Abstract
Monosodium urate, calcium pyrophosphate, and basic calcium phosphate crystals are the most common types of crystals found in the joints. Each type of crystal has been associated with the onset of different joint diseases. However, the mechanisms identified for one type of crystal are often generalized to the others; thus, overlooking the specific and distinct molecular and cellular responses activated by each type of crystal. This review describes the similarities and differences of the main molecules and mechanisms underlying the diseases associated with the three different types of crystals. Specifically, current knowledge on crystal properties and formation, on the induction and resolution of inflammation, on mechanisms involved in pain processing and senescence, and on the role of mitochondria and genomic instability are elucidated. A more complete and detailed study of the specific molecular mechanisms induced by different crystals is necessary to advance our understanding of the pathogenesis and to help identify innovative opportunities for prevention and treatment of crystal deposition disease.
1. Introduction
Crystal-induced arthropathies are a group of diseases characterized by inflammatory processes triggered by the deposition of crystals in the joints. The most common types of crystals found in articular and periarticular tissues are monosodium urate (MSU) crystals, which cause gout; calcium pyrophosphate (CPP) crystals, which are associated with calcium pyrophosphate deposition disease (CPPD); and basic calcium phosphate (BCP) crystals, which induce degenerative/destructive arthropathies or acute calcific periarthritis and have been associated with severe forms of osteoarthritis (OA). Typically, crystal-induced arthropathies can have similar clinical manifestations. For instance, inflammation induced by the three types of crystals may manifest as an acute episode that resolves spontaneously. However, the pathogenetic mechanisms leading to different types of these arthropathies may be different, and the research literature is still lacking in this regard. In this context, NLRP3 inflammasome activation is widely studied, with research focusing mainly on the MSU crystals. On the contrary, the inflammatory pathways induced by BCP crystals are poorly understood. An important issue in the study of these diseases is the tendency to generalize the mechanisms identified for one type of crystal to all others. However, this approach overlooks the fact that different types of crystals may induce specific and distinct molecular and cellular responses.
The aim of this review is to shed light on the similarities and differences in the pathogenetic mechanisms underlying arthropathies induced by different types of crystals. This will also help identify knowledge gaps where further research is currently needed.
2. Physical–Chemical Properties and Detection Methods of Crystals
Detecting the presence of MSU, CPP, and BCP crystals is of great importance for the earlier treatment of arthropathies.
The chemical structure of MSU and CPP is well defined and characterized by triclinic unit cells (Figure 1) [1,2,3].

Figure 1.
Chemical structures of MSU (left) and CPP (right).
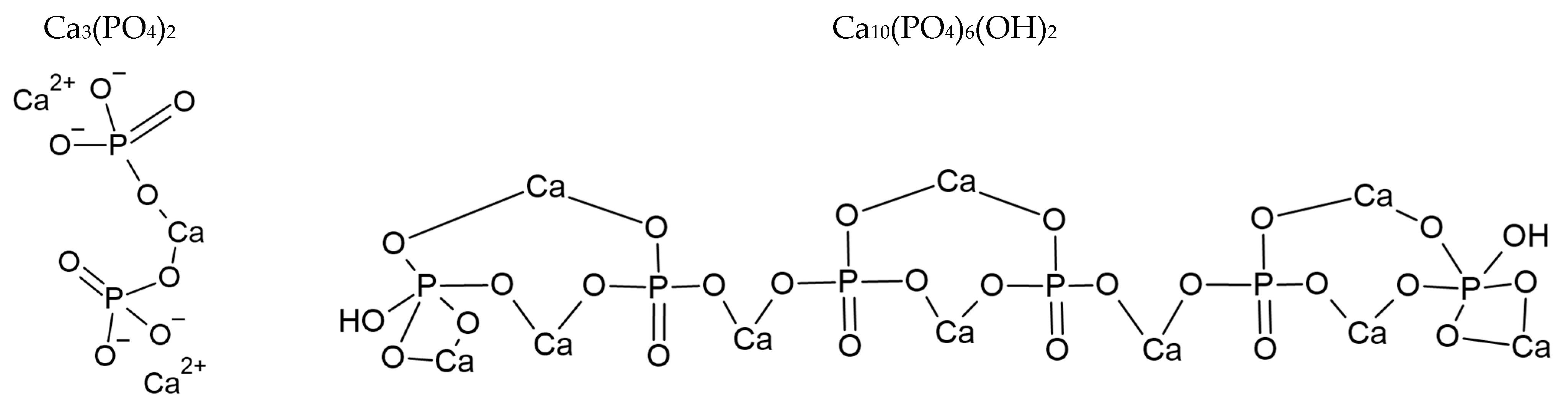
As explained by Rosenthal et al. [4] and Yavorskii et al. [5], BCP consists of different calcium phosphate crystals of hydroxyapatite (HA), octacalcium phosphate (OCP—Ca8(HPO4)2(PO4)4·5H2O), and tricalcium phosphate (TCP), or hydroxyapatite partially substituted with carbonates, as described in ref. [6,7,8]. The chemical structure of TCP and HA are given in Figure 2. The general structure of apatite crystals consists of Ca2+, PO43− and X- in hexagonal space [9]; when carbonate ions are present, they replace phosphate groups [10,11,12]. TCP presents two different phases: the low temperature β-TCP and the high temperature α-TCP phase [13].
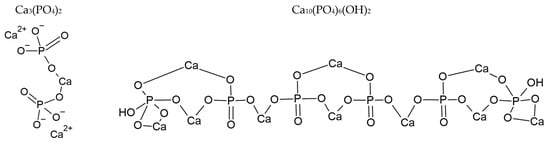
Figure 2.
Chemical structures of TCP (left) and HA (right).
Detection of crystals in the joints of patients is particularly challenging. This is due to several factors: the crystals size, the complex composition of the surrounding matrix, and the potential presence of different crystals in cases of comorbidity. Currently, the standard method for identifying joint crystals is mainly based on microscopic analysis of synovial fluid collected after arthrocentesis. Compensated polarized light microscopy (CPLM) has historically been the most widely used method for detecting MSU and CPP crystals and remains the gold standard for gout and CPPD diagnosis [14,15]. However, although this technique is sensitive and specific, it has some limitations, as it lacks the necessary resolution to identify very small crystals and is inherently subjective if not performed by properly trained expert observers. Newer techniques like Raman spectroscopy, dual energy computed tomography, and ultrasound are emerging as potential diagnostic tools, including for point-of-care applications. A comprehensive overview of these methods, is reported in the reviews by Zell et al. [16] and by Yavorskyy et al. [5], here a short resume of the differences in detecting the three types of crystals using these techniques.
2.1. Compensated Polarized Light Microscopy (CPLM)
Birefringent crystals, depending on their optical properties, interact differently with polarized light. This interaction results in the transmission of different portions of the light spectrum through the microscope, which is observed as variations in color. These color changes, along with crystal morphology, allow for crystal identification. MSU crystals exhibit strong negative birefringence and are needle-shaped, while CPP crystals show weak positive birefringence and are rod-rhomboidal shaped. BCP crystals are non-birefringent and sub-micron sized, making CPLM unsuitable for their identification (Table 1).

Table 1.
Size crystals deposited in soft tissues.
2.2. Microscopy with Staining
Alizarin red S forms a red-colored chelation complex with calcium. This makes this dye useful for detecting calcium crystals. However, it doesn’t distinguish between different types of calcium crystals, so their shape must be examined for proper identification.
2.3. Ultrasounds (US)
MSU and CPP crystals reflect ultrasound differently than surrounding soft tissues, allowing for their visualization. While some ultrasound features suggest the presence of MSU crystals, these findings are not always specific and can occur in CPPD as well. Thus, definitively differentiating between MSU and CPP crystals using only ultrasound is not straightforward [17]. In this context, dynamic assessment of the double contour (DC) sign by US helps to distinguish between gout and CPPD. Indeed, on dynamic scanning, the DC sign in the presence of CPP crystals (pseudo-DC) appeared mobile, whereas in the DC sign in gout, the MSU crystals appeared to be fixed to the cartilage [18]. Although ultrasonography is routinely used in the diagnosis and management of calcific tendonitis/periarthritis, its validation and reliability in HA deposition disease has not yet been established. The generation of acoustic shadows seems to allow for discrimination between the three types of crystals. Indeed, both HA and MSU crystals attenuated the US beam and generated acoustic shadowing, depending on the concentration and size of the aggregates. In contrast, CPP crystals generally do not attenuate the US beam [19].
2.4. Dual Energy Computed Tomography (DECT)
DECT is a variation of the CT technique that utilizes two distinct X-ray energies. A phantom study evidenced that DECT is able to differentiate MSU crystals from both calcium crystals (HA and CPP), considering DECT ratios [20]. Furthermore, an in vivo study showed that DECT is able also to distinguish CPP crystal deposits from BCP crystals. BCP calcifications have a higher dual-energy index (DEI) and effective anatomic number (Zeff) than CPP crystals, with comparable CT numbers in patients [21].
2.5. Raman Spectroscopy
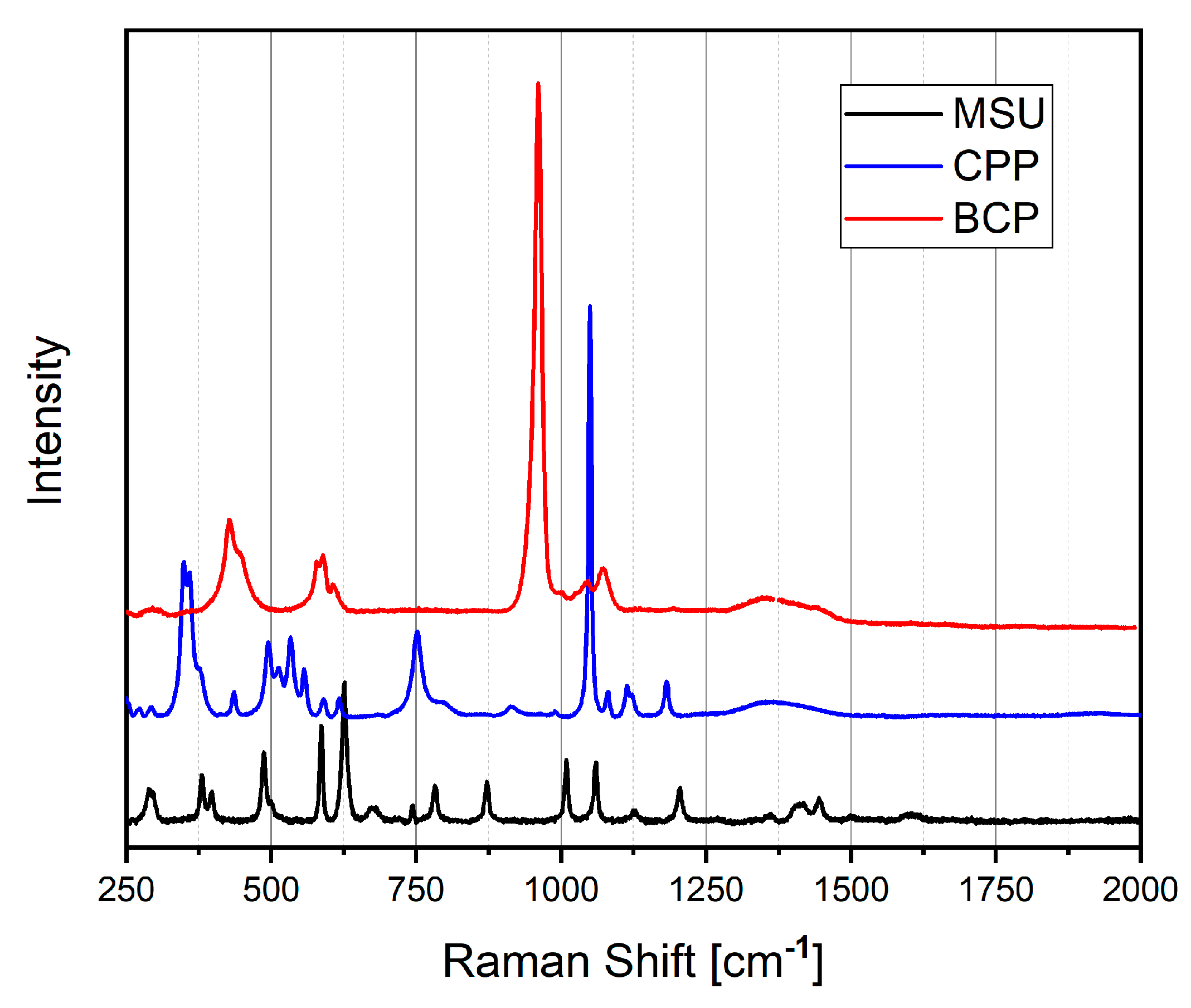
Raman spectroscopy offers significant advantages in this field. Using the microscope configuration, it can examine samples at the micrometer level. The Raman spectrum constitutes the fingerprint of the crystals and allows different species to be distinguished.
