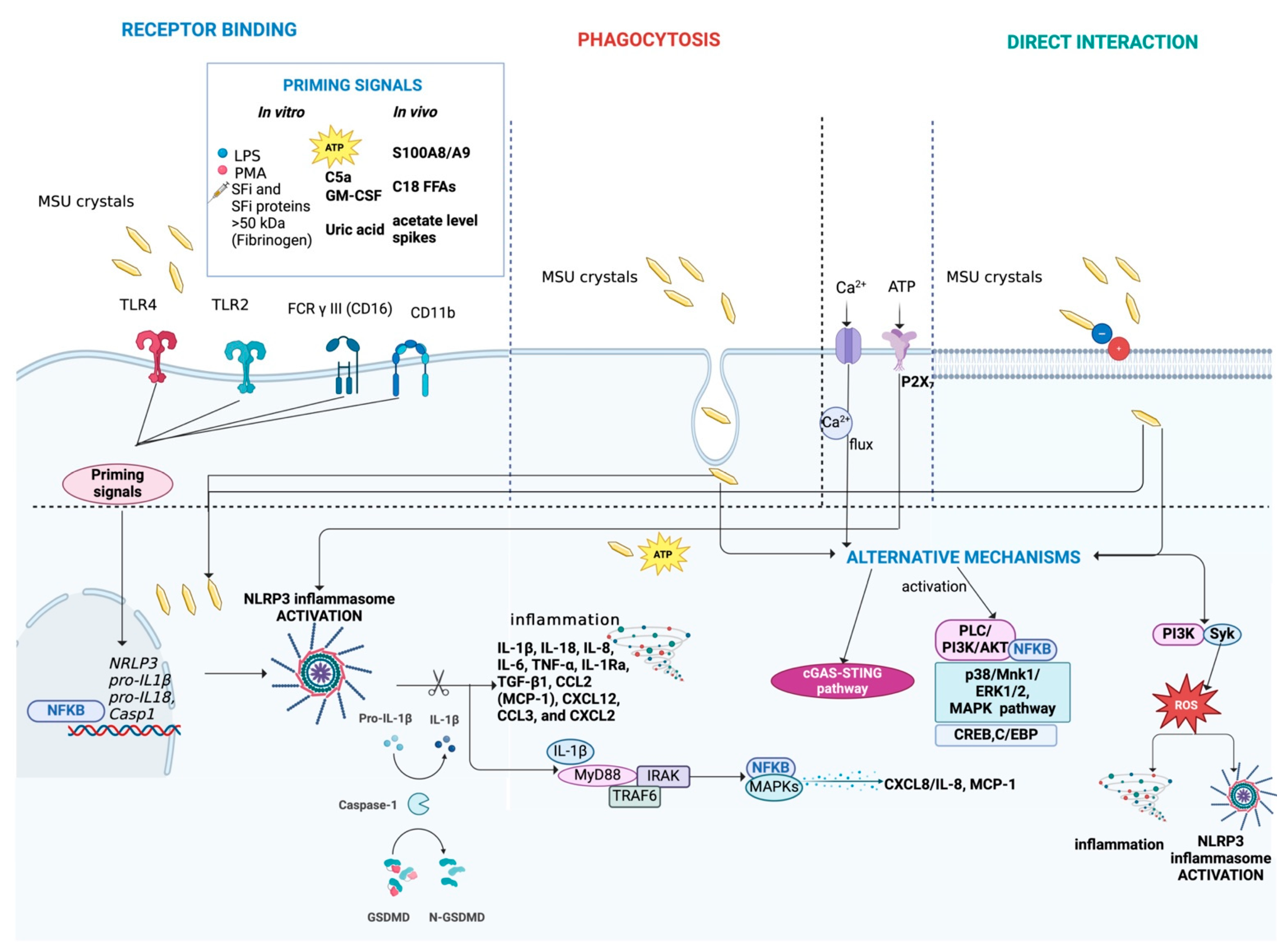
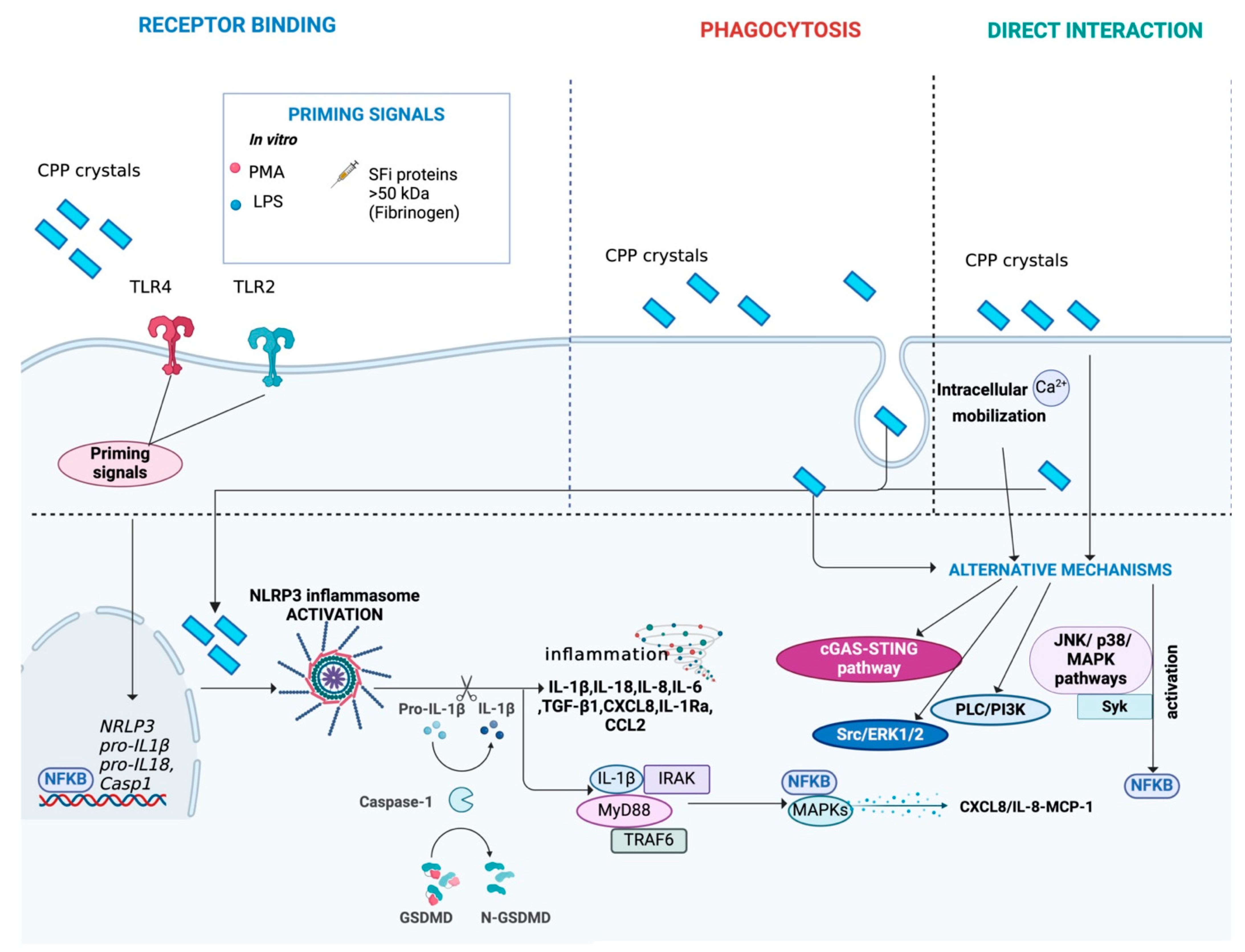
4.2. Mechanisms of Interaction Between Cells and Crystals
4.3. Activation of the NLRP3 Inflammasome
4.4. IL-1β Production After Inflammasome Activation
4.5. Mechanisms Following Inflammasome Activation
4.6. Alternative Mechanisms Activated by Crystals
4.7. Programmed Cell Deaths
5. Resolution Phase
6. Crystals and Pain
7. Crystals and Mitochondria
8. Crystals and Genomic Instability
9. Crystals and Senescence
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mandel, N.S.; Mandel, G.S. Monosodium Urate Monohydrate, the Gout Culprit. J. Am. Chem. Soc. 1976, 98, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.; Tang, W.; Gong, J. Understanding the Crystallization Pathway of Monosodium Urate Monohydrate in a Biomimetic Matrix. Cryst. Growth Des. 2020, 20, 804–812. [Google Scholar] [CrossRef]
- Mandel, N.S. The Crystal Structure of Calcium Pyrophosphate Dihydrate. Acta Crystallografica 1975, B31, 1730. [Google Scholar] [CrossRef]
- Rosenthal, A.K. Basic Calcium Phosphate Crystal-Associated Musculoskeletal Syndromes: An Update. Curr. Opin. Rheumatol. 2018, 30, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Yavorskyy, A.; Hernandez-Santana, A.; McCarthy, G.; McMahon, G. Detection of Calcium Phosphate Crystals in the Joint Fluid of Patients with Osteoarthritis—Analytical Approaches and Challenges. Analyst 2008, 133, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.S.; McCarthy, G.M. Hydroxyapatite Deposition Disease of the Joint. Curr. Rheumatol. Rep. 2003, 5, 215–221. [Google Scholar] [CrossRef]
- Tung, M.S. Calcium Phosphates: Structure, Composition, Solubility, and Stability. In Calcium Phosphates in Biological and Industrial Systems; Amjad, Z., Ed.; Springer: New York, NY, USA, 1998; pp. 1–19. [Google Scholar]
- Siddiqi, S.A.; Azhar, U. Carbonate Substituted Hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 149–173. [Google Scholar]
- Iqbal, Z.; Tomaselli, V.P.; Fahrenfeldand, O.; Miller, K.D.; Ruszalaand, F.A.; Kostiner, E. Polarized Raman scattering and low frequency infrared study of hydroxyapatite. Phys. Chem. Solids 1977, 38, 923–927. [Google Scholar] [CrossRef]
- Elliott, J.C. Calcium phosphate biominerals. Rev. Mineral. Geo-Chem. 2002, 48, 427–454. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Bleiwas, C.B.; Retino, M.; Rohanizadeh, R.; LeGeros, J.P. Zinc effect on the in vitro formation of calcium phosphates: Relevance to clinical inhibition of calculus formation. Am. J. Dent. 1999, 12, 65–71. [Google Scholar]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Jillavenkatesa, A.; Condrate, R.A., Sr. The Infrared and Raman Spectra of β-and α-Tricalcium Phosphate (Ca3(PO4)2). Spectrosc. Lett. 1998, 31, 1619–1634. [Google Scholar] [CrossRef]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castaneda, J.; Coyfish, M.; Guillo, S.; Jansen, T.; Janssens, H.; et al. 2018 updated European League Against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann. Rheum. Dis. 2020, 79, 31–38. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Bardin, T.; Barskova, V.; Guerne, P.A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: Terminology and diagnosis. Ann. Rheum. Dis. 2011, 70, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Zell, M.; Zhang, D.; Fitzgerald, J. Diagnostic Advances in Synovial Fluid Analysis and Radiographic Identification for Crystalline Arthritis. Curr. Opin. Rheumatol. 2019, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Pârvănescu, C.D.; Bărbulescu, A.L.; Bită, C.E.; Dinescu, S.C.; Tras, B.A.; Firulescu, S.C.; Vreju, F.A. Ultrasound Features in Gout: An Overview. Med. Sci. 2024, 12, 37. [Google Scholar] [CrossRef]
- Filippou, G.; Miguel-Pérez, M.; Coronel, L.; Sirotti, S.; Pacini, G.; Scanu, A.; Bong, D.; Möller, I.; EULAR Study Group on Anatomy for the Image. The ultrasonographic pseudo-double contour sign in calcium pyrophosphate deposition disease: An anatomic explanation and how to distinguish it from gout. Arthritis Rheumatol. 2023, 75, 639–640. [Google Scholar] [CrossRef] [PubMed]
- Filippou, G.; Pacini, G.; Sirotti, S.; Zadory, M.; Carboni, D.; Damiani, A.; Fiorentini, E.; Cipolletta, E.; Filippucci, E.; Froehlich, J.M.; et al. Comparison of ultrasound attenuation by calcium pyrophosphate, hydroxyapatite and monosodium urate crystals: A proof-of-concept study. Ann. Rheum. Dis. 2022, 81, 1199–1201. [Google Scholar] [CrossRef]
- Døssing, A.; Müller, F.C.; Becce, F.; Stamp, L.; Bliddal, H.; Boesen, M. Dual-Energy Computed Tomography for Detection and Characterization of Monosodium Urate, Calcium Pyrophosphate, and Hydroxyapatite: A Phantom Study on Diagnostic Performance. Investig. Radiol. 2021, 56, 417–424. [Google Scholar] [CrossRef]
- Pascart, T.; Falgayrac, G.; Norberciak, L.; Lalanne, C.; Legrand, J.; Houvenagel, E.; Ea, H.K.; Becce, F.; Budzik, J.F. Dual-energy computed-tomography-based discrimination between basic calcium phosphate and calcium pyrophosphate crystal deposition in vivo. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20936060. [Google Scholar] [CrossRef]
- Scanu, A.; Oliviero, F.; Luisetto, R.; Ramonda, R.; Doria, A.; Punzi, L.; Dayer, J.M. Effect of pathogenic crystals on the production of pro- and anti-inflammatory cytokines by different leukocyte populations. Immunobiology 2021, 226, 152042. [Google Scholar] [CrossRef]
