Regulation of Urate Homeostasis by Membrane Transporters
Abstract
1. Introduction
2. Urate Transporters Are Classified into Two Superfamilies
3. How Have the Transporters Involved in the Regulation of Urate Disposition Been Discovered?
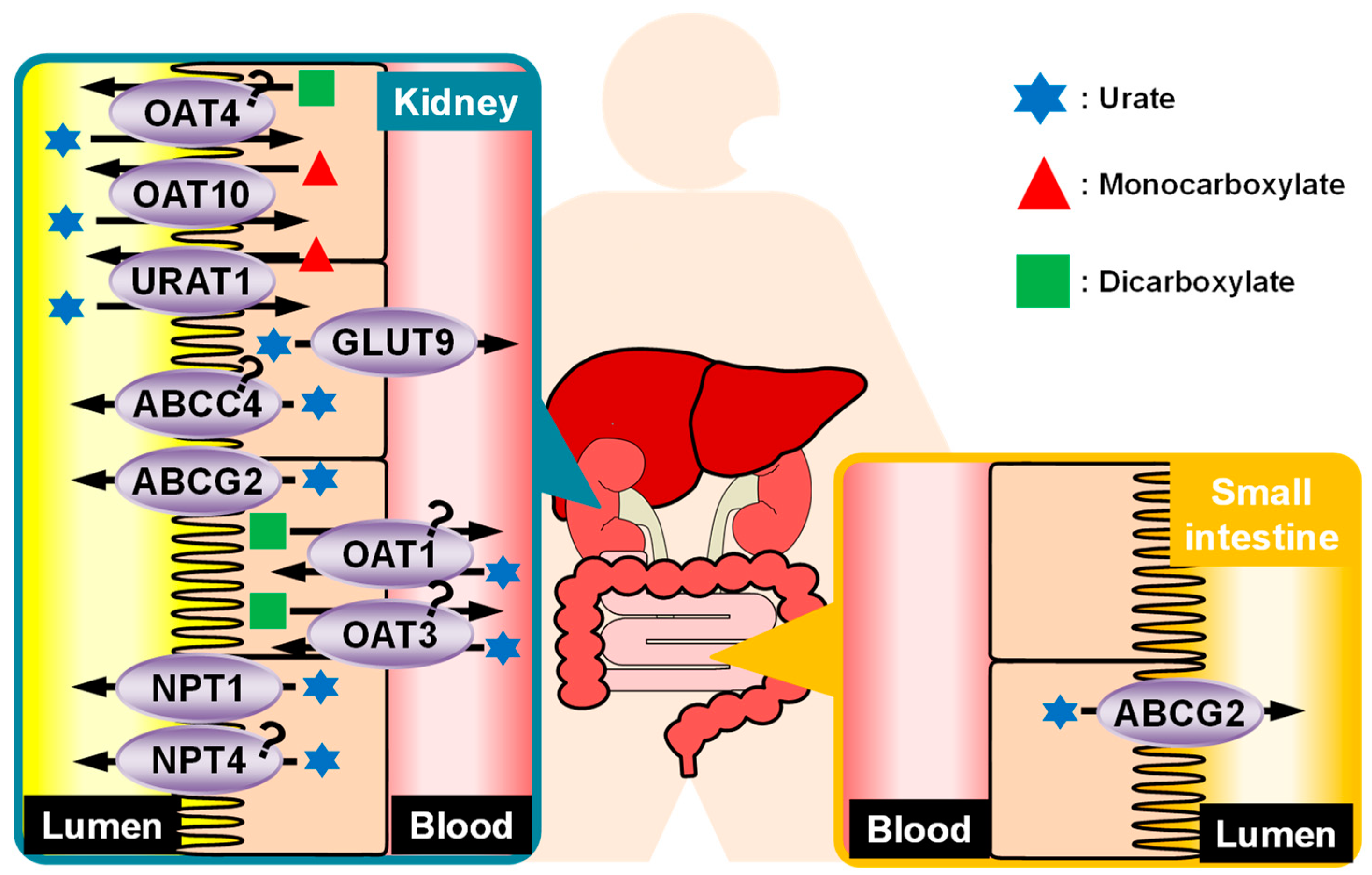
4. Regulation of Urate Kinetics in the Kidney
4.1. Reabsorption of Urate from Glomerular Filtrate
4.1.1. URAT1/SLC22A12
4.1.2. Glucose Transporter (GLUT) 9/SLC2A9
4.1.3. Organic Anion Transporter (OAT) 10/SLC22A13
4.1.4. OAT4/SLC22A11
4.2. Secretion of Urate into the Renal Tubule
4.2.1. OAT1/SLC22A6 and OAT3/SLC22A8
4.2.2. NPT1/SLC17A1 and NPT4/SLC17A3
4.2.3. Multidrug Resistance-Associated Protein (MRP) 4/ABCC4
5. BCRP/ABCG2
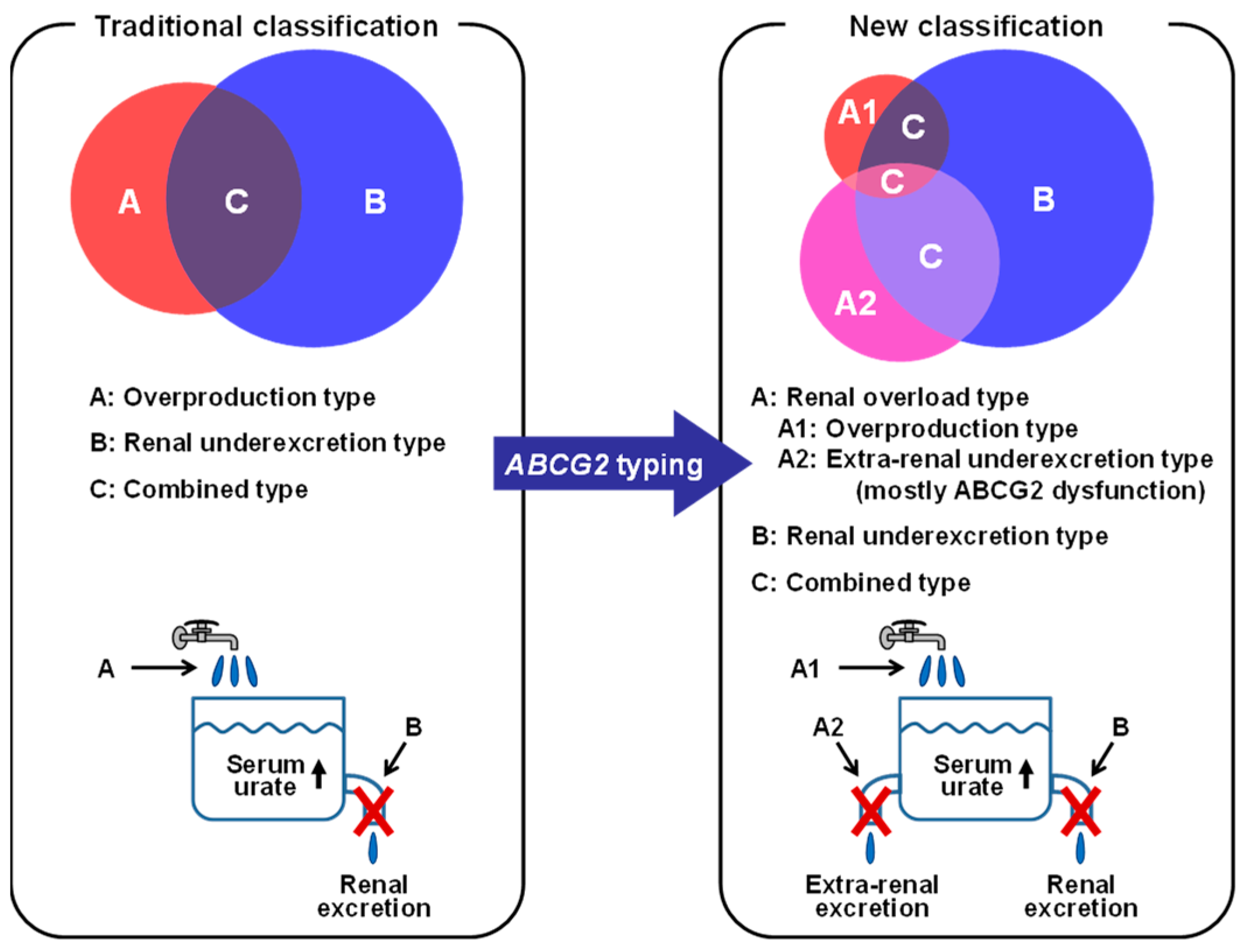
5.1. ABCG2 as a Urate Transporter
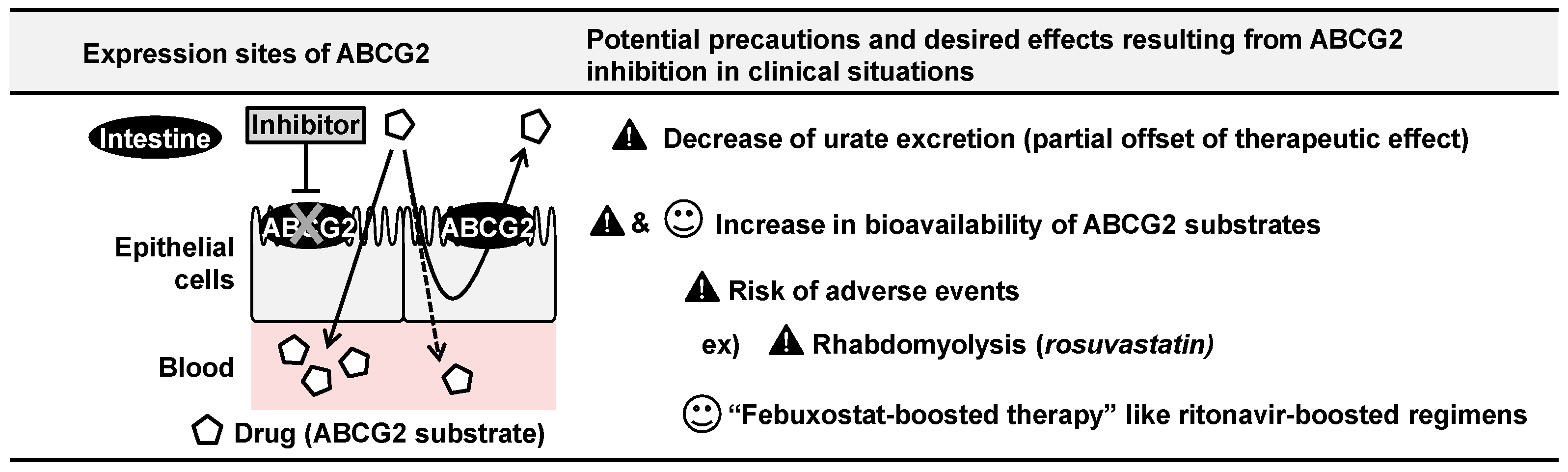
5.2. ABCG2 Inhibition by Febuxostat and Its Clinical Implications
5.3. ABCG2 Transports Uremic Toxins and other Endogenous Substrates
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, A.K.; Mount, D.B. The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 2015, 77, 323–345. [Google Scholar] [CrossRef]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef]
- Woodward, O.M.; Köttgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Köttgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef]
- Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Nakayama, A.; Ikebuchi, Y.; Ito, K.; Kusanagi, Y.; Chiba, T.; Tadokoro, S.; et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: A function-based genetic analysis in a Japanese population. Sci. Transl. Med. 2009, 1, 5ra11. [Google Scholar] [CrossRef]
- Dalbeth, N.; Choi, H.K.; Joosten, L.A.B.; Khanna, P.P.; Matsuo, H.; Perez-Ruiz, F.; Stamp, L.K. Gout. Nat. Rev. Dis. Primers 2019, 5, 69. [Google Scholar] [CrossRef]
- Ye, B.S.; Lee, W.W.; Ham, J.H.; Lee, J.J.; Lee, P.H.; Sohn, Y.H.; Alzheimer’s Disease Neuroimaging Initiative. Does serum uric acid act as a modulator of cerebrospinal fluid Alzheimer’s disease biomarker related cognitive decline? Eur. J. Neurol. 2016, 23, 948–957. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Schwid, S.R.; Marek, K.; Watts, A.; Lang, A.E.; Oakes, D.; Shoulson, I.; Ascherio, A.; Hyson, C.; Gorbold, E.; et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch. Neurol. 2008, 65, 716–723. [Google Scholar] [CrossRef]
- Ascherio, A.; LeWitt, P.A.; Xu, K.; Eberly, S.; Watts, A.; Matson, W.R.; Marras, C.; Kieburtz, K.; Rudolph, A.; Bogdanov, M.B.; et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch. Neurol. 2009, 66, 1460–1468. [Google Scholar] [CrossRef]
- Liu, B.; Shen, Y.; Xiao, K.; Tang, Y.; Cen, L.; Wei, J. Serum uric acid levels in patients with multiple sclerosis: A meta-analysis. Neurol. Res. 2012, 34, 163–171. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Nakayama, A.; Kurajoh, M.; Toyoda, Y.; Takada, T.; Ichida, K.; Matsuo, H. Dysuricemia. Biomedicines 2023, 11, 3169. [Google Scholar] [CrossRef]