Figure 3 reports the Raman spectra of artificial MSU, CPP and BCP crystals prepared in our laboratory [22]. The Raman spectra were recorded with a home-built micro-Raman system, based on a Triax-320 spectrograph (ISA Instruments, Jobin Yvon, Roma, Italy), equipped with a holographic 1200 g/mm grating and a CCD detector (Horiba Scientific Symphony II FIOE, Roma, Italy). The excitation source was a solid-state laser operating at 785 nm. A BX 40 optical microscope (Olympus, Milan, Italy) equipped with a 50× objective was optically coupled to the spectrograph. The Raman spectra were recorded between 200 and 4000 cm−1.
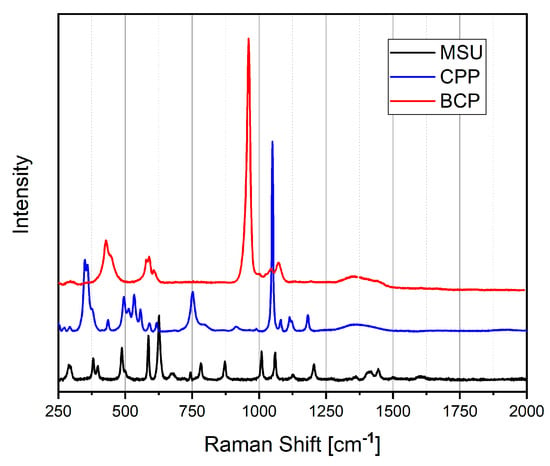
Figure 3.
Raman spectra of MSU (black line), CPP (blue line) and BCP (red line), collected by exciting at 785 nm.
The Raman spectrum of MSU shows the main Raman peaks from 386 to 1600 cm−1 [23]. All the Raman shifts are reported in Table S1, where the assignment is also outlined.
The unique Raman spectrum of each calcium phosphate phase is characterized by specific peak positions and quantities. The distinctive spectral fingerprint allows for definitive identification of the present phase.
Raman spectra of calcium phosphates are dominated by strong stretching (νs) of HPO42− and PO43− groups, whose bands appear in the 900–1000 cm−1 spectral range, and phosphate bending in the 400–600 cm−1 range.
The Raman peaks below 1200 cm−1 are due to P–O vibrations, whereas the peaks at about 3500 cm−1 are due to O–H vibrations.
The fundamental frequencies of a free PO43− ion in solution are found at 938 (ν1, corresponding to P-O symmetric stretching), 420 (ν2, corresponding to O-P-O bending), 1017 (ν3, corresponding to P-O asymmetric stretching) and 567 (ν4, corresponding to O-P-O bending) cm−1 [24]. The Raman shift of HA, TCP, OCP and CPP are reported in Table S2. The frequencies of the higher intensity signals are also highlighted in all the tables.
The comparison of Raman spectra shows that it is easy to identify and distinguish the presence of the various crystals by observing the region around 1000 cm−1.
3. Crystal Formation
Although high serum urate levels are a necessary prerequisite in the development of gout, the exact conditions that lead to MSU crystal formation in the joints remain unknown. Urate supersaturation in synovial fluid (SF) is the most important factor for MSU crystal formation [25,26]. However, studies on tiny tissue fragments recovered from gouty SFs suggest that these may be the primary site of crystal formation by templated nucleation [27]. Different factors have been found to influence MSU crystal formation, such as temperature, pH, sodium and cation concentrations, cartilage composition, mechanical stress, and SF components [25,28,29].
In contrast, CPP and BCP crystals first form in the articular cartilage vesicles (ACV) present in the extracellular matrix (ECM), and are later released into the synovial space by shedding [30,31,32]. ACVs contain nucleotide pyrophosphohydrolase (NTPPH) enzymes, such as NTPPH enzyme plasma-cell membrane glycoprotein 1 (ENPP1/PC-1), involved in the production of extracellular pyrophosphate (ePPi) by hydrolyzing ATP that is transported by ANK [33,34]. The excess PPi binds Ca2+ to form amorphous CPP precursors. Tissue-nonspecific alkaline phosphatase (TNAP) can antagonize ENPP1 by hydrolyzing ePPi to extracellular phosphate (ePi), thus favoring BCP crystal formation [35]. The Pi/PPI ratio in ACV also seems determinant for the type of crystal formed [36]. Crystal formation and growth are regulated by numerous factors such as cytokines, osteopontin, transglutaminase, different types of collagen, proteoglycans, pH, temperature, and concentrations of magnesium, citrate, and albumin. Both CPP and BCP crystals grow within or along collagen fibrils [37].
The main mechanisms that lead to the formation of crystals are reported in Table 2.

Table 2.
Summary of the most important mechanisms involved in crystal formation and crystal-induced inflammation.
4. Induction of Inflammation
MSU, CPP, and BCP crystals have been identified as danger signals that trigger inflammation through a variety of mechanisms and cell types, leading to different levels and times in the course of the inflammation. A schematic overview of the main mechanisms activated during inflammation is represented in Figure 4 for MSU crystals, in Figure 5 for CPP crystals and in Figure 6 for BCP crystals, and given in Table 2.
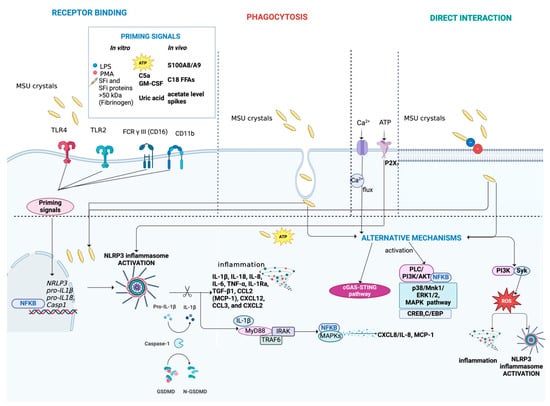
Figure 4.
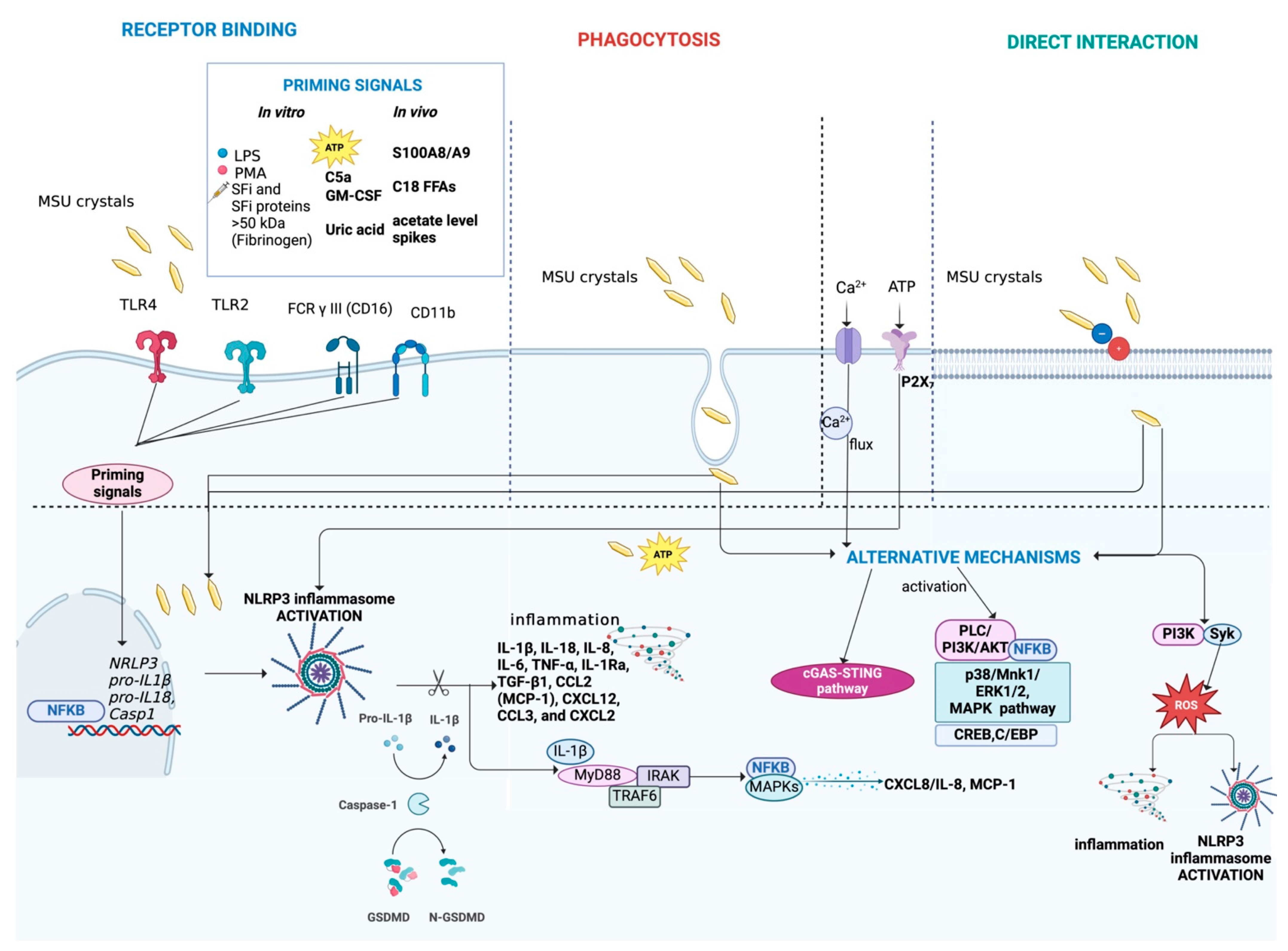
Main mechanisms activated during MSU crystal-induced inflammation. MSU crystals can interact with cells through binding to receptors, such as TLR4, TLR2, FCR γ III (CD16), and CD11b or direct interaction with the cell membrane. Alternatively, MSU crystals can be internalized by cells through phagocytosis. The complete activation of NLRP3 inflammasome induced by MSU crystals requires a second trigger, acting as a priming signal. Various endogenous molecules have been identified as priming signals, including inflammatory synovial fluid (SFi), SFi proteins > 50 kDa (e.g., fibrinogen), ATP, C5a, GM-CSF, soluble uric acid, S100A8/A9, C18 free fatty acids (FFAs), and acetate level spikes. In vitro, priming can be induced using LPS or PMA. The activation of the NLRP3 inflammasome promotes the release of pro-inflammatory cytokines and chemokines, such as IL-1β and IL-18, perpetuating low-grade inflammation. Moreover, it leads to the expression of MAPKs/NF-κB transcription factors. Additionally, MSU crystals, along with ion influx (Ca2+) and ATP, can activate alternative pathways, including the cGAS-STING pathway, MAPK signaling, ERK1/2, p38 and PI3K/PLC/Akt pathways, NF-κB signaling, and ROS production. Figure created with BioRender.com.
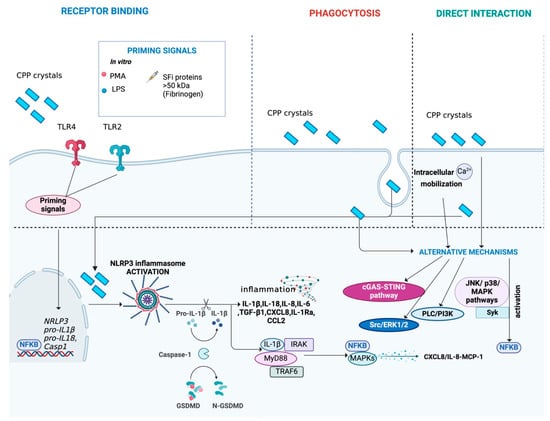
Figure 5.