- Allen, R.N.; Lipkowski, P.; Shukla, M.K.; Leszczynski, J. Vibrational analysis of complexes of urate with IA group metal cations (Li+, Na+ and K+). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 639–645. [Google Scholar] [CrossRef] [PubMed]
- de Aza, P.N.; Guitian, F.; Santos, C.; de Aza, S.; Cusco, R.; Artus, L. Vibrational Properties of Calcium Phosphate Compounds. 2. Comparison between Hydroxyapatite and b-Tricalcium Phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Punzi, L. Metabolism of crystals within the joint. Reumatismo 2011, 63, 221–229. [Google Scholar] [CrossRef]
- Pascual, E.; Addadi, L.; Andrés, M.; Sivera, F. Mechanisms of crystal formation in gout—A structural approach. Nat. Rev. Rheumatol. 2015, 11, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Chhana, A.; Lee, G.; Dalbeth, N. Factors influencing the crystallization of monosodium urate: A systematic literature review Pathophysiology of musculoskeletal disorders. BMC Musculoskelet. Disord. 2015, 16, 296. [Google Scholar] [CrossRef]
- Ahn, H.; Lee, G.; Lee, G.S. Lower temperatures exacerbate NLRP3 inflammasome activation by promoting monosodium urate crystallization, causing gout. Cells 2021, 10, 1919. [Google Scholar] [CrossRef]
- Rosenthal, A.K. Articular cartilage vesicles and calcium crystal deposition diseases. Curr. Opin. Rheumatol. 2016, 28, 127–132. [Google Scholar] [CrossRef]
- Williams, C.J.; Rosenthal, A.K. Pathogenesis of calcium pyrophosphate deposition disease. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101718. [Google Scholar] [CrossRef]
- Sirotti, S.; Scanu, A.; Pascart, T.; Niessink, T.; Maroni, P.; Lombardi, G.; Filippou, G. Calcium Pyrophosphate Crystal Formation and Deposition: Where Do we Stand and What Does the Future hold? Curr. Rheumatol. Rep. 2024, 26, 354–365. [Google Scholar] [CrossRef]
- Ryan, L.M.; Wortmann, R.L.; Karas, B.; Mccarty, D.J. Cartilage Nucleoside Triphosphate (NTP) Pyrophosphohydrolase.I. Identification as an ecto-enzyme. Arthritis Rheum. 1984, 27, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.M.; Wortmann, R.L.; Karas, B.; McCarty, D.J. Cartilage nucleoside triphosphate pyrophosphohydrolase. II. Role in extracellular pyrophosphate generation and nucleotide metabolism. Arthritis Rheum. 1985, 28, 413–418. [Google Scholar] [CrossRef]
- Johnson, K.A.; Hessle, L.; Vaingankar, S.; Wennberg, C.; Mauro, S.; Narisawa, S.; Goding, J.W.; Sano, K.; Millan, J.L.; Terkeltaub, R. Osteoblast tissue-nonspecific alkaline phosphatase antagonizes and regulates PC-1. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 279, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Thouverey, C.; Bechkoff, G.; Pikula, S.; Buchet, R. Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthr. Cartil. 2009, 17, 64–72. [Google Scholar] [CrossRef]
- Bernabei, I.; So, A.; Busso, N.; Nasi, S. Cartilage calcification in osteoarthritis: Mechanisms and clinical relevance. Nat. Rev. Rheumatol. 2023, 19, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Scanu, A.; Oliviero, F.; Gruaz, L.; Sfriso, P.; Pozzuoli, A.; Frezzato, F.; Agostini, C.; Burger, D.; Punzi, L. High-density lipoproteins downregulate CCL2 production in human fibroblast-like synoviocytes stimulated by urate crystals. Arthritis Res. Ther. 2010, 12, 23. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Fernández-Torres, J.; Loissell-Baltazar, Y.A.; Medina-Luna, D.; López-Macay, A.; Camacho-Galindo, J.; Hernández-Díaz, C.; Santamaría-Olmedo, M.G.; López-Villegas, E.O.; et al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res. Ther. 2016, 18, 117. [Google Scholar] [CrossRef]
- Oliviero, F.; Sfriso, P.; Scanu, A.; Fiocco, U.; Spinella, P.; Punzi, L. Epigallocatechin-3-gallate reduces inflammation induced by calcium pyrophosphate crystals in vitro. Front. Pharmacol. 2013, 4, 51. [Google Scholar] [CrossRef]
- Niessink, T.; Stassen, R.H.M.J.; Kischkel, B.; Vuscan, P.; Emans, P.J.; van den Akker, G.G.H.; Janssen, M.; Joosten, L.A.B.; Otto, C.; Welting, T.J.M.; et al. Discovery of calcite as a new pro-inflammatory calcium-containing crystal in human osteoarthritic synovial fluid. Osteoarthr. Cartil. 2024, 32, 1261–1272. [Google Scholar] [CrossRef]
- Meyer, F.; Dittmann, A.; Kornak, U.; Herbster, M.; Pap, T.; Lohmann, C.H.; Bertrand, J. Chondrocytes from Osteoarthritic and Chondrocalcinosis Cartilage Represent Different Phenotypes. Front. Cell Dev. Biol. 2021, 9, 622287. [Google Scholar] [CrossRef]
- Bertrand, J.; Kräft, T.; Gronau, T.; Sherwood, J.; Rutsch, F.; Lioté, F.; Dell’Accio, F.; Lohmann, C.H.; Bollmann, M.; Held, A.; et al. BCP crystals promote chondrocyte hypertrophic differentiation in OA cartilage by sequestering Wnt3a. Ann. Rheum. Dis. 2020, 79, 975–984. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Pritzker, K.; Firestein, G.S.; Terkeltaub, R. TLR2 Signaling in Chondrocytes Drives Calcium Pyrophosphate Dihydrate and Monosodium Urate Crystal-Induced Nitric Oxide Generation. J. Immunol. 2005, 174, 5016–5023. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lioté, F.; Rose, D.M.; Merz, D.; Terkeltaub, R. Proline-rich tyrosine kinase 2 and Src kinase signaling transduce monosodium urate crystal–induced nitric oxide production and matrix metalloproteinase 3 expression in chondrocytes. Arthritis Rheum. 2004, 50, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, C.H.; Tsai, H.C.; Salter, D.M. Inhibition of cyclooxygenase 2 expression by diallyl sulfide on joint inflammation induced by urate crystal and IL-1β. Osteoarthr. Cartil. 2009, 17, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Lee, C.H.; Wang, C.C.; Salter, D.M.; Lee, H.S. Pycnogenol attenuates the inflammatory and nitrosative stress on joint inflammation induced by urate crystals. Free Radic. Biol. Med. 2012, 52, 765–774. [Google Scholar] [CrossRef]