- Otani, N.; Ouchi, M.; Mizuta, E.; Morita, A.; Fujita, T.; Anzai, N.; Hisatome, I. Dysuricemia-A New Concept Encompassing Hyperuricemia and Hypouricemia. Biomedicines 2023, 11, 1255. [Google Scholar] [CrossRef]
- Borst, P.; Elferink, R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef]
- Hediger, M.A.; Clémençon, B.; Burrier, R.E.; Bruford, E.A. The ABCs of membrane transporters in health and disease (SLC series): Introduction. Mol. Asp. Med. 2013, 34, 95–107. [Google Scholar] [CrossRef]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Leask, M.P.; Merriman, T.R. The genetic basis of urate control and gout: Insights into molecular pathogenesis from follow-up study of genome-wide association study loci. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101721. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Matsuo, H.; Chiba, T.; Nagamori, S.; Nakayama, A.; Domoto, H.; Phetdee, K.; Wiriyasermkul, P.; Kikuchi, Y.; Oda, T.; Nishiyama, J.; et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am. J. Hum. Genet. 2008, 83, 744–751. [Google Scholar] [CrossRef]
- Chiba, T.; Matsuo, H.; Kawamura, Y.; Nagamori, S.; Nishiyama, T.; Wei, L.; Nakayama, A.; Nakamura, T.; Sakiyama, M.; Takada, T.; et al. NPT1/SLC17A1 is a renal urate exporter in humans and its common gain-of-function variant decreases the risk of renal underexcretion gout. Arthritis Rheumatol. 2015, 67, 281–287. [Google Scholar] [CrossRef]
- Toyoda, Y.; Kawamura, Y.; Nakayama, A.; Morimoto, K.; Shimizu, S.; Tanahashi, Y.; Tamura, T.; Kondo, T.; Kato, Y.; Ichida, K.; et al. OAT10/SLC22A13 Acts as a Renal Urate Re-Absorber: Clinico-Genetic and Functional Analyses with Pharmacological Impacts. Front. Pharmacol. 2022, 13, 842717. [Google Scholar] [CrossRef]
- Vávra, J.; Mančíková, A.; Pavelcová, K.; Hasíková, L.; Bohatá, J.; Stibůrková, B. Functional Characterization of Rare Variants in OAT1/ SLC22A6 and OAT3/ SLC22A8 Urate Transporters Identified in a Gout and Hyperuricemia Cohort. Cells 2022, 11, 1063. [Google Scholar] [CrossRef]
- Vávra, J.; Pavelcová, K.; Mašínová, J.; Hasíková, L.; Bubeníková, E.; Urbanová, A.; Mančíková, A.; Stibůrková, B. Examining the Association of Rare Allelic Variants in Urate Transporters SLC22A11, SLC22A13, and SLC17A1 with Hyperuricemia and Gout. Dis. Markers 2024, 2024, 5930566. [Google Scholar] [CrossRef]
- Tanner, C.; Boocock, J.; Stahl, E.A.; Dobbyn, A.; Mandal, A.K.; Cadzow, M.; Phipps-Green, A.J.; Topless, R.K.; Hindmarsh, J.H.; Stamp, L.K.; et al. Population-Specific Resequencing Associates the ATP-Binding Cassette Subfamily C Member 4 Gene with Gout in New Zealand Māori and Pacific Men. Arthritis Rheumatol. 2017, 69, 1461–1469. [Google Scholar] [CrossRef]