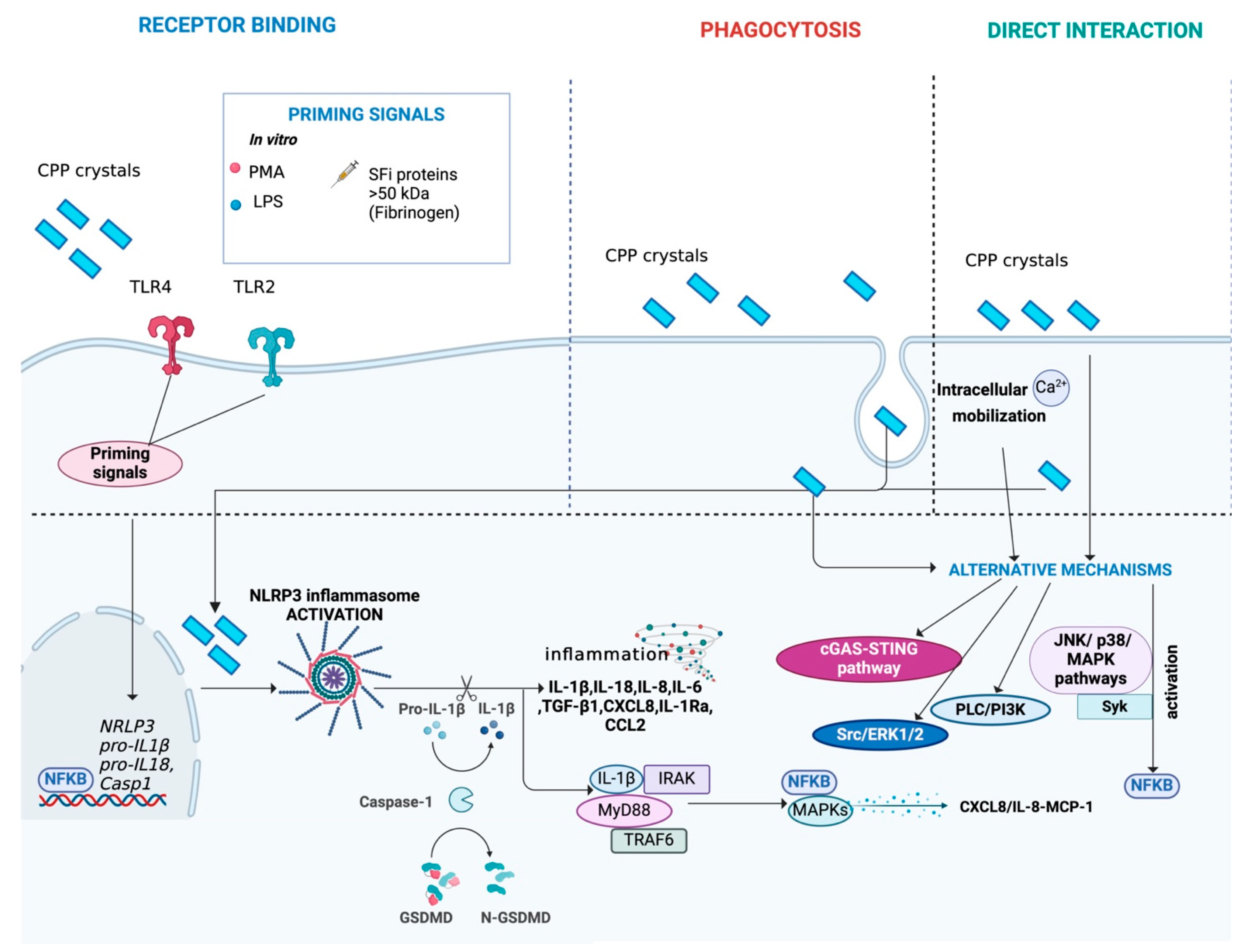
Main mechanisms activated during CPP crystal-induced inflammation. CPP crystals can interact with cells through binding to receptors, such as TLR4 and TLR2, or direct interaction with the cell membrane. Alternatively, CPP crystals can be internalized by cells through phagocytosis. The complete activation of NLRP3 inflammasome induced by CPP crystals requires a second trigger, acting as a priming signal. SFi proteins > 50 kDa (e.g., fibrinogen) have been identified as a possible CPP co-stimulus. In vitro, priming can be induced using LPS or PMA. The activation of the NLRP3 inflammasome triggers the release of pro-inflammatory cytokines and chemokines, such as IL-1β and IL-18, perpetuating low-grade inflammation. Moreover, it leads to the expression of MAPKs/NF-κB transcription factors. Additionally, CPP crystals can activate alternative pathways, including the cGAS-STING pathway, MAPK signaling (JNK and p38 pathways), PLC/PI3K, NF-κB, ERK1/2, Src and Syk signaling, facilitated by interactions with intracellular calcium. Figure created with BioRender.com.
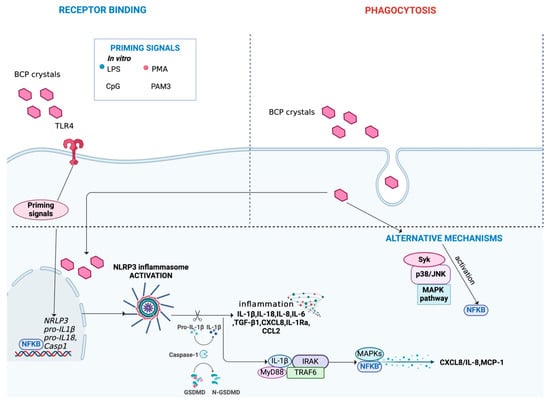
Figure 6.
Main mechanisms activated during BCP crystal-induced inflammation. BCP crystals can interact with cells through binding to receptors, such as TLR4. Alternatively, BCP crystals can be internalized by cells through phagocytosis. The complete activation of NLRP3 inflammasome induced by BCP crystals requires a second trigger, acting as a priming signal. In vitro, priming can be induced using LPS, PMA, CpG and PAM3. The activation of the NLRP3 inflammasome triggers the release of pro-inflammatory cytokines and chemokines, such as IL-1β and IL-18, perpetuating low-grade inflammation. Moreover, it leads to the expression of MAPKs/NF-κB transcription factors. Additionally, BCP crystals can activate alternative pathways, including the MAPK signaling, (JNK and p38 pathways), and Syk signaling promoting NF-κB signaling activation. Figure created with BioRender.com.
4.1. Cells Mainly Involved in Crystal-Induced Inflammatory Processes
In joints, crystals can interact with resident cells, such as chondrocytes and fibroblast-like synoviocytes (FLSs), as well as with inflammatory cells, including polymorphonuclear cells (PMNs), dendritic cells (DCs), monocytes, macrophages, and lymphocytes that are recruited during articular inflammation.
For example, in vitro studies with FLSs isolated from synovial membrane explants demonstrated that both MSU and CPP crystals induce the production of CCL2 and IL-8, while only MSU has been tested for the promotion of oxidative stress [38,39,40]. In addition, the interaction between FLSs and BCP crystals resulted in increased production of IL-6, CXCL8, and prostaglandin E2 (PGE2), and altered expression of metalloproteinases (MMPs), such as MMP-1 and MMP-3 [41]. In the same study, similar effects were observed after stimulation of human OA articular chondrocytes with BCP crystals [41]. Furthermore, BCP crystals induce hypertrophic differentiation of chondrocytes by activating the canonical Wnt signaling pathway [42,43]. Both CPP and MSU crystals activate NF-κB in chondrocytes, promoting nitric oxide (NO) production [44]. Furthermore, in articular chondrocytes, MSU crystals increased COX-2 and inducible NO synthase (iNOS) gene expression and PGE2 and IL-8 release [45,46,47].
Upon activation by MSU crystals, neutrophils release a variety of inflammatory mediators, including cytokines, degradative enzymes, and oxygen radicals, which perpetuate the inflammatory reaction and cause damage to the surrounding tissues [48]. In a previous study from our group, human PMNs released IL-8 in response to MSU, CPP, and BCP crystals in a dose- and time-dependent manner. Our experiments also demonstrated that monocytes are the main type of leukocyte involved in cytokine production after stimulation by the crystals, and that different concentrations and times are required to reach the maximum response depending on the type of crystal [22]. Both MSU and BCP crystals were found to polarize macrophages toward pro-inflammatory M1-like phenotype [49,50]. Data on macrophage polarization induced by CPP crystals are not available.
To the best of our knowledge, only one study has evaluated the effects of crystal stimulation on lymphocytes. Even though no detectable levels of cytokines were reported in crystal-stimulated lymphocytes, an indirect regulation of their function in joint tissues cannot be excluded [22]. Although the role of lymphocytes in crystal-induced arthropathies is considered marginal, further research on this cell type may be valuable to gain a more complete understanding of the mechanisms underlying these diseases.
4.2. Mechanisms of Interaction Between Cells and Crystals
The molecular mechanisms underlying the interaction between crystals and cells have not yet been fully understood. A number of reports suggest that MSU crystals can directly interact with biological phospholipid membranes thanks to their negatively charged surface and their strong surface reactivity [25,51]. However, further studies are required to fully understand the electrostatic interactions and the specific types of bonds that are formed between crystals and cells. An in vivo study using a murine model and DCs suggested that MSU crystals bind directly to cell membrane lipids of DCs, triggering lipid sorting and rearrangement that leads to SYK kinase activation and subsequent phagocytosis [52]. CPP and BCP crystals could potentially present a comparable mechanism of interaction with cells, but research on these types of crystals remains limited.
Previous studies also hypothesized an interaction mediated by the binding of crystals to receptors in the cell membranes. Among these receptors, TLR4 seems to bind to all three types of crystals [53,54,55,56,57], while TLR2 interacts with MSU and CPP [44]. Furthermore, it has been suggested that MSU crystals bind directly or indirectly to CD11b and CD16 [58,59].
Endocytosis, and in particular phagocytosis, is the primary mechanism by which crystals are internalized into cells. In vitro studies indicate that all three types of crystals are phagocytosed by different cell types, including FLSs, PMNs and monocytes/macrophages [56,60,61,62,63,64]. Evaluation of the phagocytosis index of MSU and CPP crystals in THP-1 cell cultures highlighted higher levels of phagocytosis of CPP crystals compared to MSU crystals in vitro, suggesting that this parameter may vary over time depending on the type of crystal [63]. Due to the difficult identification of BCP crystals, their phagocytosis has so far only been demonstrated indirectly. Conducting research in this field can be challenging [56,61,64].
4.3. Activation of the NLRP3 Inflammasome
After entering cells, MSU, CPP and BCP crystals can activate the NLRP3 inflammasome and promote IL-1β production. Although activation of the NLRP3 inflammasome has been extensively studied and is currently considered to play a major role in the pathogenesis of crystal-induced arthropathies, the precise mechanisms remain unclear [56,65,66]. It has been shown that a second trigger, which acts as a priming signal, is necessary for the complete activation of the inflammasome and the induction of pro-IL-1β expression. Several priming signals have been identified by both in vitro and in vivo studies. As regard MSU crystals, in vitro studies demonstrated that the complement protein C5a, the granulocyte-macrophage colony-stimulating factor (GM-CSF), and soluble uric acid can act as co-stimuli. Instead, in vivo models identified as activating factors for the same crystals the heterodimer S100A8/A9, long chain (C18) free fatty acids (FFAs) and spikes in systemic levels of acetate [67,68,69,70,71]. Furthermore, a synergic effect of ATP and MSU crystals mediated by P2X7R on inflammasome activation was found by both in vivo and in vitro experiments [72]. Interestingly, co-stimulation of THP-1 cells or human primary monocytes with MSU crystals and inflammatory synovial fluid (SFi), or with SFi proteins, particularly those with molecular weight (MW) > 50 kDa such as fibrinogen, was observed to induce IL-1β production [73]. Similar results were obtained when THP-1 cells were co-stimulated with SFi and CPP crystals instead of MSU (personal observation), and the potential cooperating role of fibrinogen was confirmed in an in vitro study with THP-1 cells pre-primed with a low dose of phorbol myristate acetate (PMA) [74].
BCP crystal-induced IL-1β secretion through activation of the NLRP3 inflammasome also requires a priming signal, although no endogenous substance has yet been identified. In this context, Pazar et al. demonstrated that all three forms of BCP crystals, OCP, carbonate substituted (CA), and HA, can stimulate the release of active IL-1β from preactivated monocytes and macrophages, and that OCP crystals are more inflammatory than either HA or CA [56].