- Popa-Nita, O.; Naccache, P.H. Crystal-induced neutrophil activation. Immunol. Cell Biol. 2010, 88, 32–40. [Google Scholar] [CrossRef]
- Mahon, O.R.; Kelly, D.J.; McCarthy, G.M.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals alter immune cell metabolism and promote M1 macrophage polarization. Osteoarthr. Cartil. 2020, 28, 603–612. [Google Scholar] [CrossRef]
- Martin, W.J.; Shaw, O.; Liu, X.; Steiger, S.; Harper, J.L. Monosodium urate monohydrate crystal-recruited noninflammatory monocytes differentiate into M1-like proinflammatory macrophages in a peritoneal murine model of gout. Arthritis Rheum. 2011, 63, 1322–1332. [Google Scholar] [CrossRef]
- McLean, L.; Dalbeth, N. Etiology and pathogenesis of gout. In Rheumatology, 6th ed.; Hochberg, M.C., Silman, A.J., Weinblatt, M.E., Smolen, J.S., Weisman, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, pp. 1555–1568. [Google Scholar]
- Ng, G.; Sharma, K.; Ward, S.M.; Desrosiers, M.D.; Stephens, L.A.; Schoel, W.M.; Li, T.; Lowell, C.A.; Ling, C.C.; Amrein, M.W.; et al. Receptor-Independent, Direct Membrane Binding Leads to Cell-Surface Lipid Sorting and Syk Kinase Activation in Dendritic Cells. Immunity 2008, 29, 807–818. [Google Scholar] [CrossRef]
- Rossato, M.F.; Hoffmeister, C.; Trevisan, G.; Bezerra, F.; Cunha, T.M.; Ferreira, J.; Silva, C.R. Monosodium urate crystal interleukin-1β release is dependent on Toll-like receptor 4 and transient receptor potential V1 activation. Rheumatology 2020, 59, 233–242. [Google Scholar] [CrossRef]
- Pascart, T.; Filippou, G.; Lioté, F.; Sirotti, S.; Jauffret, C.; Abhishek, A. Calcium pyrophosphate deposition disease. Lancet Rheumatol. 2024, 6, 791–804. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Mills, E.; Mielke, L.A.; O’Farrell, L.K.; Lavelle, E.; Mori, A.; McCarthy, G.M.; Mills, K.H.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals induce pro-inflammatory cytokines and damage-associated molecules via activation of Syk and PI3 kinase. Clin. Immunol. 2012, 144, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Pazár, B.; Ea, H.K.; Narayan, S.; Kolly, L.; Bagnoud, N.; Chobaz, V.; Roger, T.; Lioté, F.; So, A.; Busso, N. Basic Calcium Phosphate Crystals Induce Monocyte/Macrophage IL-1β Secretion through the NLRP3 Inflammasome In Vitro. J. Immunol. 2011, 186, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Grandjean-Laquerriere, A.; Tabary, O.; Jacquot, J.; Richard, D.; Frayssinet, P.; Guenounou, M.; Laurent-Maquin, D.; Laquerriere, P.; Gangloff, S. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials 2007, 28, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Ehirchiou, D.; Bernabei, I.; Pandian, V.D.; Nasi, S.; Chobaz, V.; Castelblanco, M.; So, A.; Martinon, F.; Li, X.; Acha-Orbea, H.; et al. The integrin CD11b inhibits MSU-induced NLRP3 inflammasome activation in macrophages and protects mice against MSU-induced joint inflammation. Arthritis Res. Ther. 2024, 26, 119. [Google Scholar] [CrossRef]
- Barabé, F.; Gilbert, C.; Liao, N.; Bourgoin, S.G.; Naccache, P.H. Crystal-induced neutrophil activation VI. Involvement of FcγRIIIB (CD16) and CD11b in response to inflammatory microcrystals. FASEB J. 1998, 12, 209–220. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Fernández-Torres, J.; Martínez-Nava, G.A.; Martínez-Flores, K.; Ramírez Olvera, A.; Medina-Luna, D.; Hernández Pérez, A.D.; Landa-Solís, C.; López-Reyes, A. Phagocytosis of monosodium urate crystals by human synoviocytes induces inflammation. Exp. Biol. Med. 2019, 244, 344–351. [Google Scholar] [CrossRef]
- McCarthy, G.M.; Cheung, H.S.; Abel, S.M.; Ryan, L.M. Basic calcium phosphate crystal-induced collagenase production: Role of intracellular crystal dissolution. Osteoarthr. Cartil. 1998, 6, 205–213. [Google Scholar] [CrossRef][Green Version]
- Oliviero, F.; Zamudio-Cuevas, Y.; Belluzzi, E.; Andretto, L.; Scanu, A.; Favero, M.; Ramonda, R.; Ravagnan, G.; López-Reyes, A.; Spinella, P.; et al. Polydatin and Resveratrol Inhibit the Inflammatory Process Induced by Urate and Pyrophosphate Crystals in THP-1 Cells. Foods 2019, 8, 560. [Google Scholar] [CrossRef]
- Baggio, C.; Sfriso, P.; Cignarella, A.; Galozzi, P.; Scanu, A.; Mastrotto, F.; Favero, M.; Ramonda, R.; Oliviero, F. Phagocytosis and inflammation in crystal-induced arthritis: A synovial fluid and in vitro study. Clin. Exp. Rheumatol. 2021, 39, 494–500. [Google Scholar] [CrossRef]
- Ea, H.K.; Lioté, F. Advances in understanding calcium-containing crystal disease. Curr. Opin. Rheumatol. 2009, 21, 150–157. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Mouktaroudi, M.; Bodar, E.; van der Ven, J.; Kullberg, B.J.; Netea, M.G.; van der Meer, J.W. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 βby mononuclear cells through a caspase 1-mediated process. Ann. Rheum. Dis. 2009, 68, 273–278. [Google Scholar] [CrossRef] [PubMed]