- Tin, A.; Woodward, O.M.; Kao, W.H.; Liu, C.T.; Lu, X.; Nalls, M.A.; Shriner, D.; Semmo, M.; Akylbekova, E.L.; Wyatt, S.B.; et al. Genome-wide association study for serum urate concentrations and gout among African Americans identifies genomic risk loci and a novel URAT1 loss-of-function allele. Hum. Mol. Genet. 2011, 20, 4056–4068. [Google Scholar] [CrossRef]
- Kolz, M.; Johnson, T.; Sanna, S.; Teumer, A.; Vitart, V.; Perola, M.; Mangino, M.; Albrecht, E.; Wallace, C.; Farrall, M.; et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009, 5, e1000504. [Google Scholar] [CrossRef]
- Karns, R.; Zhang, G.; Sun, G.; Rao Indugula, S.; Cheng, H.; Havas-Augustin, D.; Novokmet, N.; Rudan, D.; Durakovic, Z.; Missoni, S.; et al. Genome-wide association of serum uric acid concentration: Replication of sequence variants in an island population of the Adriatic coast of Croatia. Ann. Hum. Genet. 2012, 76, 121–127. [Google Scholar] [CrossRef]
- Lu, Y.; Nakanishi, T.; Tamai, I. Functional cooperation of SMCTs and URAT1 for renal reabsorption transport of urate. Drug Metab. Pharmacokinet. 2013, 28, 153–158. [Google Scholar] [CrossRef]
- Thangaraju, M.; Ananth, S.; Martin, P.M.; Roon, P.; Smith, S.B.; Sterneck, E.; Prasad, P.D.; Ganapathy, V. c/ebpdelta Null mouse as a model for the double knock-out of slc5a8 and slc5a12 in kidney. J. Biol. Chem. 2006, 281, 26769–26773. [Google Scholar] [CrossRef]
- Tan, P.K.; Liu, S.; Gunic, E.; Miner, J.N. Discovery and characterization of verinurad, a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout. Sci. Rep. 2017, 7, 665. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ashizawa, N.; Matsumoto, K.; Saito, R.; Motoki, K.; Sakai, M.; Chikamatsu, N.; Hagihara, C.; Hashiba, M.; Iwanaga, T. Pharmacological Evaluation of Dotinurad, a Selective Urate Reabsorption Inhibitor. J. Pharmacol. Exp. Ther. 2019, 371, 162–170. [Google Scholar] [CrossRef]
- Mandal, A.K.; Mercado, A.; Foster, A.; Zandi-Nejad, K.; Mount, D.B. Uricosuric targets of tranilast. Pharmacol. Res. Perspect. 2017, 5, e00291. [Google Scholar] [CrossRef]
- Novikov, A.; Fu, Y.; Huang, W.; Freeman, B.; Patel, R.; van Ginkel, C.; Koepsell, H.; Busslinger, M.; Onishi, A.; Nespoux, J.; et al. SGLT2 inhibition and renal urate excretion: Role of luminal glucose, GLUT9, and URAT1. Am. J. Physiol. Ren. Physiol. 2019, 316, F173–F185. [Google Scholar] [CrossRef]
- Toyoki, D.; Shibata, S.; Kuribayashi-Okuma, E.; Xu, N.; Ishizawa, K.; Hosoyamada, M.; Uchida, S. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am. J. Physiol. Ren. Physiol. 2017, 313, F826–F834. [Google Scholar] [CrossRef]
- Mandal, A.K.; Leask, M.P.; Estiverne, C.; Choi, H.K.; Merriman, T.R.; Mount, D.B. Genetic and Physiological Effects of Insulin on Human Urate Homeostasis. Front. Physiol. 2021, 12, 713710. [Google Scholar] [CrossRef]
- Quiñones Galvan, A.; Natali, A.; Baldi, S.; Frascerra, S.; Sanna, G.; Ciociaro, D.; Ferrannini, E. Effect of insulin on uric acid excretion in humans. Am. J. Physiol. 1995, 268, E1–E5. [Google Scholar] [CrossRef]