Although priming has been shown to be required for NLRP3 inflammasome activation in crystal-induced inflammation, and several primers have been identified for MSU crystals, their effect has not yet been evaluated for CPP and BCP crystals. Furthermore, the molecules identified as trigger signals have been characterized only in vitro or in vivo experimental models, without any direct confirmation from clinical studies.
4.4. IL-1β Production After Inflammasome Activation
Time course experiments with cultured monocytes isolated from blood samples of healthy volunteers revealed that different doses are required for IL-1β production, depending on the type of crystals. Specifically, at optimal concentrations of MSU, CPP or BCP crystals, the highest levels of IL-1β were observed after 18–24 h of stimulation, but cells stimulated with BCP required higher doses of crystals to achieve maximal responses compared to MSU and CPP. On the contrary, CPP crystals required the lowest concentration to achieve the same level of IL-1β at the same time point [22]. In contrast, in another in vitro study with pre-primed THP-1 or human monocytes/macrophages, BCP crystals, and in particular OCP crystals, induced higher levels of IL-1β release than MSU crystals after 6 h of stimulation, even when starting with equivalent doses [56].
4.5. Mechanisms Following Inflammasome Activation
The elicitation of IL-1β leads to an inflammatory response mediated by IL-1R activation. Indeed, IL-1 binds to the receptor complex, comprising IL-1RI and IL-1RAcP, forming the IL-1/IL-R complex. This interaction recruits adaptor proteins such as MyD88, IRAK, and TRAF6, triggering downstream signaling pathways. These pathways activate key inflammatory transcription factors, including NF-κB and MAPKs, which promote the production of chemokines such as CXCL8, IL-8, and MCP-1, along with other pro-inflammatory cytokines. These mediators amplify the IL-1-driven inflammatory cascade, facilitating neutrophil recruitment to the joints. Despite significant advances, the mechanisms underlying the IL-1 inflammatory cascade are not yet completely understood [75]. Specifically, further studies are required to elucidate the downstream pathways activated by MSU, CPP, and BCP crystals.
4.6. Alternative Mechanisms Activated by Crystals
Several studies have highlighted that crystals can activate alternative pathways in addition to the NLRP3 inflammasome. For example, neutrophils isolated from the peripheral blood of a healthy donor and cultured in vitro showed that stimulation with MSU crystals induced rapid activation of the PI3K/Akt, p38 MAPK, and ERK pathways, as well as belatedly the transcription factors NF-κB, C/EBP, and CREB, which result in the generation of different cytokine and neutrophil extracellular traps (NETs) [76]. Furthermore, as previously reported, an in vitro study using DCs demonstrated that MSU crystals interact with the plasma membrane by forming electrostatic bonds and by aggregating with cholesterol within the lipid raft, which triggers activation of the Syk/PI3K signaling pathway [52]. Syk signaling activation can lead to reactive oxygen species (ROS) production and promote the release of cytokines and chemokines that drive inflammation, potentially in conjunction with NLRP3 inflammasome activation [77]. Activation of NF-κB through the p38 MAPK pathway, Syk, and c-Jun N-terminal kinase (JNK) are also promoted by both CPP and BCP crystals [78,79,80,81]. CPP crystals can also activate the ERK1/2 and Src pathways, while conflicting results have been reported for PI3K activity during BCP crystal-induced cell stimulation [78,82,83]. It has not yet been clarified whether some transcription factors involved in the mechanisms triggered by MSU crystals, such as C/EBP and CREB, are similarly involved in the cellular processes activated by the other two types of crystals.
However, while MSU and CPP increased the level of intracellular calcium (iCa2+) through the PLC/PI3K pathway, and their effects are modulated by the protein coat, BCP crystals can directly induce oscillations in iCa2+, which in turn are involved in cellular activation and catabolic effects [84].
An in vitro study using FLSs demonstrated that MSU crystals directly induce the release of CCL2 contained in small vesicles within the cytosol independently of the IL-1 loop and inflammasome activation. Furthermore, MSU crystal stimulation triggers the replenishment of CCL2 storage within granules [38]. IL-1β and NLRP3 inflammasome-independent synovial inflammation was also observed for BCP crystals after intra-articular injection in specific knock out mice [85].
Recently, the involvement of the stimulator of interferon genes (STING), an important signaling molecule in the innate immune response to cytosolic nucleic acids, has been investigated in crystal-induced arthritis, revealing interesting findings. Indeed, high concentrations of intracellular STING were observed in SFis from patients with gout or CPP-induced arthritis and correlated with SF pro-inflammatory cytokine and cell infiltration levels, suggesting that it may play a role in the pathogenesis and progression of these arthropathies [86]. Further studies are needed to determine the effective role of STING in these diseases. Moreover, it may be interesting to evaluate the expression profile and activity of STING in the presence of BCP crystals.
Due to the complex nature of these signaling mechanisms and the lack of studies conducted with all crystal types, it is difficult to determine which pathways are preferentially activated by one crystal type over another.
4.7. Programmed Cell Deaths
Crystals induce programmed cell death through various mechanisms. Cell death patterns may play different roles in either promoting disease progression or facilitating disease resolution.
Most research has focused on the role of MSU crystals in preprogrammed cell death, whereas relatively fewer studies have investigated the effects of CPP and BCP crystals. In vitro studies using chondrocytes or FLSs suggested that MSU crystals may promote apoptosis through ROS generation and oxidative response [39,87]. BCP crystals also induce apoptosis in chondrocytes, but cell–crystal contact and intracellular crystal dissolution are required [88]. Conversely, CPP crystals were observed to reduce TNF-α-induced neutrophil apoptosis [89]. Furthermore, MSU crystals can induce programmed cell necrosis, including necroptosis and pyroptosis, in macrophages and neutrophils [90,91,92,93]. However, no studies have described necrosis induced by CPP and BCP crystals. Finally, MSU and CPP crystals were found to induce NETosis that resulted in the release of NETs typically composed of chromatin [94,95,96]. In vitro experiments demonstrated that increasing concentrations of MSU and CPP crystals induce a similar amount of NET release in a dose-dependent manner regardless of neutrophil numbers [97]. Although MSU crystal-induced NETs have been found to exacerbate inflammation by promoting activation and polarization of macrophages toward the proinflammatory M1 phenotype [98], it has also been suggested that NETs are involved in the resolution of neutrophilic inflammation [99]. Studies evaluating the NETosis induced by BCP should be carried out.
5. Resolution Phase
Crystal-induced arthritis is characterized by acute inflammatory episodes that usually resolve spontaneously, although there are marked differences in the length of inflammation depending on the type of crystal. For example, untreated gout flares caused by MSU crystals usually resolve within a few days, whereas acute CPP-induced arthritis episodes can persist for up to 1–3 weeks [100]. BCP crystals can also cause acute periarticular attacks in patients, with a rapid onset of symptoms lasting 4–7 days and resolving spontaneously within 3–4 weeks [56,101]. In vivo experimental models of inflammation induced by MSU crystals injected into the ankle of mice demonstrated that inflammation peaks after 24 h and completely resolves within 5 days [102]. In contrast, CPP-induced inflammation reaches its maximum intensity at 48 h and remains elevated even after 6 days [103,104]. Currently, there are no in vivo studies reporting the duration of the acute inflammatory response induced by BCP crystals.
A variety of mechanisms and factors are involved in the spontaneous resolution of inflammation [105]. Among them, a number of plasma proteins and lipoproteins identified in SF, such as apolipoproteins, LDL, and HDL, have been shown to inhibit the acute inflammatory attack induced by both MSU and CPP crystals [38,106,107,108,109,110]. No studies have been conducted to evaluate the effect of lipoproteins in BCP crystal-induced inflammation. The differentiation of infiltrated monocytes into macrophages, and in particular the switch to the anti-inflammatory M2 phenotype, could also play an important role in the clearance of apoptotic neutrophils and MSU and CPP crystals [111,112].
As mentioned above, NETs can participate in the resolution of inflammation. In particular, it was observed that aggNET, resulting from the aggregation of NETs, can facilitate the degradation of cytokines and chemokines, limit neutrophil recruitment and activation, and promote neutrophil polarization toward the anti-inflammatory N2 phenotype [99]. In addition to NETs, neutrophils release microvesicles (PMN-Ecto) that attenuate MSU-induced inflammation by inhibiting C5a-mediated priming of the inflammasome [113].
Even some cytokines display anti-inflammatory action in crystal-induced inflammation. In particular, high levels of TGFβ1 in SF from patients during the late stage of acute gout attacks suggest a key role for this cytokine in promoting auto-remission of inflammation [114]. In addition to TGFβ1, elevated concentrations of IL-1Ra, IL-10, and sTNFR-I/II were also detected in the SF of patients during an acute gout attack and were associated with spontaneous resolution [115].
Even though no data have been reported on the role of these cytokines in the spontaneous resolution of CPP or BCP crystal-induced inflammation, an in vitro study revealed that the three types of crystals induced a rapid production of pro-inflammatory cytokines, while a longer time was required to release high levels of IL-1Ra and TGFβ1 [22].
Other mechanisms are reported to be involved in the resolution phase only for MSU crystal-induced inflammation, but it could be interesting to investigate their possible contribution with the other two crystals. They include the induction of the peroxisome proliferator-activated receptor γ [116], activation of the CD300a receptor [117], and production of IL-37 [118].
The main mechanisms involved in spontaneous resolution for the three types of crystals are summarized in Table 2.
6. Crystals and Pain
Intense pain occurs in acute attacks of gout and CPPD, whereas it is usually absent in chronic gout and chondrocalcinosis. Some BCP-associated conditions, such as Milwaukee shoulder syndrome, can be characterized by severe pain, while others are mostly asymptomatic, such as calcific tendinitis [4,119,120]. The role of CPP or BCP crystals in OA pain is still debated. Their presence in the SF of patients with OA is however often associated with more intense pain [37,121].
Even though pain is one of the main clinical features of crystal-associated arthropathies, the exact molecular mechanisms underlying this symptom are not yet fully understood. It has been suggested that crystals do not induce nociception directly, but rather through the interaction between specific pain receptors and pro-nociceptive mediators released after crystal-induced activation of different cells. The most important of these molecules are cytokines (especially IL-1β), PGE2, bradykinin, and substance P [122].
The main receptors involved in nociception in MSU crystal-induced inflammation are TRP vanilloid 1 (TRPV1) and TRP ankyrin 1 (TRPA1). In vivo experiments have demonstrated that MSU crystals injected in the paw of rats increase the immunoreactivity of the TRPV1 channel and nociception, which was significantly reduced after treatment with selective TRPV1 receptor antagonists [123,124]. Furthermore, in a rodent model of acute gout, TRPA1 antagonism reduced the MSU-induced nociceptive response, and TRPA1 in sensory neurons was indirectly activated by H2O2 production [125]. TRPA1 involvement was confirmed in experiments using TRPA1 deficient mice, which demonstrated reduced pain after intra-articular injection of MSU crystals compared to wild-type animals [126]. The expression of both receptors is increased by MSU crystals [125]. Recently, Lan et al. demonstrated that TRPV4 expression is upregulated in human PBMCs from patients with acute gout flares and that TRPV4 is required for MSU crystal-induced reflexive pain-related responses in a mouse model of gout [127].
To our knowledge, no studies have investigated the involvement of pain receptors in CPP and BCP associated arthropathies.
7. Crystals and Mitochondria
Recent research has suggested that mitochondria may play a multifunctional role in the pathogenesis of crystal-induced arthropathies.
An in vitro study conducted on primary mouse bone marrow-derived macrophages (BMDMs) demonstrated that MSU induced a decrease in intracellular K+, which in turn caused mitochondrial membrane collapse and organelle fragmentation via SIRT3 ubiquitination. Loss of membrane integrity decreases mitochondrial antioxidant activity and promotes massive ROS release, activation of NRLP3, and strengthening of the inflammatory cycle [128]. Furthermore, MSU crystals can induce the release of mitochondrial DNA that can act as a DAMP signal leading to the activation of NLRP3 through TLR9 and exacerbating inflammation [129].
Crystals also have effects on mitochondrial bioenergetics. Indeed, crystal stimulation decreases both oxidative phosphorylation and tricarboxylic acid cycling by favoring glycolysis at the expense of cellular respiration, as demonstrated in macrophages from patients with gout and pseudogout and confirmed by in vivo studies [130]. These metabolic changes alter the presence of substrates such as α-ketoglutarate, succinate and stimulate glutaminolysis with a decrease in glutamate and glutamine. Notably α-ketoglutarate is produced in the mitochondrial matrix by isocitrate dehydrogenase whose IDH2 gene is associated with gout [131]. Changes in mitochondrial α-ketoglutarate significantly influence epigenetic regulation in immune cells by altering the expression levels of various DNA methylases, histone-modifying enzymes, and ten-eleven translocation (Tet) methylcytosine dioxygenase [132].