- An, L.L.; Mehta, P.; Xu, L.; Turman, S.; Reimer, T.; Naiman, B.; Connor, J.; Sanjuan, M.; Kolbeck, R.; Fung, M. Complement C5a potentiates uric acid crystal-induced IL-1β production. Eur. J. Immunol. 2014, 44, 3669–3679. [Google Scholar] [CrossRef]
- Shaw, O.M.; Steiger, S.; Liu, X.; Hamilton, J.A.; Harper, J.L. Brief Report: Granulocyte-Macrophage Colony-Stimulating Factor Drives Monosodium Urate Monohydrate Crystal–Induced Inflammatory Macrophage Differentiation and NLRP3 Inflammasome Up-Regulation in an in Vivo Mouse Model. Arthritis Rheumatol. 2014, 66, 2423–2428. [Google Scholar] [CrossRef]
- Crișan, T.O.; Cleophas, M.C.; Oosting, M.; Lemmers, H.; Toenhake-Dijkstra, H.; Netea, M.G.; Jansen, T.L.; Joosten, L.A. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 2016, 75, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Nippe, N.; Vogl, T.; Marketon, K.; Mysore, V.; Weinhage, T.; Dalbeth, N.; Pool, B.; Merriman, T.; Baeten, D.; et al. Myeloid-Related Proteins 8 and 14 Contribute to Monosodium Urate Monohydrate Crystal–Induced Inflammation in Gout. Arthritis Rheumatol. 2014, 66, 1327–1339. [Google Scholar] [CrossRef]
- Vieira, A.T.; Macia, L.; Galvão, I.; Martins, F.S.; Canesso, M.C.; Amaral, F.A.; Garcia, C.C.; Maslowski, K.M.; De Leon, E.; Shim, D.; et al. A Role for Gut Microbiota and the Metabolite-Sensing Receptor GPR43 in a Murine Model of Gout. Arthritis Rheumatol. 2015, 67, 1646–1656. [Google Scholar] [CrossRef]
- Li, X.; Wan, A.; Liu, Y.; Li, M.; Zhu, Z.; Luo, C.; Tao, J. P2X7R Mediates the Synergistic Effect of ATP and MSU Crystals to Induce Acute Gouty Arthritis. Oxidative Med. Cell. Longev. 2023, 2023, 1–12. [Google Scholar] [CrossRef]
- Scanu, A.; Oliviero, F.; Gruaz, L.; Galozzi, P.; Luisetto, R.; Ramonda, R.; Burger, D.; Punzi, L. Synovial fluid proteins are required for the induction of interleukin-1β production by monosodium urate crystals. Scand. J. Rheumatol. 2016, 45, 384–393. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Dayer, J.M.; Fiocco, U.; Sfriso, P.; Punzi, L. Response to ‘Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, 405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.K. The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout. J. Rheum. Dis. 2022, 29, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Tatsiy, O.; Mayer, T.Z.; de Carvalho Oliveira, V.; Sylvain-Prévost, S.; Isabel, M.; Dubois, C.M.; McDonald, P.P. Cytokine Production and NET Formation by Monosodium Urate-Activated Human Neutrophils Involves Early and Late Events, and Requires Upstream TAK1 and Syk. Front. Immunol. 2020, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wei, K.; Jiang, P.; Chang, C.; Xu, L.; Xu, L.; Shi, Y.; Guo, S.; Xue, Y.; He, D. Inflammatory Response to Regulated Cell Death in Gout and Its Functional Implications. Front. Immunol. 2022, 6, 888306. [Google Scholar] [CrossRef]
- Corr, E.M.; Cunningham, C.C.; Helbert, L.; McCarthy, G.M.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res. Ther. 2017, 19, 23. [Google Scholar] [CrossRef]
- Campillo-Gimenez, L.; Renaudin, F.; Jalabert, M.; Gras, P.; Gosset, M.; Rey, C.; Sarda, S.; Collet, C.; Cohen-Solal, M.; Combes, C.; et al. Inflammatory Potential of Four Different Phases of Calcium Pyrophosphate Relies on NF-κB Activation and MAPK Pathways. Front. Immunol. 2018, 9, 2248. [Google Scholar] [CrossRef]
- Chang, C.C.; Tsai, Y.H.; Liu, Y.; Lin, S.Y.; Liang, Y.C. Calcium-containing crystals enhance receptor activator of nuclear factor κB ligand/macrophage colony-stimulating factor–mediated osteoclastogenesis via extracellular-signal-regulated kinase and p38 pathways. Rheumatology 2015, 54, 1913–1922. [Google Scholar] [CrossRef]
- Ea, H.K.; Uzan, B.; Rey, C.; Lioté, F. Octacalcium phosphate crystals directly stimulate expression of inducible nitric oxide synthase through p38 and JNK mitogen-activated protein kinases in articular chondrocytes. Arthritis Res. Ther. 2005, 7, R915. [Google Scholar] [CrossRef]
- McCarthy, G.M.; Augustine, J.A.; Baldwin, A.S.; Christopherson, P.A.; Cheung, H.S.; Westfall, P.R.; Scheinman, R.I. Molecular mechanism of basic calcium phosphate crystal-induced activation of human fibroblasts. Role of nuclear factor kappab, activator protein 1, and protein kinase C. J. Biol. Chem. 1998, 273, 35161–35169. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Corr, E.M.; McCarthy, G.M.; Dunne, A. Intra-articular basic calcium phosphate and monosodium urate crystals inhibit anti-osteoclastogenic cytokine signalling. Osteoarthr. Cartil. 2016, 24, 2141–2152. [Google Scholar] [CrossRef]
- Nguyen, C.; Lieberherr, M.; Bordat, C.; Velard, F.; Côme, D.; Lioté, F.; Ea, H.K. Intracellular calcium oscillations in articular chondrocytes induced by basic calcium phosphate crystals lead to cartilage degradation. Osteoarthr. Cartil. 2012, 20, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Ea, H.K.; Chobaz, V.; Nguyen, C.; Nasi, S.; van Lent, P.; Daudon, M.; Dessombz, A.; Bazin, D.; McCarthy, G.; Jolles-Haeberli, B.; et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PLoS ONE. 2013, 8, e57352. [Google Scholar] [CrossRef]
