Atherogenic Activation of Human Vascular Smooth Muscle Cells by Monosodium Urate Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Culture of Human VSMCs
2.3. VSMC Proliferation Assay
2.4. ELISA Analysis
2.5. Western Blot Analysis
2.6. Immunostaining
2.7. RNA Sequencing
2.8. Targeted Lipidomics
2.9. Statistical Analyses
3. Results
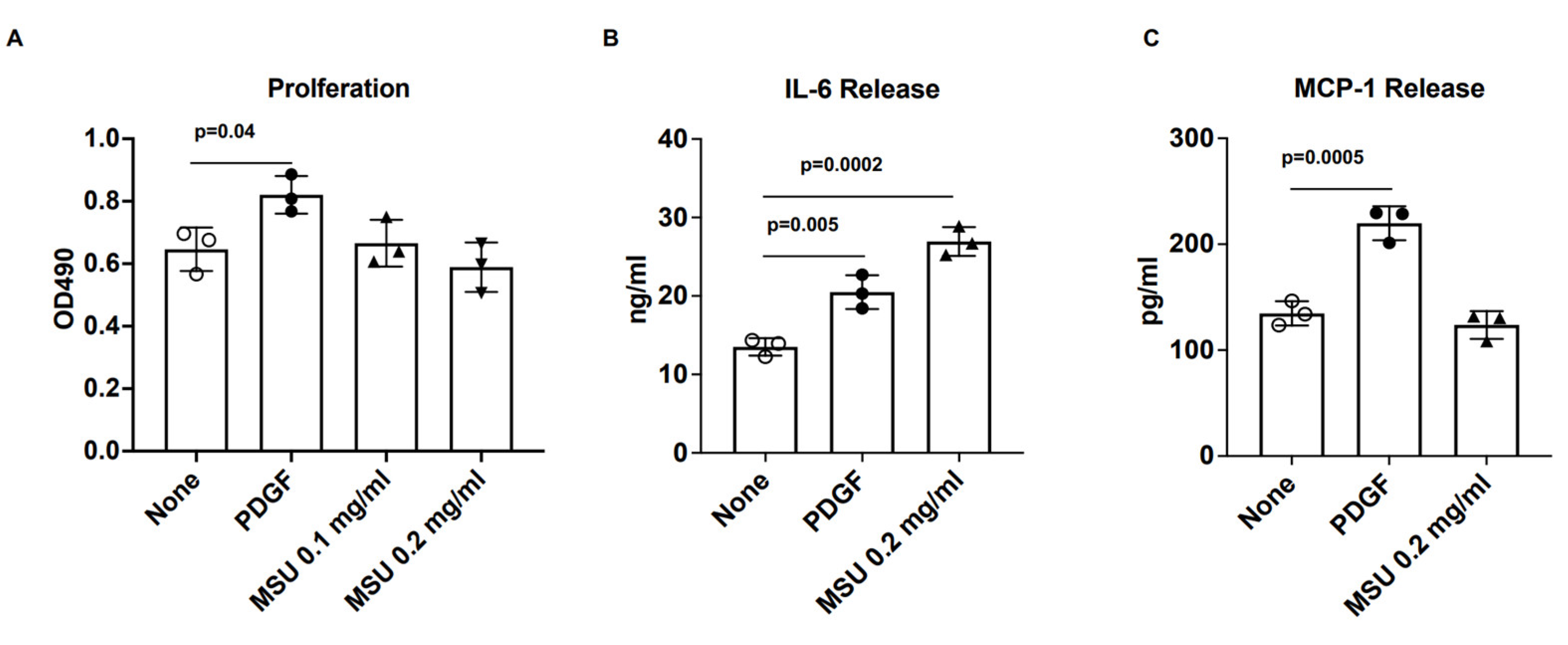