As regard to calcium crystals, mitochondria seem to play a role in crystal formation. In the calcification of the articular cartilage associated with OA, BCP crystals are formed by mitochondrial autophagy from hypertrophic chondrocytes. Mitochondria play a key role in maintaining Ca2+ and inorganic P homeostasis. Under inflammatory conditions, the influx of Ca2+ and inorganic P into the organelle increases by depolarizing the outer mitochondrial membrane. This depolarization activates PTEN induced putative kinase 1 (PINK1), which leads to the formation of the mitochondrial autolysosome rich in ACP (amorphous calcium phosphate precursor), a precursor to BCP crystals. After being secreted by exocytosis, it infiltrates the extracellular matrix of collagen and then transforms into crystals [37,133]. Chondrocyte mitochondria are involved in the production of BCP crystals through the intrinsic pathway of apoptosis. Indeed, in an inflammatory, hypoxia, or strong oxidative stress environment, a significant loss of mitochondrial transmembrane potential is generated. This change stimulates the inducers of apoptosis such as DIABLO, cytochrome C, and HTRA2 that activate caspases and leads to the formation of apoptotic bodies. Apoptotic bodies possess TNAP and ENPP1, which are essential for crystal formation [37,134].
8. Crystals and Genomic Instability
Although crystals are known to trigger a wide range of cellular events, including cytokine release, mitochondrial damage, and massive ROS production, which can lead to genomic DNA damage, the number of studies in this context is extremely limited.
In BMDCs and leukocytes derived from the MSU crystal-induced peritonitis mouse model, Licandro et al. demonstrated that NLRP3 supports ROS-mediated DNA oxidation with increased expression of several genes involved in double strand and base-excision DNA repair [135]. However, a genome-wide association study (GWAS) of 2.6 million participants showed 377 loci associated with gout but did not detect any evidence of an association between genes related to DNA replication or DNA sensing and repairing pathways [131].
Critical telomere shortening is recognized as DNA damage. Telomeres are cap-like structures composed of repetitive genomic DNA sequences that protect chromosome ends from DNA damage sensors and oxidative damage, and ensure proper replication. High levels of pro-inflammatory cytokines, such as IL-6 and TNF-α, appear to trigger accelerated telomere shortening by suppressing telomerase activity [136]. A study in New Zealand and Dutch patients with gout demonstrated that, in immune cells, shorter telomere length is associated with gout and significant telomere attrition is associated with the number of gouty flares with cardiovascular disease [137].
Finally, a higher percentage of cells with micronuclei was found in the SF of patients with crystal-induced arthritis compared to other arthropathies [138]. Micronuclei are small DNA-containing nuclear structures in nuclear proximity and are considered important biomarkers for mutagenic and genotoxic damage.
Although few observations have demonstrated the presence of genomic dysfunction in crystal-related diseases, the concomitant action of inflammatory processes, oxidative stress, and DNA damage indirectly demonstrates that these mechanisms may be involved in the pathogenesis of gout and other crystal-induced inflammatory arthritis.
9. Crystals and Senescence
Several studies have shown that persistent MSU crystal-induced inflammation contributes to both cellular senescence and inflammageing, a chronic low-grade inflammation that has been associated with age-related conditions.
Chronic exposure to MSU crystals induces a cellular phenotype, defined as SASP (senescence-associated secretory phenotype), characterized by massive secretion of pro-inflammatory cytokines, cell cycle blockage, ROS accumulation, significant genomic DNA damage, and telomere shortening [139].
Furthermore, in vitro stimulation of monocytes and macrophages with MSU crystals can disrupt cellular metabolism by promoting the degradation of coenzyme NAD and inhibition of nucleotide synthesis by overexpression of CD38. Research suggests that the decline in NAD+ levels is involved in several age-related conditions [140].
Using a genome-wide transcriptome analysis approach comparing mouse and human inflamed joints, it has been shown that acute inflammation induced by MSU crystals promotes alternative splicing variants that are associated with ageing [141]. Although the link between crystals and cellular senescence in cartilage is unclear, some studies show that CPP crystals are more strongly associated with senescence than BCP [37]. Furthermore, an increased expression of chondrocyte p16 and p21, e.g., cell cycle inhibitors that are considered biomarkers of cell senescence, was observed in patients with chondrocalcinosis than in those with OA and healthy controls [42,142,143].
Despite this evidence, more in-depth and systematic studies are needed to determine whether crystals promote senescence or are instead an effect of ageing.
10. Conclusions
Crystal-associated arthropathies are the result of a complex biological response that may depend on the type of crystal involved. A better understanding of the various inflammatory response pathways and molecular mechanisms induced by different crystals is necessary to improve our understanding of the pathogenesis, as well as contribute to identifying innovative opportunities for prevention and treatment of crystal deposition diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gucdd3020007/s1, Table S1: Raman peak position of MSU crystals; Table S2: Raman peak position of HA, TCP, OCP and CPP crystals. References [144,145,146,147,148,149,150,151,152,153,154] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, R.L., S.M. and A.S.; writing—original draft preparation, M.Z., R.L., R.P., R.S. and A.S.; writing—review and editing, P.C. and A.S.; supervision, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mandel, N.S.; Mandel, G.S. Monosodium Urate Monohydrate, the Gout Culprit. J. Am. Chem. Soc. 1976, 98, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.; Tang, W.; Gong, J. Understanding the Crystallization Pathway of Monosodium Urate Monohydrate in a Biomimetic Matrix. Cryst. Growth Des. 2020, 20, 804–812. [Google Scholar] [CrossRef]
- Mandel, N.S. The Crystal Structure of Calcium Pyrophosphate Dihydrate. Acta Crystallografica 1975, B31, 1730. [Google Scholar] [CrossRef]
- Rosenthal, A.K. Basic Calcium Phosphate Crystal-Associated Musculoskeletal Syndromes: An Update. Curr. Opin. Rheumatol. 2018, 30, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Yavorskyy, A.; Hernandez-Santana, A.; McCarthy, G.; McMahon, G. Detection of Calcium Phosphate Crystals in the Joint Fluid of Patients with Osteoarthritis—Analytical Approaches and Challenges. Analyst 2008, 133, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.S.; McCarthy, G.M. Hydroxyapatite Deposition Disease of the Joint. Curr. Rheumatol. Rep. 2003, 5, 215–221. [Google Scholar] [CrossRef]
- Tung, M.S. Calcium Phosphates: Structure, Composition, Solubility, and Stability. In Calcium Phosphates in Biological and Industrial Systems; Amjad, Z., Ed.; Springer: New York, NY, USA, 1998; pp. 1–19. [Google Scholar]
- Siddiqi, S.A.; Azhar, U. Carbonate Substituted Hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 149–173. [Google Scholar]
- Iqbal, Z.; Tomaselli, V.P.; Fahrenfeldand, O.; Miller, K.D.; Ruszalaand, F.A.; Kostiner, E. Polarized Raman scattering and low frequency infrared study of hydroxyapatite. Phys. Chem. Solids 1977, 38, 923–927. [Google Scholar] [CrossRef]
- Elliott, J.C. Calcium phosphate biominerals. Rev. Mineral. Geo-Chem. 2002, 48, 427–454. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Bleiwas, C.B.; Retino, M.; Rohanizadeh, R.; LeGeros, J.P. Zinc effect on the in vitro formation of calcium phosphates: Relevance to clinical inhibition of calculus formation. Am. J. Dent. 1999, 12, 65–71. [Google Scholar]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Jillavenkatesa, A.; Condrate, R.A., Sr. The Infrared and Raman Spectra of β-and α-Tricalcium Phosphate (Ca3(PO4)2). Spectrosc. Lett. 1998, 31, 1619–1634. [Google Scholar] [CrossRef]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castaneda, J.; Coyfish, M.; Guillo, S.; Jansen, T.; Janssens, H.; et al. 2018 updated European League Against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann. Rheum. Dis. 2020, 79, 31–38. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Bardin, T.; Barskova, V.; Guerne, P.A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: Terminology and diagnosis. Ann. Rheum. Dis. 2011, 70, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Zell, M.; Zhang, D.; Fitzgerald, J. Diagnostic Advances in Synovial Fluid Analysis and Radiographic Identification for Crystalline Arthritis. Curr. Opin. Rheumatol. 2019, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Pârvănescu, C.D.; Bărbulescu, A.L.; Bită, C.E.; Dinescu, S.C.; Tras, B.A.; Firulescu, S.C.; Vreju, F.A. Ultrasound Features in Gout: An Overview. Med. Sci. 2024, 12, 37. [Google Scholar] [CrossRef]
- Filippou, G.; Miguel-Pérez, M.; Coronel, L.; Sirotti, S.; Pacini, G.; Scanu, A.; Bong, D.; Möller, I.; EULAR Study Group on Anatomy for the Image. The ultrasonographic pseudo-double contour sign in calcium pyrophosphate deposition disease: An anatomic explanation and how to distinguish it from gout. Arthritis Rheumatol. 2023, 75, 639–640. [Google Scholar] [CrossRef] [PubMed]
- Filippou, G.; Pacini, G.; Sirotti, S.; Zadory, M.; Carboni, D.; Damiani, A.; Fiorentini, E.; Cipolletta, E.; Filippucci, E.; Froehlich, J.M.; et al. Comparison of ultrasound attenuation by calcium pyrophosphate, hydroxyapatite and monosodium urate crystals: A proof-of-concept study. Ann. Rheum. Dis. 2022, 81, 1199–1201. [Google Scholar] [CrossRef]
- Døssing, A.; Müller, F.C.; Becce, F.; Stamp, L.; Bliddal, H.; Boesen, M. Dual-Energy Computed Tomography for Detection and Characterization of Monosodium Urate, Calcium Pyrophosphate, and Hydroxyapatite: A Phantom Study on Diagnostic Performance. Investig. Radiol. 2021, 56, 417–424. [Google Scholar] [CrossRef]
- Pascart, T.; Falgayrac, G.; Norberciak, L.; Lalanne, C.; Legrand, J.; Houvenagel, E.; Ea, H.K.; Becce, F.; Budzik, J.F. Dual-energy computed-tomography-based discrimination between basic calcium phosphate and calcium pyrophosphate crystal deposition in vivo. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20936060. [Google Scholar] [CrossRef]
- Scanu, A.; Oliviero, F.; Luisetto, R.; Ramonda, R.; Doria, A.; Punzi, L.; Dayer, J.M. Effect of pathogenic crystals on the production of pro- and anti-inflammatory cytokines by different leukocyte populations. Immunobiology 2021, 226, 152042. [Google Scholar] [CrossRef]