- Scanu, A.; Lorenzin, M.; Luisetto, R.; Galozzi, P.; Ortolan, A.; Oliviero, F.; Doria, A.; Ramonda, R. Identification in synovial fluid of a new potential pathogenic player in arthropathies. Exp. Biol. Med. 2022, 247, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J.; Sun, Y.; Pearlman, E.; Ginley, N.M.; Awadallah, A.; Wisler, B.A.; Dennis, J.E. Monosodium Urate and Tumor Necrosis Factor-α Increase Apoptosis in Human Chondrocyte Cultures. Rheumatology 2012, 2, 113. [Google Scholar] [CrossRef]
- Ea, H.K.; Monceau, V.; Camors, E.; Cohen-Solal, M.; Charlemagne, D.; Lioté, F. Annexin 5 overexpression increased articular chondrocyte apoptosis induced by basic calcium phosphate crystals. Ann. Rheum. Dis. 2008, 67, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Tudan, C.; Jackson, J.K.; Blanis, L.; Pelech, S.L.; Burt, H.M. Inhibition of TNF-α-Induced Neutrophil Apoptosis by Crystals of Calcium Pyrophosphate Dihydrate Is Mediated by the Extracellular Signal-Regulated Kinase and Phosphatidylinositol 3-Kinase/Akt Pathways Up-Stream of Caspase 3. J. Immunol. 2000, 165, 5798–5806. [Google Scholar] [CrossRef]
- Zhong, C.S.; Zeng, B.; Qiu, J.H.; Xu, L.H.; Zhong, M.Y.; Huang, Y.T.; Xu, R.; Liu, S.Y.; Zha, Q.B.; Hu, B.; et al. Gout-associated monosodium urate crystal-induced necrosis is independent of NLRP3 activity but can be suppressed by combined inhibitors for multiple signaling pathways. Acta Pharmacol. Sin. 2022, 43, 1324–1336. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Guo, Y.; Li, M.; Yang, K.; Liu, Y.; Ge, D.; Liu, Y.; Xue, C.; Xia, T.; et al. Monosodium Urate Crystal-Induced Pyroptotic Cell Death in Neutrophil and Macrophage Facilitates the Pathological Progress of Gout. Small 2024, 20, e2308749. [Google Scholar] [CrossRef]
- Xu, X.; Qiu, H. BRD4 promotes gouty arthritis through MDM2-mediated PPARγ degradation and pyroptosis. Mol. Med. 2024, 30, 67. [Google Scholar] [CrossRef]
- Hwang, H.; Yang, C.; Park, S.; Kim, H. Monosodium Urate Crystal-Induced Chondrocyte Death via Autophagic Process. Int. J. Mol. Sci. 2015, 16, 29265–29277. [Google Scholar] [CrossRef]
- Schorn, C.; Janko, C.; Krenn, V.; Zhao, Y.; Munoz, L.E.; Schett, G.; Herrmann, M. Bonding the foe—NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front. Immunol. 2012, 3, 376. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, S.M.; Grebe, K.; Whitehead, L.W.; Rogers, K.L.; Nebl, T.; Murphy, J.M.; Wicks, I.P. Monosodium Urate Crystals Generate Nuclease-Resistant Neutrophil Extracellular Traps via a Distinct Molecular Pathway. J. Immunol. 2018, 200, 1802–1816. [Google Scholar] [CrossRef]
- Pang, L.; Hayes, C.P.; Buac, K.; Yoo, D.; Rada, B. Pseudogout-Associated Inflammatory Calcium Pyrophosphate Dihydrate Microcrystals Induce Formation of Neutrophil Extracellular Traps. J. Immunol. 2013, 190, 6488–6500. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, E.; Gamberucci, A.; Lucherini, O.M.; Alì, A.; Simpatico, A.; Lorenzini, S.; Lazzerini, P.E.; Tripodi, S.; Frediani, B.; Selvi, E. Neutrophil extracellular traps release in gout and pseudogout depends on the number of crystals regardless of leukocyte count. Rheumatology 2021, 60, 4920–4928. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhang, S.; Liao, J.; Qiu, X.; Zhang, Z.; Wang, Z.; Geng, H.; Zhang, J.; Jia, E. Mechanism of macrophages in gout: Recent progress and perspective. Heliyon 2024, 10, e38288. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Abhishek, A.; Doherty, M. Update on calcium pyrophosphate deposition. Clin. Exp. Rheumatol. 2016, 34, 32–38. [Google Scholar] [PubMed]
- Dimmick, S.; Hayter, C.; Linklater, J. Acute calcific periarthritis—A commonly misdiagnosed pathology. Skelet. Radiol. 2022, 51, 1553–1561. [Google Scholar] [CrossRef]
- Reber, L.L.; Marichal, T.; Sokolove, J.; Starkl, P.; Gaudenzio, N.; Iwakura, Y.; Karasuyama, H.; Schwartz, L.B.; Robinson, W.H.; Tsai, M.; et al. Contribution of mast cell-derived interleukin-1β to uric acid crystal-induced acute arthritis in mice. Arthritis Rheumatol. 2014, 66, 2881–2891. [Google Scholar] [CrossRef]
- Oliviero, F.; Galozzi, P.; Scanu, A.; Galuppini, F.; Lazzarin, V.; Brocco, S.; Ravagnan, G.; Sfriso, P.; Ramonda, R.; Spinella, P.; et al. Polydatin Prevents Calcium Pyrophosphate Crystal-Induced Arthritis in Mice. Nutrients 2021, 13, 929. [Google Scholar] [CrossRef]
- Luisetto, R.; Scanu, A. The translational value of calcium pyrophosphate deposition disease experimental mouse models. Front. Med. 2024, 11, 1417318. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Scanu, A. How Factors Involved in the Resolution of Crystal-Induced Inflammation Target IL-1β. Front. Pharmacol. 2017, 8, 164. [Google Scholar] [CrossRef][Green Version]
- Terkeltaub, R.; Martin, J.; Curtiss, L.K.; Ginsberg, M.H. Apolipoprotein B mediates the capacity of low density lipoprotein to suppress neutrophil stimulation by particulates. J. Biol. Chem. 1986, 261, 15662–15667. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.A.; Dyer, C.A.; Martin, J.; Curtiss, L.K. Apolipoprotein (apo) E inhibits the capacity of monosodium urate crystals to stimulate neutrophils. Characterization of intraarticular apo E and demonstration of apo E binding to urate crystals in vivo. J. Clin. Investig. 1991, 87, 20–26. [Google Scholar] [CrossRef]
- Burt, H.M.; Jackson, J.K.; Rowell, J. Calcium pyrophosphate and monosodium urate crystal interactions with neutrophils: Effect of crystal size and lipoprotein binding to crystals. J. Rheumatol. 1989, 16, 809–817. [Google Scholar]