- Muscelli, E.; Natali, A.; Bianchi, S.; Bigazzi, R.; Galvan, A.Q.; Sironi, A.M.; Frascerra, S.; Ciociaro, D.; Ferrannini, E. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am. J. Hypertens. 1996, 9, 746–752. [Google Scholar] [CrossRef]
- Ter Maaten, J.C.; Voorburg, A.; Heine, R.J.; Ter Wee, P.M.; Donker, A.J.; Gans, R.O. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin. Sci. 1997, 92, 51–58. [Google Scholar] [CrossRef]
- Dehghan, A.; Köttgen, A.; Yang, Q.; Hwang, S.J.; Kao, W.L.; Rivadeneira, F.; Boerwinkle, E.; Levy, D.; Hofman, A.; Astor, B.C.; et al. Association of three genetic loci with uric acid concentration and risk of gout: A genome-wide association study. Lancet 2008, 372, 1953–1961. [Google Scholar] [CrossRef]
- Augustin, R.; Carayannopoulos, M.O.; Dowd, L.O.; Phay, J.E.; Moley, J.F.; Moley, K.H. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): Alternative splicing alters trafficking. J. Biol. Chem. 2004, 279, 16229–16236. [Google Scholar] [CrossRef]
- Manolescu, A.R.; Augustin, R.; Moley, K.; Cheeseman, C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol. Membr. Biol. 2007, 24, 455–463. [Google Scholar] [CrossRef]
- Doblado, M.; Moley, K.H. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E831–E835. [Google Scholar] [CrossRef]
- Anzai, N.; Ichida, K.; Jutabha, P.; Kimura, T.; Babu, E.; Jin, C.J.; Srivastava, S.; Kitamura, K.; Hisatome, I.; Endou, H.; et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J. Biol. Chem. 2008, 283, 26834–26838. [Google Scholar] [CrossRef]
- Phay, J.E.; Hussain, H.B.; Moley, J.F. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9). Genomics 2000, 66, 217–220. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Huang, C.; Feng, W.; Cui, H.; Li, M. Xanthoceras sorbifolium leaves alleviate hyperuricemic nephropathy by inhibiting the PI3K/AKT signaling pathway to regulate uric acid transport. J. Ethnopharmacol. 2024, 327, 117946. [Google Scholar] [CrossRef]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J.; et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437–442. [Google Scholar] [CrossRef]
- Cummings, N.; Dyer, T.D.; Kotea, N.; Kowlessur, S.; Chitson, P.; Zimmet, P.; Blangero, J.; Jowett, J.B. Genome-wide scan identifies a quantitative trait locus at 4p15.3 for serum urate. Eur. J. Hum. Genet. 2010, 18, 1243–1247. [Google Scholar] [CrossRef]
- Tin, A.; Marten, J.; Halperin Kuhns, V.L.; Li, Y.; Wuttke, M.; Kirsten, H.; Sieber, K.B.; Qiu, C.; Gorski, M.; Yu, Z.; et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat. Genet. 2019, 51, 1459–1474. [Google Scholar] [CrossRef]
- Dinour, D.; Gray, N.K.; Campbell, S.; Shu, X.; Sawyer, L.; Richardson, W.; Rechavi, G.; Amariglio, N.; Ganon, L.; Sela, B.A.; et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J. Am. Soc. Nephrol. 2010, 21, 64–72. [Google Scholar] [CrossRef]