- Allen, R.N.; Lipkowski, P.; Shukla, M.K.; Leszczynski, J. Vibrational analysis of complexes of urate with IA group metal cations (Li+, Na+ and K+). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 639–645. [Google Scholar] [CrossRef] [PubMed]
- de Aza, P.N.; Guitian, F.; Santos, C.; de Aza, S.; Cusco, R.; Artus, L. Vibrational Properties of Calcium Phosphate Compounds. 2. Comparison between Hydroxyapatite and b-Tricalcium Phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Punzi, L. Metabolism of crystals within the joint. Reumatismo 2011, 63, 221–229. [Google Scholar] [CrossRef]
- Pascual, E.; Addadi, L.; Andrés, M.; Sivera, F. Mechanisms of crystal formation in gout—A structural approach. Nat. Rev. Rheumatol. 2015, 11, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Chhana, A.; Lee, G.; Dalbeth, N. Factors influencing the crystallization of monosodium urate: A systematic literature review Pathophysiology of musculoskeletal disorders. BMC Musculoskelet. Disord. 2015, 16, 296. [Google Scholar] [CrossRef]
- Ahn, H.; Lee, G.; Lee, G.S. Lower temperatures exacerbate NLRP3 inflammasome activation by promoting monosodium urate crystallization, causing gout. Cells 2021, 10, 1919. [Google Scholar] [CrossRef]
- Rosenthal, A.K. Articular cartilage vesicles and calcium crystal deposition diseases. Curr. Opin. Rheumatol. 2016, 28, 127–132. [Google Scholar] [CrossRef]
- Williams, C.J.; Rosenthal, A.K. Pathogenesis of calcium pyrophosphate deposition disease. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101718. [Google Scholar] [CrossRef]
- Sirotti, S.; Scanu, A.; Pascart, T.; Niessink, T.; Maroni, P.; Lombardi, G.; Filippou, G. Calcium Pyrophosphate Crystal Formation and Deposition: Where Do we Stand and What Does the Future hold? Curr. Rheumatol. Rep. 2024, 26, 354–365. [Google Scholar] [CrossRef]
- Ryan, L.M.; Wortmann, R.L.; Karas, B.; Mccarty, D.J. Cartilage Nucleoside Triphosphate (NTP) Pyrophosphohydrolase.I. Identification as an ecto-enzyme. Arthritis Rheum. 1984, 27, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.M.; Wortmann, R.L.; Karas, B.; McCarty, D.J. Cartilage nucleoside triphosphate pyrophosphohydrolase. II. Role in extracellular pyrophosphate generation and nucleotide metabolism. Arthritis Rheum. 1985, 28, 413–418. [Google Scholar] [CrossRef]
- Johnson, K.A.; Hessle, L.; Vaingankar, S.; Wennberg, C.; Mauro, S.; Narisawa, S.; Goding, J.W.; Sano, K.; Millan, J.L.; Terkeltaub, R. Osteoblast tissue-nonspecific alkaline phosphatase antagonizes and regulates PC-1. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 279, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Thouverey, C.; Bechkoff, G.; Pikula, S.; Buchet, R. Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthr. Cartil. 2009, 17, 64–72. [Google Scholar] [CrossRef]
- Bernabei, I.; So, A.; Busso, N.; Nasi, S. Cartilage calcification in osteoarthritis: Mechanisms and clinical relevance. Nat. Rev. Rheumatol. 2023, 19, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Scanu, A.; Oliviero, F.; Gruaz, L.; Sfriso, P.; Pozzuoli, A.; Frezzato, F.; Agostini, C.; Burger, D.; Punzi, L. High-density lipoproteins downregulate CCL2 production in human fibroblast-like synoviocytes stimulated by urate crystals. Arthritis Res. Ther. 2010, 12, 23. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Fernández-Torres, J.; Loissell-Baltazar, Y.A.; Medina-Luna, D.; López-Macay, A.; Camacho-Galindo, J.; Hernández-Díaz, C.; Santamaría-Olmedo, M.G.; López-Villegas, E.O.; et al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res. Ther. 2016, 18, 117. [Google Scholar] [CrossRef]
- Oliviero, F.; Sfriso, P.; Scanu, A.; Fiocco, U.; Spinella, P.; Punzi, L. Epigallocatechin-3-gallate reduces inflammation induced by calcium pyrophosphate crystals in vitro. Front. Pharmacol. 2013, 4, 51. [Google Scholar] [CrossRef]
- Niessink, T.; Stassen, R.H.M.J.; Kischkel, B.; Vuscan, P.; Emans, P.J.; van den Akker, G.G.H.; Janssen, M.; Joosten, L.A.B.; Otto, C.; Welting, T.J.M.; et al. Discovery of calcite as a new pro-inflammatory calcium-containing crystal in human osteoarthritic synovial fluid. Osteoarthr. Cartil. 2024, 32, 1261–1272. [Google Scholar] [CrossRef]
- Meyer, F.; Dittmann, A.; Kornak, U.; Herbster, M.; Pap, T.; Lohmann, C.H.; Bertrand, J. Chondrocytes from Osteoarthritic and Chondrocalcinosis Cartilage Represent Different Phenotypes. Front. Cell Dev. Biol. 2021, 9, 622287. [Google Scholar] [CrossRef]
- Bertrand, J.; Kräft, T.; Gronau, T.; Sherwood, J.; Rutsch, F.; Lioté, F.; Dell’Accio, F.; Lohmann, C.H.; Bollmann, M.; Held, A.; et al. BCP crystals promote chondrocyte hypertrophic differentiation in OA cartilage by sequestering Wnt3a. Ann. Rheum. Dis. 2020, 79, 975–984. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Pritzker, K.; Firestein, G.S.; Terkeltaub, R. TLR2 Signaling in Chondrocytes Drives Calcium Pyrophosphate Dihydrate and Monosodium Urate Crystal-Induced Nitric Oxide Generation. J. Immunol. 2005, 174, 5016–5023. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lioté, F.; Rose, D.M.; Merz, D.; Terkeltaub, R. Proline-rich tyrosine kinase 2 and Src kinase signaling transduce monosodium urate crystal–induced nitric oxide production and matrix metalloproteinase 3 expression in chondrocytes. Arthritis Rheum. 2004, 50, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, C.H.; Tsai, H.C.; Salter, D.M. Inhibition of cyclooxygenase 2 expression by diallyl sulfide on joint inflammation induced by urate crystal and IL-1β. Osteoarthr. Cartil. 2009, 17, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Lee, C.H.; Wang, C.C.; Salter, D.M.; Lee, H.S. Pycnogenol attenuates the inflammatory and nitrosative stress on joint inflammation induced by urate crystals. Free Radic. Biol. Med. 2012, 52, 765–774. [Google Scholar] [CrossRef]
- Popa-Nita, O.; Naccache, P.H. Crystal-induced neutrophil activation. Immunol. Cell Biol. 2010, 88, 32–40. [Google Scholar] [CrossRef]
- Mahon, O.R.; Kelly, D.J.; McCarthy, G.M.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals alter immune cell metabolism and promote M1 macrophage polarization. Osteoarthr. Cartil. 2020, 28, 603–612. [Google Scholar] [CrossRef]
- Martin, W.J.; Shaw, O.; Liu, X.; Steiger, S.; Harper, J.L. Monosodium urate monohydrate crystal-recruited noninflammatory monocytes differentiate into M1-like proinflammatory macrophages in a peritoneal murine model of gout. Arthritis Rheum. 2011, 63, 1322–1332. [Google Scholar] [CrossRef]
- McLean, L.; Dalbeth, N. Etiology and pathogenesis of gout. In Rheumatology, 6th ed.; Hochberg, M.C., Silman, A.J., Weinblatt, M.E., Smolen, J.S., Weisman, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, pp. 1555–1568. [Google Scholar]
- Ng, G.; Sharma, K.; Ward, S.M.; Desrosiers, M.D.; Stephens, L.A.; Schoel, W.M.; Li, T.; Lowell, C.A.; Ling, C.C.; Amrein, M.W.; et al. Receptor-Independent, Direct Membrane Binding Leads to Cell-Surface Lipid Sorting and Syk Kinase Activation in Dendritic Cells. Immunity 2008, 29, 807–818. [Google Scholar] [CrossRef]
- Rossato, M.F.; Hoffmeister, C.; Trevisan, G.; Bezerra, F.; Cunha, T.M.; Ferreira, J.; Silva, C.R. Monosodium urate crystal interleukin-1β release is dependent on Toll-like receptor 4 and transient receptor potential V1 activation. Rheumatology 2020, 59, 233–242. [Google Scholar] [CrossRef]
- Pascart, T.; Filippou, G.; Lioté, F.; Sirotti, S.; Jauffret, C.; Abhishek, A. Calcium pyrophosphate deposition disease. Lancet Rheumatol. 2024, 6, 791–804. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Mills, E.; Mielke, L.A.; O’Farrell, L.K.; Lavelle, E.; Mori, A.; McCarthy, G.M.; Mills, K.H.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals induce pro-inflammatory cytokines and damage-associated molecules via activation of Syk and PI3 kinase. Clin. Immunol. 2012, 144, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Pazár, B.; Ea, H.K.; Narayan, S.; Kolly, L.; Bagnoud, N.; Chobaz, V.; Roger, T.; Lioté, F.; So, A.; Busso, N. Basic Calcium Phosphate Crystals Induce Monocyte/Macrophage IL-1β Secretion through the NLRP3 Inflammasome In Vitro. J. Immunol. 2011, 186, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Grandjean-Laquerriere, A.; Tabary, O.; Jacquot, J.; Richard, D.; Frayssinet, P.; Guenounou, M.; Laurent-Maquin, D.; Laquerriere, P.; Gangloff, S. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials 2007, 28, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Ehirchiou, D.; Bernabei, I.; Pandian, V.D.; Nasi, S.; Chobaz, V.; Castelblanco, M.; So, A.; Martinon, F.; Li, X.; Acha-Orbea, H.; et al. The integrin CD11b inhibits MSU-induced NLRP3 inflammasome activation in macrophages and protects mice against MSU-induced joint inflammation. Arthritis Res. Ther. 2024, 26, 119. [Google Scholar] [CrossRef]
- Barabé, F.; Gilbert, C.; Liao, N.; Bourgoin, S.G.; Naccache, P.H. Crystal-induced neutrophil activation VI. Involvement of FcγRIIIB (CD16) and CD11b in response to inflammatory microcrystals. FASEB J. 1998, 12, 209–220. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Fernández-Torres, J.; Martínez-Nava, G.A.; Martínez-Flores, K.; Ramírez Olvera, A.; Medina-Luna, D.; Hernández Pérez, A.D.; Landa-Solís, C.; López-Reyes, A. Phagocytosis of monosodium urate crystals by human synoviocytes induces inflammation. Exp. Biol. Med. 2019, 244, 344–351. [Google Scholar] [CrossRef]
- McCarthy, G.M.; Cheung, H.S.; Abel, S.M.; Ryan, L.M. Basic calcium phosphate crystal-induced collagenase production: Role of intracellular crystal dissolution. Osteoarthr. Cartil. 1998, 6, 205–213. [Google Scholar] [CrossRef][Green Version]
- Oliviero, F.; Zamudio-Cuevas, Y.; Belluzzi, E.; Andretto, L.; Scanu, A.; Favero, M.; Ramonda, R.; Ravagnan, G.; López-Reyes, A.; Spinella, P.; et al. Polydatin and Resveratrol Inhibit the Inflammatory Process Induced by Urate and Pyrophosphate Crystals in THP-1 Cells. Foods 2019, 8, 560. [Google Scholar] [CrossRef]
- Baggio, C.; Sfriso, P.; Cignarella, A.; Galozzi, P.; Scanu, A.; Mastrotto, F.; Favero, M.; Ramonda, R.; Oliviero, F. Phagocytosis and inflammation in crystal-induced arthritis: A synovial fluid and in vitro study. Clin. Exp. Rheumatol. 2021, 39, 494–500. [Google Scholar] [CrossRef]
- Ea, H.K.; Lioté, F. Advances in understanding calcium-containing crystal disease. Curr. Opin. Rheumatol. 2009, 21, 150–157. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Mouktaroudi, M.; Bodar, E.; van der Ven, J.; Kullberg, B.J.; Netea, M.G.; van der Meer, J.W. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 βby mononuclear cells through a caspase 1-mediated process. Ann. Rheum. Dis. 2009, 68, 273–278. [Google Scholar] [CrossRef] [PubMed]
- An, L.L.; Mehta, P.; Xu, L.; Turman, S.; Reimer, T.; Naiman, B.; Connor, J.; Sanjuan, M.; Kolbeck, R.; Fung, M. Complement C5a potentiates uric acid crystal-induced IL-1β production. Eur. J. Immunol. 2014, 44, 3669–3679. [Google Scholar] [CrossRef]