3.1. VSMC IL-6 Release Induced by Human VSMCs
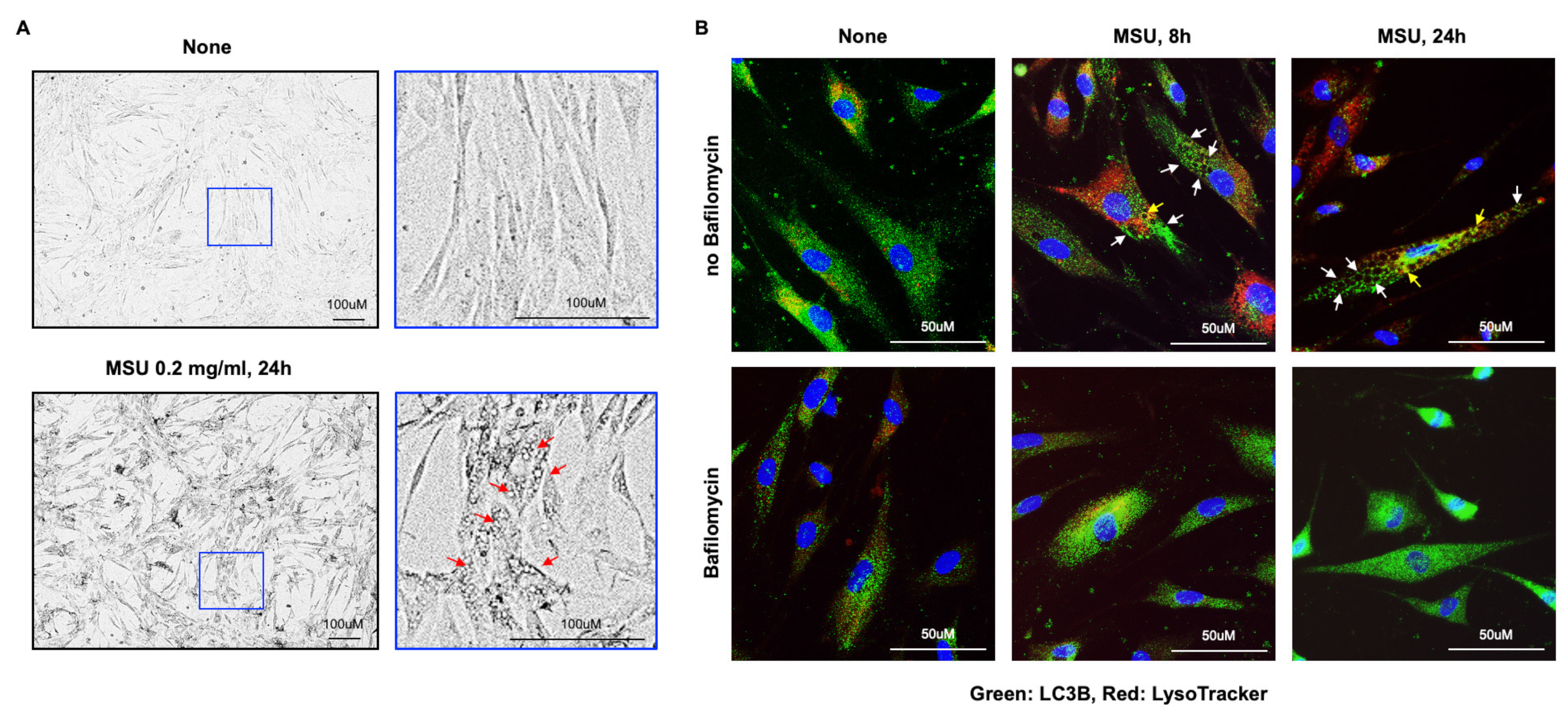
3.2. Marked VSMC Vacuolar Changes Induced by MSU Crystals
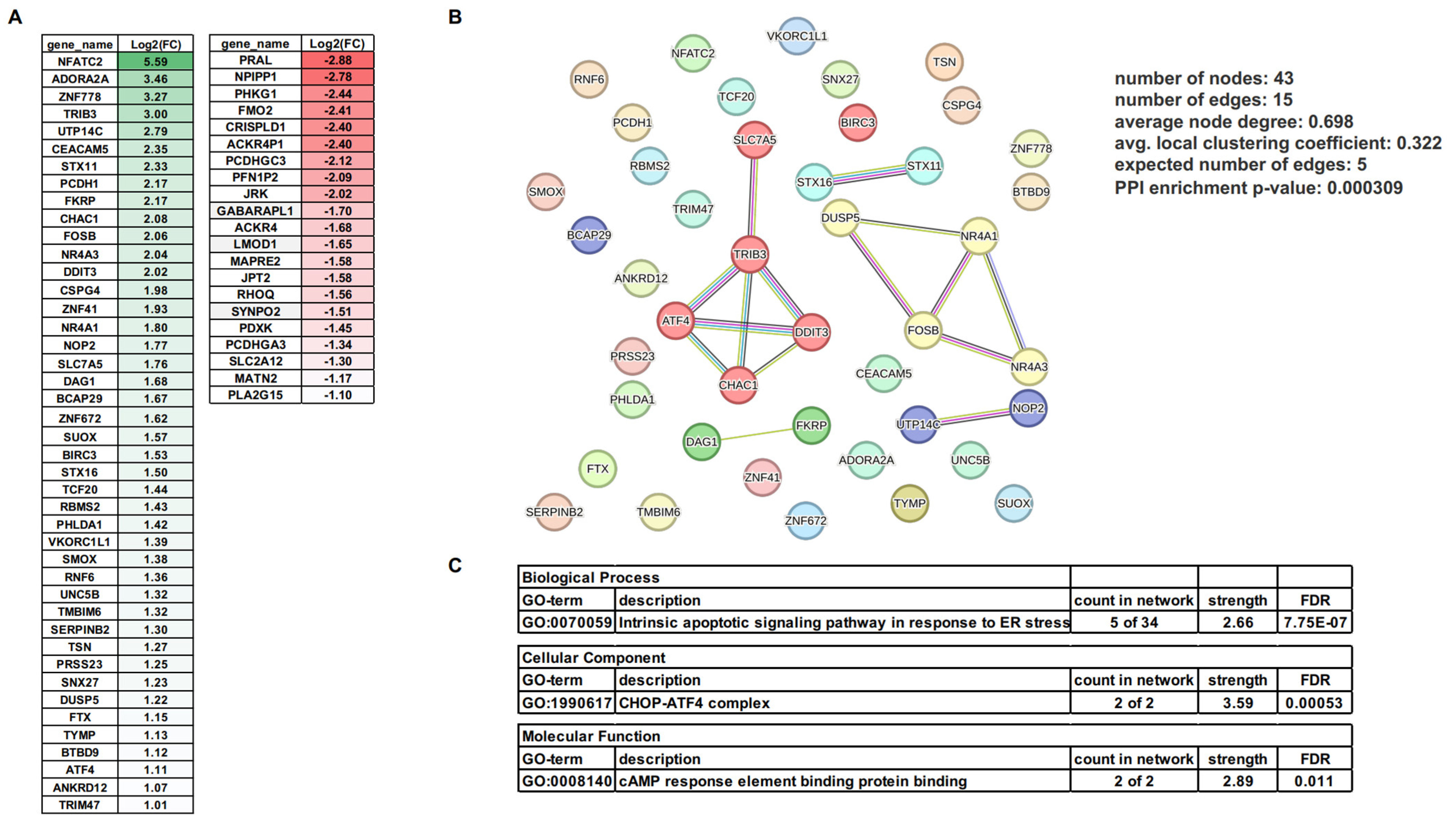