- Preitner, F.; Bonny, O.; Laverrière, A.; Rotman, S.; Firsov, D.; Da Costa, A.; Metref, S.; Thorens, B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc. Natl. Acad. Sci. USA 2009, 106, 15501–15506. [Google Scholar] [CrossRef]
- Bahn, A.; Hagos, Y.; Reuter, S.; Balen, D.; Brzica, H.; Krick, W.; Burckhardt, B.C.; Sabolic, I.; Burckhardt, G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J. Biol. Chem. 2008, 283, 16332–16341. [Google Scholar] [CrossRef]
- Higashino, T.; Morimoto, K.; Nakaoka, H.; Toyoda, Y.; Kawamura, Y.; Shimizu, S.; Nakamura, T.; Hosomichi, K.; Nakayama, A.; Ooyama, K.; et al. Dysfunctional missense variant of OAT10/SLC22A13 decreases gout risk and serum uric acid levels. Ann. Rheum. Dis. 2020, 79, 164–166. [Google Scholar] [CrossRef]
- Ohtsu, N.; Ohgaki, R.; Jin, C.; Xu, M.; Okanishi, H.; Takahashi, R.; Matsui, A.; Kishimoto, W.; Ishiguro, N.; Kanai, Y. Functional coupling of organic anion transporter OAT10 (SLC22A13) and monocarboxylate transporter MCT1 (SLC16A1) influencing the transport function of OAT10. J. Pharmacol. Sci. 2022, 150, 41–48. [Google Scholar] [CrossRef]
- Ekaratanawong, S.; Anzai, N.; Jutabha, P.; Miyazaki, H.; Noshiro, R.; Takeda, M.; Kanai, Y.; Sophasan, S.; Endou, H. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J. Pharmacol. Sci. 2004, 94, 297–304. [Google Scholar] [CrossRef]
- Hagos, Y.; Stein, D.; Ugele, B.; Burckhardt, G.; Bahn, A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J. Am. Soc. Nephrol. 2007, 18, 430–439. [Google Scholar] [CrossRef]
- Köttgen, A.; Albrecht, E.; Teumer, A.; Vitart, V.; Krumsiek, J.; Hundertmark, C.; Pistis, G.; Ruggiero, D.; O’Seaghdha, C.M.; Haller, T.; et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 2013, 45, 145–154. [Google Scholar] [CrossRef]
- Sinnott-Armstrong, N.; Tanigawa, Y.; Amar, D.; Mars, N.; Benner, C.; Aguirre, M.; Venkataraman, G.R.; Wainberg, M.; Ollila, H.M.; Kiiskinen, T.; et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 2021, 53, 185–194. [Google Scholar] [CrossRef]
- Gutman, A.B.; Yu, T.F.; Berger, L. Tubular secretion of urate in man. J. Clin. Investig. 1959, 38, 1778–1781. [Google Scholar] [CrossRef]
- Henjakovic, M.; Hagos, Y.; Krick, W.; Burckhardt, G.; Burckhardt, B.C. Human organic anion transporter 2 is distinct from organic anion transporters 1 and 3 with respect to transport function. Am. J. Physiol. Ren. Physiol. 2015, 309, F843–F851. [Google Scholar] [CrossRef]
- Race, J.E.; Grassl, S.M.; Williams, W.J.; Holtzman, E.J. Molecular cloning and characterization of two novel human renal organic anion transporters (hOAT1 and hOAT3). Biochem. Biophys. Res. Commun. 1999, 255, 508–514. [Google Scholar] [CrossRef]
- Hosoyamada, M.; Sekine, T.; Kanai, Y.; Endou, H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am. J. Physiol. 1999, 276, F122–F128. [Google Scholar] [CrossRef]