- Shaw, O.M.; Steiger, S.; Liu, X.; Hamilton, J.A.; Harper, J.L. Brief Report: Granulocyte-Macrophage Colony-Stimulating Factor Drives Monosodium Urate Monohydrate Crystal–Induced Inflammatory Macrophage Differentiation and NLRP3 Inflammasome Up-Regulation in an in Vivo Mouse Model. Arthritis Rheumatol. 2014, 66, 2423–2428. [Google Scholar] [CrossRef]
- Crișan, T.O.; Cleophas, M.C.; Oosting, M.; Lemmers, H.; Toenhake-Dijkstra, H.; Netea, M.G.; Jansen, T.L.; Joosten, L.A. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 2016, 75, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Nippe, N.; Vogl, T.; Marketon, K.; Mysore, V.; Weinhage, T.; Dalbeth, N.; Pool, B.; Merriman, T.; Baeten, D.; et al. Myeloid-Related Proteins 8 and 14 Contribute to Monosodium Urate Monohydrate Crystal–Induced Inflammation in Gout. Arthritis Rheumatol. 2014, 66, 1327–1339. [Google Scholar] [CrossRef]
- Vieira, A.T.; Macia, L.; Galvão, I.; Martins, F.S.; Canesso, M.C.; Amaral, F.A.; Garcia, C.C.; Maslowski, K.M.; De Leon, E.; Shim, D.; et al. A Role for Gut Microbiota and the Metabolite-Sensing Receptor GPR43 in a Murine Model of Gout. Arthritis Rheumatol. 2015, 67, 1646–1656. [Google Scholar] [CrossRef]
- Li, X.; Wan, A.; Liu, Y.; Li, M.; Zhu, Z.; Luo, C.; Tao, J. P2X7R Mediates the Synergistic Effect of ATP and MSU Crystals to Induce Acute Gouty Arthritis. Oxidative Med. Cell. Longev. 2023, 2023, 1–12. [Google Scholar] [CrossRef]
- Scanu, A.; Oliviero, F.; Gruaz, L.; Galozzi, P.; Luisetto, R.; Ramonda, R.; Burger, D.; Punzi, L. Synovial fluid proteins are required for the induction of interleukin-1β production by monosodium urate crystals. Scand. J. Rheumatol. 2016, 45, 384–393. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Dayer, J.M.; Fiocco, U.; Sfriso, P.; Punzi, L. Response to ‘Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, 405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.K. The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout. J. Rheum. Dis. 2022, 29, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Tatsiy, O.; Mayer, T.Z.; de Carvalho Oliveira, V.; Sylvain-Prévost, S.; Isabel, M.; Dubois, C.M.; McDonald, P.P. Cytokine Production and NET Formation by Monosodium Urate-Activated Human Neutrophils Involves Early and Late Events, and Requires Upstream TAK1 and Syk. Front. Immunol. 2020, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wei, K.; Jiang, P.; Chang, C.; Xu, L.; Xu, L.; Shi, Y.; Guo, S.; Xue, Y.; He, D. Inflammatory Response to Regulated Cell Death in Gout and Its Functional Implications. Front. Immunol. 2022, 6, 888306. [Google Scholar] [CrossRef]
- Corr, E.M.; Cunningham, C.C.; Helbert, L.; McCarthy, G.M.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res. Ther. 2017, 19, 23. [Google Scholar] [CrossRef]
- Campillo-Gimenez, L.; Renaudin, F.; Jalabert, M.; Gras, P.; Gosset, M.; Rey, C.; Sarda, S.; Collet, C.; Cohen-Solal, M.; Combes, C.; et al. Inflammatory Potential of Four Different Phases of Calcium Pyrophosphate Relies on NF-κB Activation and MAPK Pathways. Front. Immunol. 2018, 9, 2248. [Google Scholar] [CrossRef]
- Chang, C.C.; Tsai, Y.H.; Liu, Y.; Lin, S.Y.; Liang, Y.C. Calcium-containing crystals enhance receptor activator of nuclear factor κB ligand/macrophage colony-stimulating factor–mediated osteoclastogenesis via extracellular-signal-regulated kinase and p38 pathways. Rheumatology 2015, 54, 1913–1922. [Google Scholar] [CrossRef]
- Ea, H.K.; Uzan, B.; Rey, C.; Lioté, F. Octacalcium phosphate crystals directly stimulate expression of inducible nitric oxide synthase through p38 and JNK mitogen-activated protein kinases in articular chondrocytes. Arthritis Res. Ther. 2005, 7, R915. [Google Scholar] [CrossRef]
- McCarthy, G.M.; Augustine, J.A.; Baldwin, A.S.; Christopherson, P.A.; Cheung, H.S.; Westfall, P.R.; Scheinman, R.I. Molecular mechanism of basic calcium phosphate crystal-induced activation of human fibroblasts. Role of nuclear factor kappab, activator protein 1, and protein kinase C. J. Biol. Chem. 1998, 273, 35161–35169. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Corr, E.M.; McCarthy, G.M.; Dunne, A. Intra-articular basic calcium phosphate and monosodium urate crystals inhibit anti-osteoclastogenic cytokine signalling. Osteoarthr. Cartil. 2016, 24, 2141–2152. [Google Scholar] [CrossRef]
- Nguyen, C.; Lieberherr, M.; Bordat, C.; Velard, F.; Côme, D.; Lioté, F.; Ea, H.K. Intracellular calcium oscillations in articular chondrocytes induced by basic calcium phosphate crystals lead to cartilage degradation. Osteoarthr. Cartil. 2012, 20, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Ea, H.K.; Chobaz, V.; Nguyen, C.; Nasi, S.; van Lent, P.; Daudon, M.; Dessombz, A.; Bazin, D.; McCarthy, G.; Jolles-Haeberli, B.; et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PLoS ONE. 2013, 8, e57352. [Google Scholar] [CrossRef]
- Scanu, A.; Lorenzin, M.; Luisetto, R.; Galozzi, P.; Ortolan, A.; Oliviero, F.; Doria, A.; Ramonda, R. Identification in synovial fluid of a new potential pathogenic player in arthropathies. Exp. Biol. Med. 2022, 247, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J.; Sun, Y.; Pearlman, E.; Ginley, N.M.; Awadallah, A.; Wisler, B.A.; Dennis, J.E. Monosodium Urate and Tumor Necrosis Factor-α Increase Apoptosis in Human Chondrocyte Cultures. Rheumatology 2012, 2, 113. [Google Scholar] [CrossRef]
- Ea, H.K.; Monceau, V.; Camors, E.; Cohen-Solal, M.; Charlemagne, D.; Lioté, F. Annexin 5 overexpression increased articular chondrocyte apoptosis induced by basic calcium phosphate crystals. Ann. Rheum. Dis. 2008, 67, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Tudan, C.; Jackson, J.K.; Blanis, L.; Pelech, S.L.; Burt, H.M. Inhibition of TNF-α-Induced Neutrophil Apoptosis by Crystals of Calcium Pyrophosphate Dihydrate Is Mediated by the Extracellular Signal-Regulated Kinase and Phosphatidylinositol 3-Kinase/Akt Pathways Up-Stream of Caspase 3. J. Immunol. 2000, 165, 5798–5806. [Google Scholar] [CrossRef]
- Zhong, C.S.; Zeng, B.; Qiu, J.H.; Xu, L.H.; Zhong, M.Y.; Huang, Y.T.; Xu, R.; Liu, S.Y.; Zha, Q.B.; Hu, B.; et al. Gout-associated monosodium urate crystal-induced necrosis is independent of NLRP3 activity but can be suppressed by combined inhibitors for multiple signaling pathways. Acta Pharmacol. Sin. 2022, 43, 1324–1336. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Guo, Y.; Li, M.; Yang, K.; Liu, Y.; Ge, D.; Liu, Y.; Xue, C.; Xia, T.; et al. Monosodium Urate Crystal-Induced Pyroptotic Cell Death in Neutrophil and Macrophage Facilitates the Pathological Progress of Gout. Small 2024, 20, e2308749. [Google Scholar] [CrossRef]
- Xu, X.; Qiu, H. BRD4 promotes gouty arthritis through MDM2-mediated PPARγ degradation and pyroptosis. Mol. Med. 2024, 30, 67. [Google Scholar] [CrossRef]
- Hwang, H.; Yang, C.; Park, S.; Kim, H. Monosodium Urate Crystal-Induced Chondrocyte Death via Autophagic Process. Int. J. Mol. Sci. 2015, 16, 29265–29277. [Google Scholar] [CrossRef]
- Schorn, C.; Janko, C.; Krenn, V.; Zhao, Y.; Munoz, L.E.; Schett, G.; Herrmann, M. Bonding the foe—NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front. Immunol. 2012, 3, 376. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, S.M.; Grebe, K.; Whitehead, L.W.; Rogers, K.L.; Nebl, T.; Murphy, J.M.; Wicks, I.P. Monosodium Urate Crystals Generate Nuclease-Resistant Neutrophil Extracellular Traps via a Distinct Molecular Pathway. J. Immunol. 2018, 200, 1802–1816. [Google Scholar] [CrossRef]
- Pang, L.; Hayes, C.P.; Buac, K.; Yoo, D.; Rada, B. Pseudogout-Associated Inflammatory Calcium Pyrophosphate Dihydrate Microcrystals Induce Formation of Neutrophil Extracellular Traps. J. Immunol. 2013, 190, 6488–6500. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, E.; Gamberucci, A.; Lucherini, O.M.; Alì, A.; Simpatico, A.; Lorenzini, S.; Lazzerini, P.E.; Tripodi, S.; Frediani, B.; Selvi, E. Neutrophil extracellular traps release in gout and pseudogout depends on the number of crystals regardless of leukocyte count. Rheumatology 2021, 60, 4920–4928. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhang, S.; Liao, J.; Qiu, X.; Zhang, Z.; Wang, Z.; Geng, H.; Zhang, J.; Jia, E. Mechanism of macrophages in gout: Recent progress and perspective. Heliyon 2024, 10, e38288. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Abhishek, A.; Doherty, M. Update on calcium pyrophosphate deposition. Clin. Exp. Rheumatol. 2016, 34, 32–38. [Google Scholar] [PubMed]
- Dimmick, S.; Hayter, C.; Linklater, J. Acute calcific periarthritis—A commonly misdiagnosed pathology. Skelet. Radiol. 2022, 51, 1553–1561. [Google Scholar] [CrossRef]
- Reber, L.L.; Marichal, T.; Sokolove, J.; Starkl, P.; Gaudenzio, N.; Iwakura, Y.; Karasuyama, H.; Schwartz, L.B.; Robinson, W.H.; Tsai, M.; et al. Contribution of mast cell-derived interleukin-1β to uric acid crystal-induced acute arthritis in mice. Arthritis Rheumatol. 2014, 66, 2881–2891. [Google Scholar] [CrossRef]
- Oliviero, F.; Galozzi, P.; Scanu, A.; Galuppini, F.; Lazzarin, V.; Brocco, S.; Ravagnan, G.; Sfriso, P.; Ramonda, R.; Spinella, P.; et al. Polydatin Prevents Calcium Pyrophosphate Crystal-Induced Arthritis in Mice. Nutrients 2021, 13, 929. [Google Scholar] [CrossRef]
- Luisetto, R.; Scanu, A. The translational value of calcium pyrophosphate deposition disease experimental mouse models. Front. Med. 2024, 11, 1417318. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Scanu, A. How Factors Involved in the Resolution of Crystal-Induced Inflammation Target IL-1β. Front. Pharmacol. 2017, 8, 164. [Google Scholar] [CrossRef]
- Terkeltaub, R.; Martin, J.; Curtiss, L.K.; Ginsberg, M.H. Apolipoprotein B mediates the capacity of low density lipoprotein to suppress neutrophil stimulation by particulates. J. Biol. Chem. 1986, 261, 15662–15667. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.A.; Dyer, C.A.; Martin, J.; Curtiss, L.K. Apolipoprotein (apo) E inhibits the capacity of monosodium urate crystals to stimulate neutrophils. Characterization of intraarticular apo E and demonstration of apo E binding to urate crystals in vivo. J. Clin. Investig. 1991, 87, 20–26. [Google Scholar] [CrossRef]
- Burt, H.M.; Jackson, J.K.; Rowell, J. Calcium pyrophosphate and monosodium urate crystal interactions with neutrophils: Effect of crystal size and lipoprotein binding to crystals. J. Rheumatol. 1989, 16, 809–817. [Google Scholar]
- Kumagai, Y.; Watanabe, W.; Kobayashi, A.; Sato, K.; Onuma, S.; Sakamoto, H. Inhibitory Effect of Low Density Lipoprotein on the Inflammation-Inducing Activity of Calcium Pyrophosphate Dihydrate Crystals. J. Rheumatol. 2001, 28, 2674–2680. [Google Scholar]