- Kumagai, Y.; Watanabe, W.; Kobayashi, A.; Sato, K.; Onuma, S.; Sakamoto, H. Inhibitory Effect of Low Density Lipoprotein on the Inflammation-Inducing Activity of Calcium Pyrophosphate Dihydrate Crystals. J. Rheumatol. 2001, 28, 2674–2680. [Google Scholar]
- Scanu, A.; Luisetto, R.; Oliviero, F.; Gruaz, L.; Sfriso, P.; Burger, D.; Punzi, L. High-density lipoproteins inhibit urate crystal-induced inflammation in mice. Ann. Rheum. Dis. 2015, 74, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Landis, R.C.; Yagnik, D.R.; Florey, O.; Philippidis, P.; Emons, V.; Mason, J.C.; Haskard, D.O. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum. 2002, 46, 3026–3033. [Google Scholar] [CrossRef]
- Galozzi, P.; Maschio, L.; Carraro, S.; Scanu, A.; Facco, M.; Oliviero, F. M2 macrophages as resolvers of crystal-induced inflammation. Rheumatology 2021, 60, 2480–2483. [Google Scholar] [CrossRef]
- Cumpelik, A.; Ankli, B.; Zecher, D.; Schifferli, J.A. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann. Rheum. Dis. 2016, 75, 1236–1245. [Google Scholar] [CrossRef]
- Scanu, A.; Oliviero, F.; Ramonda, R.; Frallonardo, P.; Dayer, J.M.; Punzi, L. Cytokine levels in human synovial fluid during the different stages of acute gout: Role of transforming growth factor β1 in the resolution phase. Ann. Rheum. Dis. 2012, 71, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Hsieh, S.C.; Chen, W.Y.; Li, K.J.; Wu, C.H.; Wu, P.C.; Tsai, C.Y.; Yu, C.L. Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGFβ1, IL-10 and soluble TNF receptors and the intracellular cytokine negative regulators CIS and SOCS3. Ann. Rheum. Dis. 2011, 70, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, T.; Namai, R.; Murakami, Y.; Watanabe, M.; Matsui, T.; Nishimura, A.; Kitasato, H.; Kameya, T.; Kondo, H. Rapid induction of peroxisome proliferator-activated receptor gamma expression in human monocytes by monosodium urate monohydrate crystals. Arthritis Rheum. 2003, 48, 231–239. [Google Scholar] [CrossRef]
- Valiate, B.V.S.; Queiroz-Junior, C.M.; Levi-Schaffer, F.; Galvão, I.; Teixeira, M.M. CD300a contributes to the resolution of articular inflammation triggered by MSU crystals by controlling neutrophil apoptosis. Immunology 2021, 164, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Dang, W.; Chen, B.; Qing, Y.; Xie, W.; Zhao, M.; Zhou, J. IL-37 inhibits the production of pro-inflammatory cytokines in MSU crystal-induced inflammatory response. Clin. Rheumatol. 2016, 35, 2251–2258. [Google Scholar] [CrossRef]
- Speed, C.A.; Hazleman, B.L. Calcific Tendinitis of the Shoulder. N. Engl. J. Med. 1999, 340, 1582–1584. [Google Scholar] [CrossRef]
- Epis, O.; Viola, E.; Bruschi, E.; Benazzo, F.; Montecucco, C. Milwaukee shoulder syndrome (apatite associated destructive arthritis): Therapeutic aspects. Reumatismo 2005, 57, 69–77. [Google Scholar] [CrossRef]
- Frallonardo, P.; Ramonda, R.; Peruzzo, L.; Scanu, A.; Galozzi, P.; Tauro, L.; Punzi, L.; Oliviero, F. Basic calcium phosphate and pyrophosphate crystals in early and late osteoarthritis: Relationship with clinical indices and inflammation. Clin. Rheumatol. 2018, 37, 2847–2853. [Google Scholar] [CrossRef]
- Ramonda, R.; Oliviero, F.; Galozzi, P.; Frallonardo, P.; Lorenzin, M.; Ortolan, A.; Scanu, A.; Punzi, L. Molecular mechanisms of pain in crystal-induced arthritis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 98–110. [Google Scholar] [CrossRef]
- Hoffmeister, C.; Silva, M.A.; Rossato, M.F.; Trevisan, G.; Oliveira, S.M.; Guerra, G.P.; Silva, C.R.; Ferreira, J. Participation of the TRPV1 receptor in the development of acute gout attacks. Rheumatology 2014, 53, 240–249. [Google Scholar] [CrossRef]
- Hoffmeister, C.; Trevisan, G.; Rossato, M.F.; de Oliveira, S.M.; Gomez, M.V.; Ferreira, J. Role of TRPV1 in nociception and edema induced by monosodium urate crystals in rats. Pain 2011, 152, 1777–1788. [Google Scholar] [CrossRef]
- Trevisan, G.; Hoffmeister, C.; Rossato, M.F.; Oliveira, S.M.; Silva, M.A.; Ineu, R.P.; Guerra, G.P.; Materazzi, S.; Fusi, C.; Nassini, R.; et al. Transient receptor potential ankyrin 1 receptor stimulation by hydrogen peroxide is critical to trigger pain during monosodium urate-induced inflammation in rodents. Arthritis Rheum. 2013, 65, 2984–2995. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, L.J.; Hämäläinen, M.; Lehtimäki, L.; Nieminen, R.M.; Moilanen, E. Urate Crystal Induced Inflammation and Joint Pain Are Reduced in Transient Receptor Potential Ankyrin 1 Deficient Mice—Potential Role for Transient Receptor Potential Ankyrin 1 in Gout. PLoS ONE 2015, 10, e0117770. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, L.; Feng, J.; Xie, Z.; Liu, Z.; Wang, F.; Liu, P.; Yue, X.; Du, L.; Zhao, Y.; et al. Mechanosensitive TRPV4 is required for crystal-induced inflammation. Ann. Rheum. Dis. 2021, 80, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhou, X.; Yu, Q.; Li, R.; Dai, Q.; Zeng, M. ML335 inhibits TWIK2 channel-mediated potassium efflux and attenuates mitochondrial damage in MSU crystal-induced inflammation. J. Transl. Med. 2024, 22, 785. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Park, K.Y.; Choe, J.Y. Toll-Like Receptor 9 Is Involved in NLRP3 Inflammasome Activation and IL-1β Production Through Monosodium Urate-Induced Mitochondrial DNA. Inflammation 2020, 43, 2301–2311. [Google Scholar] [CrossRef]