- Cha, S.H.; Sekine, T.; Fukushima, J.I.; Kanai, Y.; Kobayashi, Y.; Goya, T.; Endou, H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol. Pharmacol. 2001, 59, 1277–1286. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef]
- Reimer, R.J. SLC17: A functionally diverse family of organic anion transporters. Mol. Asp. Med. 2013, 34, 350–359. [Google Scholar] [CrossRef]
- Iharada, M.; Miyaji, T.; Fujimoto, T.; Hiasa, M.; Anzai, N.; Omote, H.; Moriyama, Y. Type 1 sodium-dependent phosphate transporter (SLC17A1 Protein) is a Cl(-)-dependent urate exporter. J. Biol. Chem. 2010, 285, 26107–26113. [Google Scholar] [CrossRef]
- Stark, K.; Reinhard, W.; Grassl, M.; Erdmann, J.; Schunkert, H.; Illig, T.; Hengstenberg, C. Common polymorphisms influencing serum uric acid levels contribute to susceptibility to gout, but not to coronary artery disease. PLoS ONE 2009, 4, e7729. [Google Scholar] [CrossRef]
- Urano, W.; Taniguchi, A.; Anzai, N.; Inoue, E.; Kanai, Y.; Yamanaka, M.; Endou, H.; Kamatani, N.; Yamanaka, H. Sodium-dependent phosphate cotransporter type 1 sequence polymorphisms in male patients with gout. Ann. Rheum. Dis. 2010, 69, 1232–1234. [Google Scholar] [CrossRef]
- Yang, Q.; Köttgen, A.; Dehghan, A.; Smith, A.V.; Glazer, N.L.; Chen, M.H.; Chasman, D.I.; Aspelund, T.; Eiriksdottir, G.; Harris, T.B.; et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ. Cardiovasc. Genet. 2010, 3, 523–530. [Google Scholar] [CrossRef]
- Hollis-Moffatt, J.E.; Phipps-Green, A.J.; Chapman, B.; Jones, G.T.; van Rij, A.; Gow, P.J.; Harrison, A.A.; Highton, J.; Jones, P.B.; Montgomery, G.W.; et al. The renal urate transporter SLC17A1 locus: Confirmation of association with gout. Arthritis Res. Ther. 2012, 14, R92. [Google Scholar] [CrossRef]
- Jutabha, P.; Anzai, N.; Kimura, T.; Taniguchi, A.; Urano, W.; Yamanaka, H.; Endou, H.; Sakurai, H. Functional Analysis of Human Sodium-Phosphate Transporter 4 (NPT4/SLC17A3) Polymorphisms. J. Pharmacol. Sci. 2011, 115, 249–253. [Google Scholar] [CrossRef]
- van Aubel, R.A.M.H.; Smeets, P.H.E.; Peters, J.G.P.; Bindels, R.J.M.; Russel, F.G.M. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: Putative efflux pump for urinary cAMP and cGMP. J. Am. Soc. Nephrol. 2002, 13, 595–603. [Google Scholar] [CrossRef]
- Russel, F.G.; Koenderink, J.B.; Masereeuw, R. Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol. Sci. 2008, 29, 200–207. [Google Scholar] [CrossRef]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef]
- Miyake, K.; Mickley, L.; Litman, T.; Zhan, Z.; Robey, R.; Cristensen, B.; Brangi, M.; Greenberger, L.; Dean, M.; Fojo, T.; et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999, 59, 8–13. [Google Scholar]
- Allikmets, R.; Schriml, L.M.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339. [Google Scholar]
- Vlaming, M.L.; Lagas, J.S.; Schinkel, A.H. Physiological and pharmacological roles of ABCG2 (BCRP): Recent findings in Abcg2 knockout mice. Adv. Drug Deliv. Rev. 2009, 61, 14–25. [Google Scholar] [CrossRef]