- Scanu, A.; Luisetto, R.; Oliviero, F.; Gruaz, L.; Sfriso, P.; Burger, D.; Punzi, L. High-density lipoproteins inhibit urate crystal-induced inflammation in mice. Ann. Rheum. Dis. 2015, 74, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Landis, R.C.; Yagnik, D.R.; Florey, O.; Philippidis, P.; Emons, V.; Mason, J.C.; Haskard, D.O. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum. 2002, 46, 3026–3033. [Google Scholar] [CrossRef]
- Galozzi, P.; Maschio, L.; Carraro, S.; Scanu, A.; Facco, M.; Oliviero, F. M2 macrophages as resolvers of crystal-induced inflammation. Rheumatology 2021, 60, 2480–2483. [Google Scholar] [CrossRef]
- Cumpelik, A.; Ankli, B.; Zecher, D.; Schifferli, J.A. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann. Rheum. Dis. 2016, 75, 1236–1245. [Google Scholar] [CrossRef]
- Scanu, A.; Oliviero, F.; Ramonda, R.; Frallonardo, P.; Dayer, J.M.; Punzi, L. Cytokine levels in human synovial fluid during the different stages of acute gout: Role of transforming growth factor β1 in the resolution phase. Ann. Rheum. Dis. 2012, 71, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Hsieh, S.C.; Chen, W.Y.; Li, K.J.; Wu, C.H.; Wu, P.C.; Tsai, C.Y.; Yu, C.L. Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGFβ1, IL-10 and soluble TNF receptors and the intracellular cytokine negative regulators CIS and SOCS3. Ann. Rheum. Dis. 2011, 70, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, T.; Namai, R.; Murakami, Y.; Watanabe, M.; Matsui, T.; Nishimura, A.; Kitasato, H.; Kameya, T.; Kondo, H. Rapid induction of peroxisome proliferator-activated receptor gamma expression in human monocytes by monosodium urate monohydrate crystals. Arthritis Rheum. 2003, 48, 231–239. [Google Scholar] [CrossRef]
- Valiate, B.V.S.; Queiroz-Junior, C.M.; Levi-Schaffer, F.; Galvão, I.; Teixeira, M.M. CD300a contributes to the resolution of articular inflammation triggered by MSU crystals by controlling neutrophil apoptosis. Immunology 2021, 164, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Dang, W.; Chen, B.; Qing, Y.; Xie, W.; Zhao, M.; Zhou, J. IL-37 inhibits the production of pro-inflammatory cytokines in MSU crystal-induced inflammatory response. Clin. Rheumatol. 2016, 35, 2251–2258. [Google Scholar] [CrossRef]
- Speed, C.A.; Hazleman, B.L. Calcific Tendinitis of the Shoulder. N. Engl. J. Med. 1999, 340, 1582–1584. [Google Scholar] [CrossRef]
- Epis, O.; Viola, E.; Bruschi, E.; Benazzo, F.; Montecucco, C. Milwaukee shoulder syndrome (apatite associated destructive arthritis): Therapeutic aspects. Reumatismo 2005, 57, 69–77. [Google Scholar] [CrossRef]
- Frallonardo, P.; Ramonda, R.; Peruzzo, L.; Scanu, A.; Galozzi, P.; Tauro, L.; Punzi, L.; Oliviero, F. Basic calcium phosphate and pyrophosphate crystals in early and late osteoarthritis: Relationship with clinical indices and inflammation. Clin. Rheumatol. 2018, 37, 2847–2853. [Google Scholar] [CrossRef]
- Ramonda, R.; Oliviero, F.; Galozzi, P.; Frallonardo, P.; Lorenzin, M.; Ortolan, A.; Scanu, A.; Punzi, L. Molecular mechanisms of pain in crystal-induced arthritis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 98–110. [Google Scholar] [CrossRef]
- Hoffmeister, C.; Silva, M.A.; Rossato, M.F.; Trevisan, G.; Oliveira, S.M.; Guerra, G.P.; Silva, C.R.; Ferreira, J. Participation of the TRPV1 receptor in the development of acute gout attacks. Rheumatology 2014, 53, 240–249. [Google Scholar] [CrossRef]
- Hoffmeister, C.; Trevisan, G.; Rossato, M.F.; de Oliveira, S.M.; Gomez, M.V.; Ferreira, J. Role of TRPV1 in nociception and edema induced by monosodium urate crystals in rats. Pain 2011, 152, 1777–1788. [Google Scholar] [CrossRef]
- Trevisan, G.; Hoffmeister, C.; Rossato, M.F.; Oliveira, S.M.; Silva, M.A.; Ineu, R.P.; Guerra, G.P.; Materazzi, S.; Fusi, C.; Nassini, R.; et al. Transient receptor potential ankyrin 1 receptor stimulation by hydrogen peroxide is critical to trigger pain during monosodium urate-induced inflammation in rodents. Arthritis Rheum. 2013, 65, 2984–2995. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, L.J.; Hämäläinen, M.; Lehtimäki, L.; Nieminen, R.M.; Moilanen, E. Urate Crystal Induced Inflammation and Joint Pain Are Reduced in Transient Receptor Potential Ankyrin 1 Deficient Mice—Potential Role for Transient Receptor Potential Ankyrin 1 in Gout. PLoS ONE 2015, 10, e0117770. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, L.; Feng, J.; Xie, Z.; Liu, Z.; Wang, F.; Liu, P.; Yue, X.; Du, L.; Zhao, Y.; et al. Mechanosensitive TRPV4 is required for crystal-induced inflammation. Ann. Rheum. Dis. 2021, 80, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhou, X.; Yu, Q.; Li, R.; Dai, Q.; Zeng, M. ML335 inhibits TWIK2 channel-mediated potassium efflux and attenuates mitochondrial damage in MSU crystal-induced inflammation. J. Transl. Med. 2024, 22, 785. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Park, K.Y.; Choe, J.Y. Toll-Like Receptor 9 Is Involved in NLRP3 Inflammasome Activation and IL-1β Production Through Monosodium Urate-Induced Mitochondrial DNA. Inflammation 2020, 43, 2301–2311. [Google Scholar] [CrossRef]
- Renaudin, F.; Orliaguet, L.; Castelli, F.; Fenaille, F.; Prignon, A.; Alzaid, F.; Combes, C.; Delvaux, A.; Adimy, Y.; Cohen-Solal, M.; et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Ann. Rheum. Dis. 2020, 79, 1506–1514. [Google Scholar] [CrossRef]
- Major, T.J.; Takei, R.; Matsuo, H.; Leask, M.P.; Sumpter, N.A.; Topless, R.K.; Shirai, Y.; Wang, W.; Cadzow, M.J.; Phipps-Green, A.J.; et al. A genome-wide association analysis reveals new pathogenic pathways in gout. Nat. Genet. 2024, 56, 2392–2406. [Google Scholar] [CrossRef]
- Leask, M.P.; Crișan, T.O.; Ji, A.; Matsuo, H.; Köttgen, A.; Merriman, T.R. The pathogenesis of gout: Molecular insights from genetic, epigenomic and transcriptomic studies. Nat. Rev. Rheumatol. 2024, 20, 510–523. [Google Scholar] [CrossRef]
- Pei, D.D.; Sun, J.L.; Zhu, C.H.; Tian, F.C.; Jiao, K.; Anderson, M.R.; Yiu, C.; Huang, C.; Jin, C.X.; Bergeron, B.E.; et al. Contribution of Mitophagy to Cell-Mediated Mineralization: Revisiting a 50-Year-Old Conundrum. Adv. Sci. 2018, 5, 1800873. [Google Scholar] [CrossRef]
- Hashimoto, S.; Ochs, R.L.; Rosen, F.; Quach, J.; McCabe, G.; Solan, J.; Seegmiller, J.E.; Terkeltaub, R.; Lotz, M. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc. Natl. Acad. Sci. USA 1998, 95, 3094–3099. [Google Scholar] [CrossRef] [PubMed]
- Licandro, G.; Ling Khor, H.; Beretta, O.; Lai, J.; Derks, H.; Laudisi, F.; Conforti-Andreoni, C.; Liang Qian, H.; Teng, G.G.; Ricciardi-Castagnoli, P.; et al. The NLRP3 inflammasome affects DNA damage responses after oxidative and genotoxic stress in dendritic cells. Eur. J. Immunol. 2013, 43, 2126–2137. [Google Scholar] [CrossRef]
- Deo, P.; Dhillon, V.S.; Lim, W.M.; Jaunay, E.L.; Donnellan, L.; Peake, B.; McCullough, C.; Fenech, M. Advanced glycation end-products accelerate telomere attrition and increase pro-inflammatory mediators in human WIL2-NS cells. Mutagenesis 2020, 35, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Vazirpanah, N.; Kienhorst, L.B.E.; Van Lochem, E.; Wichers, C.; Rossato, M.; Shiels, P.G.; Dalbeth, N.; Stamp, L.K.; Merriman, T.R.; Janssen, M.; et al. Patients with gout have short telomeres compared with healthy participants: Association of telomere length with flare frequency and cardiovascular disease in gout. Ann. Rheum. Dis. 2017, 76, 1313–1319. [Google Scholar] [CrossRef]
- Baggio, C.; Luisetto, R.; Boscaro, C.; Scanu, A.; Ramonda, R.; Albiero, M.; Sfriso, P.; Oliviero, F. Leucocyte Abnormalities in Synovial Fluid of Degenerative and Inflammatory Arthropathies. Int. J. Mol. Sci. 2023, 24, 5450. [Google Scholar] [CrossRef] [PubMed]
- Vazirpanah, N.; Radstake, T.; Broen, J. Inflamm-ageing and senescence in gout: The tale of an old king’s disease. Curr. Aging Sci. 2015, 8, 186–201. [Google Scholar] [CrossRef]
- Wen, S.; Arakawa, H.; Tamai, I. CD38 activation by monosodium urate crystals contributes to inflammatory responses in human and murine macrophages. Biochem. Biophys. Res. Commun. 2021, 581, 6–11. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, J.; Xie, S.; He, J.; Huang, S.; Chen, J.; Jiang, S.; Yu, L.; Zhou, Y.; Cao, X.; et al. Systematic analysis of inflammation and pain pathways in a mouse model of gout. Mol. Pain 2022, 18, 17448069221097760. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Stücker, S.; Bollmann, M.; Garbers, C.; Bertrand, J. The role of calcium crystals and their effect on osteoarthritis pathogenesis. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101722. [Google Scholar] [CrossRef]
- Karampasa, I.A.; Kontoyannisa, C.G. Characterization of calcium phosphates mixtures. Vib. Spectrosc. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, G.; Rey, C.; Bres, E. MicroRaman Spectral Study of the PO4 and CO3 Vibrational Modes in Synthetic and Biological Apatites. Calcif. Tissue Int. 1998, 63, 475–481. [Google Scholar] [CrossRef]
- Chen, K.-O.; Lià, M.J.; Chengà, W.T.; Balic-Zunic, T.; Lin, S.Y. Identification of monoclinic calcium pyrophosphate dihydrate and hydroxyapatite in human sclera using Raman microspectroscopy. Int. J. Exp. Path. 2009, 90, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Sauer, G.R.; Zunic, W.B.; Durig, J.R.; Wuthier, R.E. Fourier Transform Raman Spectroscopy of Synthetic and Biological Calcium Phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef]
- Xu, J.; Gilson, D.F.R.; Butler, I.S.; Stangel, I. Effect of high external pressures on the vibrational sDectra of biomedical materials: Calcium hydroxyapatite and calcium fluoroapatite. J. Biomed. Mater. Res. 1996, 30, 239–244. [Google Scholar] [CrossRef]
- Chen, K.H.; Cheng, W.H.; Li, M.J.; Yang, D.M.; Lin, S.Y. Calcification of senile cataractous lens determined by Fourier transform infrared (FTIR) and Raman microspectroscopies. J. Microsc. 2005, 219, 36–41. [Google Scholar] [CrossRef]
- Gordon, C.; Swan, A.; Dieppe, P. Detection of Crystals in Synovial Fluids by Light Microscopy: Sensitivity and Reliability. Ann. Rheum. Dis. 1989, 48, 737–742. [Google Scholar] [CrossRef]
- Paul, H.; Reginato, A.J.; Ralph Schumacher, H. Alizarin Red s Staining as a Screening Test to Detect Calcium Compounds in Synovial Fluid. Arthritis Rheum. 1983, 26, 191–200. [Google Scholar] [CrossRef]
- Li, B.; Yang, S.; Akkus, O. A Customized Raman System for Point-of-Care Detection of Arthropathic Crystals in the Synovial Fluid. Analyst 2014, 139, 823–830. [Google Scholar] [CrossRef]
- Miura, K.; Fukuda, H.; Mineta, H.; Yamaguchi, K.; Harada, H.; Yusa, H.; Tsutsui, Y. Phosphoglyceride crystal deposition disease. Pathol. Int. 2000, 50, 992–998. [Google Scholar] [CrossRef]
- Cheng, X.; Haggins, D.G.; York, R.H.; Yeni, Y.N.; Akkus, O. Analysis of Crystals Leading to Joint Arthropathies by Raman Spectroscopy: Comparison with Compensated Polarized Imaging. Appl. Spectrosc. 2009, 63, 381–386. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Gout, Hyperuricemia and Crystal Associated Disease Network. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).