- Renaudin, F.; Orliaguet, L.; Castelli, F.; Fenaille, F.; Prignon, A.; Alzaid, F.; Combes, C.; Delvaux, A.; Adimy, Y.; Cohen-Solal, M.; et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Ann. Rheum. Dis. 2020, 79, 1506–1514. [Google Scholar] [CrossRef]
- Major, T.J.; Takei, R.; Matsuo, H.; Leask, M.P.; Sumpter, N.A.; Topless, R.K.; Shirai, Y.; Wang, W.; Cadzow, M.J.; Phipps-Green, A.J.; et al. A genome-wide association analysis reveals new pathogenic pathways in gout. Nat. Genet. 2024, 56, 2392–2406. [Google Scholar] [CrossRef]
- Leask, M.P.; Crișan, T.O.; Ji, A.; Matsuo, H.; Köttgen, A.; Merriman, T.R. The pathogenesis of gout: Molecular insights from genetic, epigenomic and transcriptomic studies. Nat. Rev. Rheumatol. 2024, 20, 510–523. [Google Scholar] [CrossRef]
- Pei, D.D.; Sun, J.L.; Zhu, C.H.; Tian, F.C.; Jiao, K.; Anderson, M.R.; Yiu, C.; Huang, C.; Jin, C.X.; Bergeron, B.E.; et al. Contribution of Mitophagy to Cell-Mediated Mineralization: Revisiting a 50-Year-Old Conundrum. Adv. Sci. 2018, 5, 1800873. [Google Scholar] [CrossRef]
- Hashimoto, S.; Ochs, R.L.; Rosen, F.; Quach, J.; McCabe, G.; Solan, J.; Seegmiller, J.E.; Terkeltaub, R.; Lotz, M. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc. Natl. Acad. Sci. USA 1998, 95, 3094–3099. [Google Scholar] [CrossRef] [PubMed]
- Licandro, G.; Ling Khor, H.; Beretta, O.; Lai, J.; Derks, H.; Laudisi, F.; Conforti-Andreoni, C.; Liang Qian, H.; Teng, G.G.; Ricciardi-Castagnoli, P.; et al. The NLRP3 inflammasome affects DNA damage responses after oxidative and genotoxic stress in dendritic cells. Eur. J. Immunol. 2013, 43, 2126–2137. [Google Scholar] [CrossRef]
- Deo, P.; Dhillon, V.S.; Lim, W.M.; Jaunay, E.L.; Donnellan, L.; Peake, B.; McCullough, C.; Fenech, M. Advanced glycation end-products accelerate telomere attrition and increase pro-inflammatory mediators in human WIL2-NS cells. Mutagenesis 2020, 35, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Vazirpanah, N.; Kienhorst, L.B.E.; Van Lochem, E.; Wichers, C.; Rossato, M.; Shiels, P.G.; Dalbeth, N.; Stamp, L.K.; Merriman, T.R.; Janssen, M.; et al. Patients with gout have short telomeres compared with healthy participants: Association of telomere length with flare frequency and cardiovascular disease in gout. Ann. Rheum. Dis. 2017, 76, 1313–1319. [Google Scholar] [CrossRef]
- Baggio, C.; Luisetto, R.; Boscaro, C.; Scanu, A.; Ramonda, R.; Albiero, M.; Sfriso, P.; Oliviero, F. Leucocyte Abnormalities in Synovial Fluid of Degenerative and Inflammatory Arthropathies. Int. J. Mol. Sci. 2023, 24, 5450. [Google Scholar] [CrossRef] [PubMed]
- Vazirpanah, N.; Radstake, T.; Broen, J. Inflamm-ageing and senescence in gout: The tale of an old king’s disease. Curr. Aging Sci. 2015, 8, 186–201. [Google Scholar] [CrossRef]
- Wen, S.; Arakawa, H.; Tamai, I. CD38 activation by monosodium urate crystals contributes to inflammatory responses in human and murine macrophages. Biochem. Biophys. Res. Commun. 2021, 581, 6–11. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, J.; Xie, S.; He, J.; Huang, S.; Chen, J.; Jiang, S.; Yu, L.; Zhou, Y.; Cao, X.; et al. Systematic analysis of inflammation and pain pathways in a mouse model of gout. Mol. Pain 2022, 18, 17448069221097760. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Stücker, S.; Bollmann, M.; Garbers, C.; Bertrand, J. The role of calcium crystals and their effect on osteoarthritis pathogenesis. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101722. [Google Scholar] [CrossRef]
- Karampasa, I.A.; Kontoyannisa, C.G. Characterization of calcium phosphates mixtures. Vib. Spectrosc. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, G.; Rey, C.; Bres, E. MicroRaman Spectral Study of the PO4 and CO3 Vibrational Modes in Synthetic and Biological Apatites. Calcif. Tissue Int. 1998, 63, 475–481. [Google Scholar] [CrossRef]
- Chen, K.-O.; Lià, M.J.; Chengà, W.T.; Balic-Zunic, T.; Lin, S.Y. Identification of monoclinic calcium pyrophosphate dihydrate and hydroxyapatite in human sclera using Raman microspectroscopy. Int. J. Exp. Path. 2009, 90, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Sauer, G.R.; Zunic, W.B.; Durig, J.R.; Wuthier, R.E. Fourier Transform Raman Spectroscopy of Synthetic and Biological Calcium Phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef]
- Xu, J.; Gilson, D.F.R.; Butler, I.S.; Stangel, I. Effect of high external pressures on the vibrational sDectra of biomedical materials: Calcium hydroxyapatite and calcium fluoroapatite. J. Biomed. Mater. Res. 1996, 30, 239–244. [Google Scholar] [CrossRef]
- Chen, K.H.; Cheng, W.H.; Li, M.J.; Yang, D.M.; Lin, S.Y. Calcification of senile cataractous lens determined by Fourier transform infrared (FTIR) and Raman microspectroscopies. J. Microsc. 2005, 219, 36–41. [Google Scholar] [CrossRef]
- Gordon, C.; Swan, A.; Dieppe, P. Detection of Crystals in Synovial Fluids by Light Microscopy: Sensitivity and Reliability. Ann. Rheum. Dis. 1989, 48, 737–742. [Google Scholar] [CrossRef]
- Paul, H.; Reginato, A.J.; Ralph Schumacher, H. Alizarin Red s Staining as a Screening Test to Detect Calcium Compounds in Synovial Fluid. Arthritis Rheum. 1983, 26, 191–200. [Google Scholar] [CrossRef]