- Woodward, O.M.; Tukaye, D.N.; Cui, J.; Greenwell, P.; Constantoulakis, L.M.; Parker, B.S.; Rao, A.; Köttgen, M.; Maloney, P.C.; Guggino, W.B. Gout-causing Q141K mutation in ABCG2 leads to instability of the nucleotide-binding domain and can be corrected with small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, 5223–5228. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, B.N.; He, Y.J.; Fan, L.; Li, Q.; Liu, Z.Q.; Wang, A.; Liu, Y.L.; Tan, Z.R.; Fen, J.; et al. Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin. Chim. Acta 2006, 373, 99–103. [Google Scholar] [CrossRef]
- Keskitalo, J.E.; Zolk, O.; Fromm, M.F.; Kurkinen, K.J.; Neuvonen, P.J.; Niemi, M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. Ther. 2009, 86, 197–203. [Google Scholar] [CrossRef]
- Cheng, L.S.; Chiang, S.L.; Tu, H.P.; Chang, S.J.; Wang, T.N.; Ko, A.M.; Chakraborty, R.; Ko, Y.C. Genomewide scan for gout in taiwanese aborigines reveals linkage to chromosome 4q25. Am. J. Hum. Genet. 2004, 75, 498–503. [Google Scholar] [CrossRef]
- Hoque, K.M.; Dixon, E.E.; Lewis, R.M.; Allan, J.; Gamble, G.D.; Phipps-Green, A.J.; Halperin Kuhns, V.L.; Horne, A.M.; Stamp, L.K.; Merriman, T.R.; et al. The ABCG2 Q141K hyperuricemia and gout associated variant illuminates the physiology of human urate excretion. Nat. Commun. 2020, 11, 2767. [Google Scholar] [CrossRef]
- Zhao, T.; Cao, L.; Lin, C.; Xu, R.; Du, X.; Zhou, M.; Yang, X.; Wan, W.; Zou, H.; Zhu, X. Intestinal uric acid excretion contributes to serum uric acid decrease during acute gout attack. Rheumatology 2023, 62, 3984–3992. [Google Scholar] [CrossRef]
- Matsuo, H.; Nakayama, A.; Sakiyama, M.; Chiba, T.; Shimizu, S.; Kawamura, Y.; Nakashima, H.; Nakamura, T.; Takada, Y.; Oikawa, Y.; et al. ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci. Rep. 2014, 4, 3755. [Google Scholar] [CrossRef]
- Huls, M.; Brown, C.D.; Windass, A.S.; Sayer, R.; van den Heuvel, J.J.; Heemskerk, S.; Russel, F.G.; Masereeuw, R. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008, 73, 220–225. [Google Scholar] [CrossRef]
- Miyata, H.; Takada, T.; Toyoda, Y.; Matsuo, H.; Ichida, K.; Suzuki, H. Identification of Febuxostat as a New Strong ABCG2 Inhibitor: Potential Applications and Risks in Clinical Situations. Front. Pharmacol. 2016, 7, 518. [Google Scholar] [CrossRef]
- Lehtisalo, M.; Keskitalo, J.E.; Tornio, A.; Lapatto-Reiniluoto, O.; Deng, F.; Jaatinen, T.; Viinamäki, J.; Neuvonen, M.; Backman, J.T.; Niemi, M. Febuxostat, But Not Allopurinol, Markedly Raises the Plasma Concentrations of the Breast Cancer Resistance Protein Substrate Rosuvastatin. Clin. Transl. Sci. 2020, 13, 1236–1243. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Suzuki, H. Inhibitors of Human ABCG2: From Technical Background to Recent Updates with Clinical Implications. Front. Pharmacol. 2019, 10, 208. [Google Scholar] [CrossRef]
- Takada, T.; Yamamoto, T.; Matsuo, H.; Tan, J.K.; Ooyama, K.; Sakiyama, M.; Miyata, H.; Yamanashi, Y.; Toyoda, Y.; Higashino, T.; et al. Identification of ABCG2 as an Exporter of Uremic Toxin Indoxyl Sulfate in Mice and as a Crucial Factor Influencing CKD Progression. Sci. Rep. 2018, 8, 11147. [Google Scholar] [CrossRef]
- Taniguchi, T.; Omura, K.; Motoki, K.; Sakai, M.; Chikamatsu, N.; Ashizawa, N.; Takada, T.; Iwanaga, T. Hypouricemic agents reduce indoxyl sulfate excretion by inhibiting the renal transporters OAT1/3 and ABCG2. Sci. Rep. 2021, 11, 7232. [Google Scholar] [CrossRef]
- Jonker, J.W.; Buitelaar, M.; Wagenaar, E.; Van Der Valk, M.A.; Scheffer, G.L.; Scheper, R.J.; Plosch, T.; Kuipers, F.; Elferink, R.P.; Rosing, H.; et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. USA 2002, 99, 15649–15654. [Google Scholar] [CrossRef]
- van Herwaarden, A.E.; Wagenaar, E.; Merino, G.; Jonker, J.W.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell Biol. 2007, 27, 1247–1253. [Google Scholar] [CrossRef]
- Álvarez-Fernández, L.; Gomez-Gomez, A.; Haro, N.; García-Lino, A.M.; Álvarez, A.I.; Pozo, O.J.; Merino, G. ABCG2 transporter plays a key role in the biodistribution of melatonin and its main metabolites. J. Pineal Res. 2023, 74, e12849. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.; Liu, C.; Sun, P.; Liu, P.; Li, X. ABCG2 is an itaconate exporter that limits antibacterial innate immunity by alleviating TFEB-dependent lysosomal biogenesis. Cell Metab. 2024, 36, 498–510.e411. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Miyata, H.; Matsuo, H.; Kassai, H.; Nakao, K.; Nakatochi, M.; Kawamura, Y.; Shimizu, S.; Shinomiya, N.; et al. Identification of GLUT12/SLC2A12 as a urate transporter that regulates the blood urate level in hyperuricemia model mice. Proc. Natl. Acad. Sci. USA 2020, 117, 18175–18177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, T.; Miyata, H.; Toyoda, Y.; Nakayama, A.; Ichida, K.; Matsuo, H. Regulation of Urate Homeostasis by Membrane Transporters. Gout Urate Cryst. Depos. Dis. 2024, 2, 206-219. https://doi.org/10.3390/gucdd2020016
Takada T, Miyata H, Toyoda Y, Nakayama A, Ichida K, Matsuo H. Regulation of Urate Homeostasis by Membrane Transporters. Gout, Urate, and Crystal Deposition Disease. 2024; 2(2):206-219. https://doi.org/10.3390/gucdd2020016
Chicago/Turabian StyleTakada, Tappei, Hiroshi Miyata, Yu Toyoda, Akiyoshi Nakayama, Kimiyoshi Ichida, and Hirotaka Matsuo. 2024. "Regulation of Urate Homeostasis by Membrane Transporters" Gout, Urate, and Crystal Deposition Disease 2, no. 2: 206-219. https://doi.org/10.3390/gucdd2020016
APA StyleTakada, T., Miyata, H., Toyoda, Y., Nakayama, A., Ichida, K., & Matsuo, H. (2024). Regulation of Urate Homeostasis by Membrane Transporters. Gout, Urate, and Crystal Deposition Disease, 2(2), 206-219. https://doi.org/10.3390/gucdd2020016