- Li, B.; Yang, S.; Akkus, O. A Customized Raman System for Point-of-Care Detection of Arthropathic Crystals in the Synovial Fluid. Analyst 2014, 139, 823–830. [Google Scholar] [CrossRef]
- Miura, K.; Fukuda, H.; Mineta, H.; Yamaguchi, K.; Harada, H.; Yusa, H.; Tsutsui, Y. Phosphoglyceride crystal deposition disease. Pathol. Int. 2000, 50, 992–998. [Google Scholar] [CrossRef]
- Cheng, X.; Haggins, D.G.; York, R.H.; Yeni, Y.N.; Akkus, O. Analysis of Crystals Leading to Joint Arthropathies by Raman Spectroscopy: Comparison with Compensated Polarized Imaging. Appl. Spectrosc. 2009, 63, 381–386. [Google Scholar] [CrossRef] [PubMed]

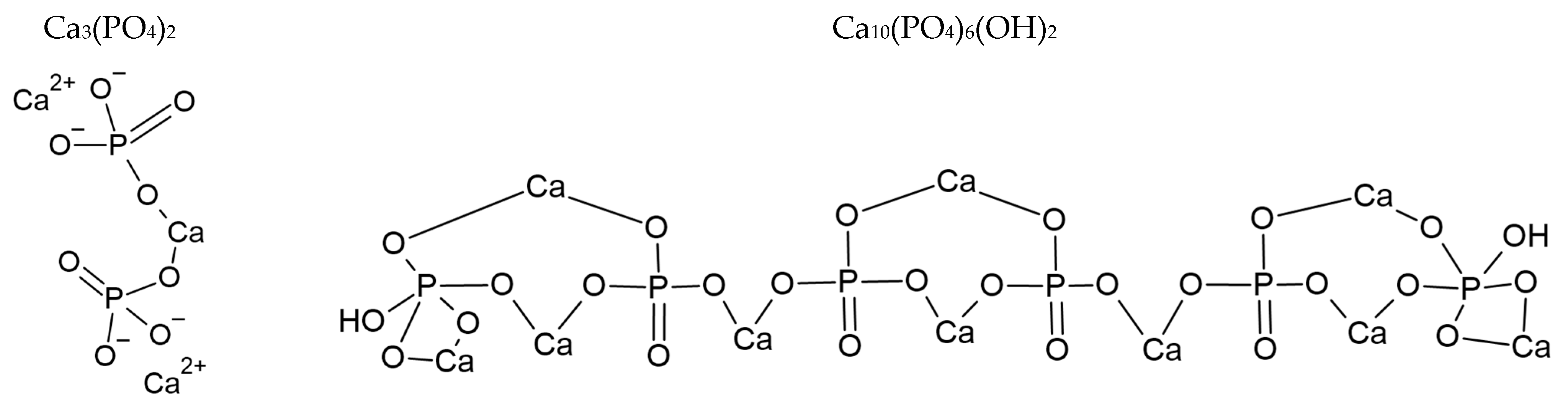
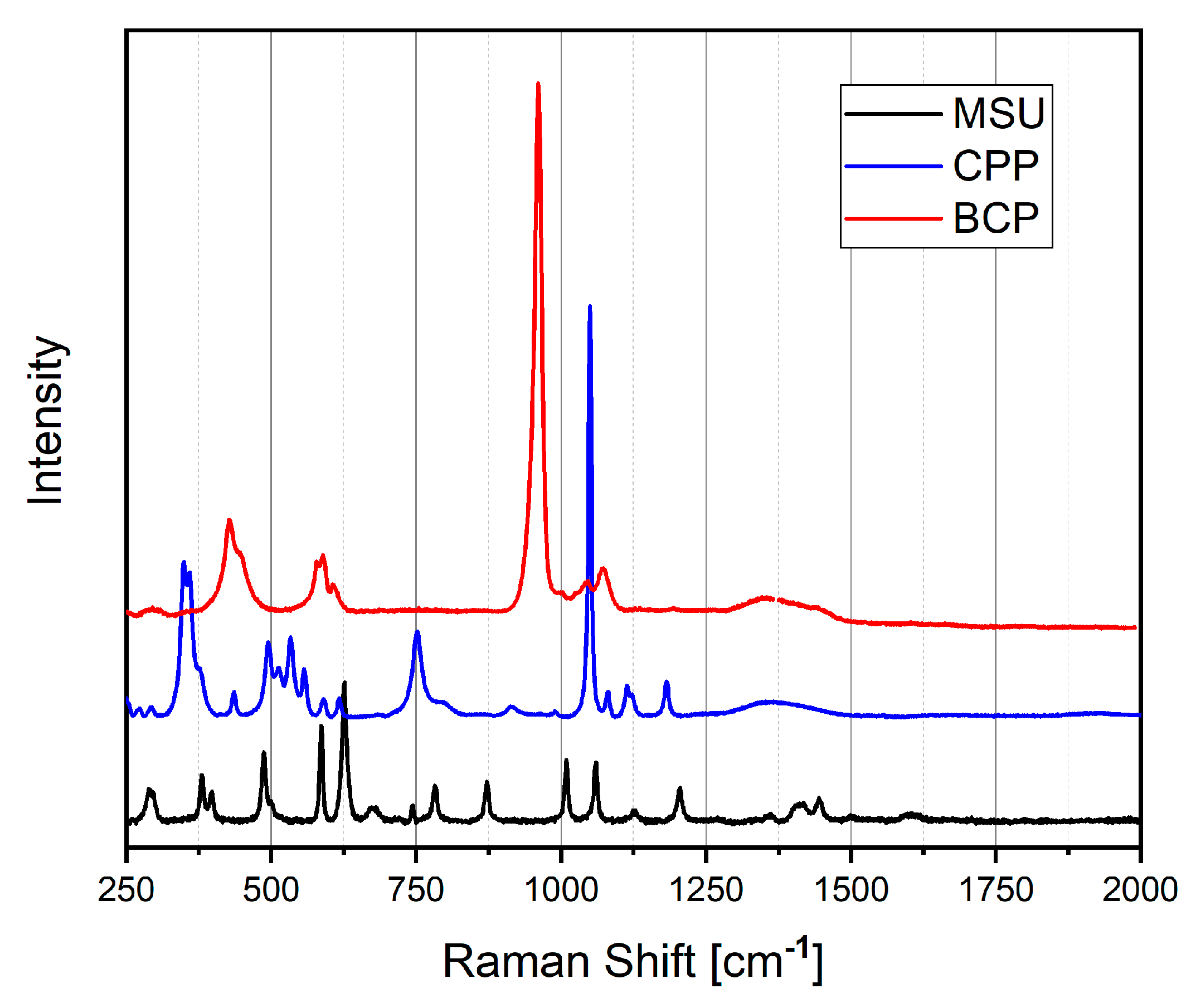

| Crystal | Size Dimension [μm] | Morphology |
|---|---|---|
| MSU | 2–10 | Needle, rods |
| CPP | 2–10 | Rhomboids, rods |
| BCP HA, OCP, TCP | <1 | Round, irregular clumps |
| MSU Crystals | CPP Crystals | BCP Crystals | |||
|---|---|---|---|---|---|
| Crystal formation | Supersaturation of monosodium urate in SF leads to MSU crystal formation | The excess PPi, formed by ENPP1/PC-1, binds Ca2+ leading to CPP precursors formation in ACV in the ECM | The TNAP enzyme hydrolyzes ePPi to ePi leading to BCP formation in ACV in the ECM | ||
| Cells involved in crystal-induced inflammation | FLSs, chondrocytes, PMNs, monocytes, lymphocytes and DCs | FLSs, chondrocytes, PMNs, monocytes and lymphocytes | FLSs, chondrocytes, PMNs, monocytes and lymphocytes | ||
| Crystal-induced macrophages polarization | M1-pro-inflammatory phenotype | No data available | M1-pro-inflammatory phenotype | ||
| Mechanisms of interaction between cells and crystals | Receptor binding (TLR4, TLR2, CD16 and CD11b), direct interaction or internalized through phagocytosis | Receptor binding (TLR4 and TLR2), direct interaction or internalized through phagocytosis | Receptor binding (TLR4) or internalized through phagocytosis | ||
| Activation of the NLRP3 inflammasome | NLRP3 activation and IL-1β production | NLRP3 activation and IL-1β production | NLRP3 activation and IL-1β production | ||
| Cytokines and chemokines released after NLRP3 inflammasome activation | IL-1β, IL-18, CXCL8/IL-8, IL-6, TNF-α, IL-1Ra, TGF-β1, CCL2 (MCP-1), CXCL12, CCL3 and CXCL2 | IL-1β, IL-18, IL-6, TNF-α, CXCL8/IL-8, IL-1Ra, TGF-β1 and CCL2 (MCP-1) | IL-1β, IL-18, CXCL8/IL-8, IL-6, TNF-α, TGF-β1, IL-1Ra and CCL2 (MCP-1) | ||
| Priming signals | In vitro | LPS, PMA, SFi and SFi proteins (>50 kDa, such as fibrinogen), C5a, GM-CSF, soluble uric acid and ATP | LPS, PMA and SFi proteins (>50 kDa, such as fibrinogen) | LPS, PMA, CpG and PAM3 | |
| In vivo | S100A8/A9, C18 FFAs and spikes in systemic levels of acetate | No studies available | No studies available | ||
| Mechanisms following Inflammasome activation | MyD88, IRAK and TRAF6 recruitment, NF-κB/MAPKs expression | MyD88, IRAK and TRAF6 recruitment, NF-κB /MAPKs expression | MyD88, IRAK and TRAF6 recruitment, NF-κB /MAPKs expression | ||
| Alternative mechanisms activated by crystals | Ca2+ and ATP influx, p38 MAPK, ERK1/2, PI3K/Akt pathway, NF-κB, CREB, C/EBP, ROS production, Syk/PI3K, increased iCa2+ levels induced by PL3/PI3K | NF-κB, Syk, p38/JNK MAPKs, ERK1/2, Src, increased iCa2+ levels induced by PL3/PI3K | NF-κB, Syk, p38/JNK MAPKs, iCa2+ oscillations directly induced by BCP | ||
| Programmed cell deaths | Apoptosis, Necrosis (necroptosis and pyroptosis), NETosis | NETosis | Apoptosis | ||
| Duration of the acute inflammatory response | In patients | Few days | 1–3 weeks | Rapid onset of symptoms lasting 4–7 days, resolution within 3–4 weeks | |
| In vivo experimental models | Peak after 24 h and completely resolution within 5 days | Peak after 48 h and persistence after 6 days | No studies available | ||
| Mechanisms involved in the spontaneous resolution | Apolipoproteins, (LDL and HDL), aggNETs, M2-macrophage polarization, PMN-Ecto, high levels of TGF-β1, IL-1Ra, IL-10, sTNFR-I/II, IL-37, activation of PPAR γ and CD300a receptor | Apolipoproteins (LDL and HDL) and M2 macrophage polarization | No studies available | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Gout, Hyperuricemia and Crystal Associated Disease Network. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangari, M.; Luisetto, R.; Pilot, R.; Contessa, P.; Signorini, R.; Masiero, S.; Scanu, A. Comparison of Pathophysiological Mechanisms Among Crystal-Induced Arthropathies. Gout Urate Cryst. Depos. Dis. 2025, 3, 7. https://doi.org/10.3390/gucdd3020007
Zangari M, Luisetto R, Pilot R, Contessa P, Signorini R, Masiero S, Scanu A. Comparison of Pathophysiological Mechanisms Among Crystal-Induced Arthropathies. Gout, Urate, and Crystal Deposition Disease. 2025; 3(2):7. https://doi.org/10.3390/gucdd3020007
Chicago/Turabian StyleZangari, Maddalena, Roberto Luisetto, Roberto Pilot, Paola Contessa, Raffaella Signorini, Stefano Masiero, and Anna Scanu. 2025. "Comparison of Pathophysiological Mechanisms Among Crystal-Induced Arthropathies" Gout, Urate, and Crystal Deposition Disease 3, no. 2: 7. https://doi.org/10.3390/gucdd3020007
APA StyleZangari, M., Luisetto, R., Pilot, R., Contessa, P., Signorini, R., Masiero, S., & Scanu, A. (2025). Comparison of Pathophysiological Mechanisms Among Crystal-Induced Arthropathies. Gout, Urate, and Crystal Deposition Disease, 3(2), 7. https://doi.org/10.3390/gucdd3020007