3.3. mRNA Changes Induced by MSU Crystals in Human VSMCs
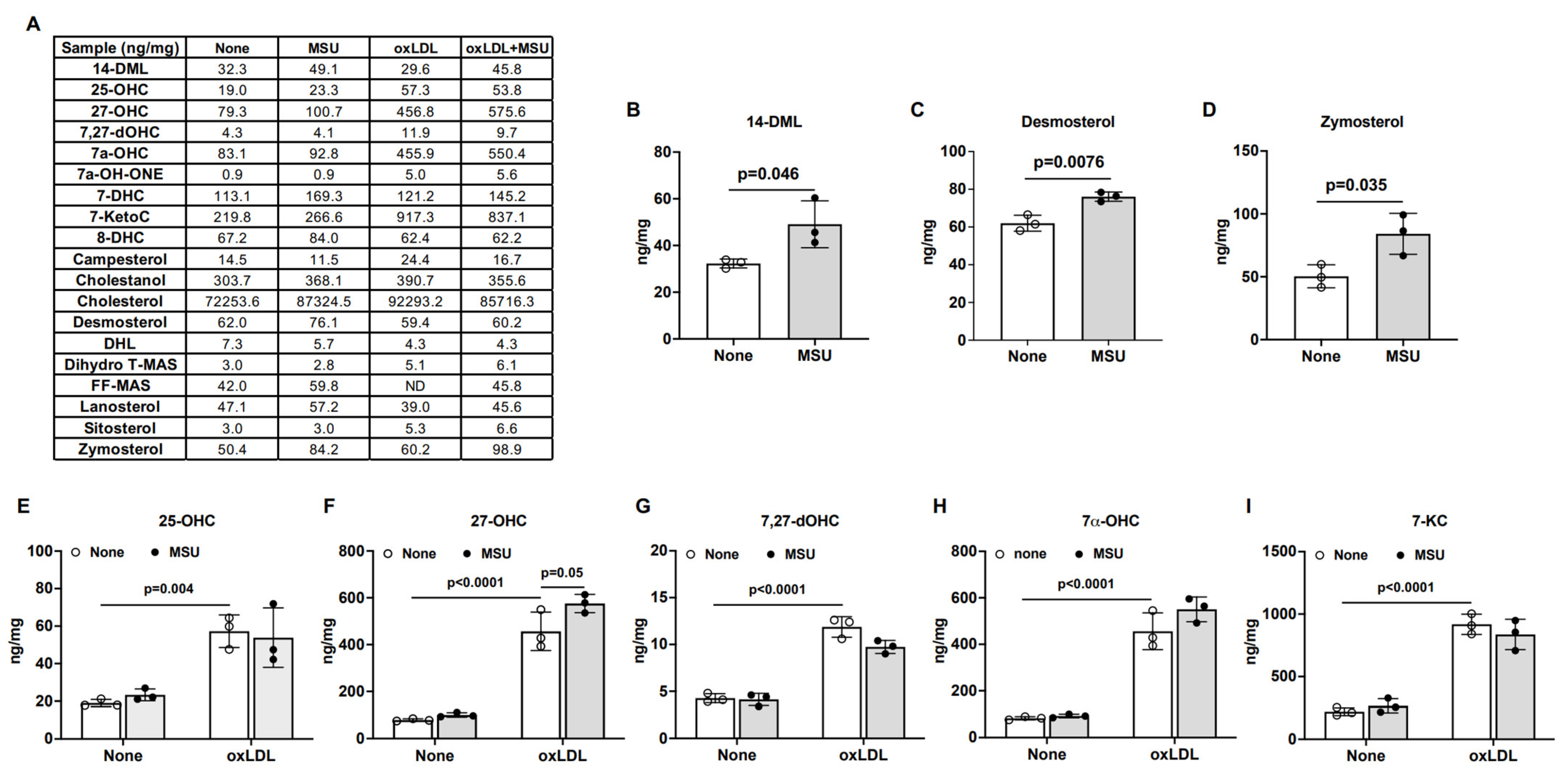
3.4. Effects of MSU Crystals on Intracellular Cholesterol Metabolism in Human VSMCs
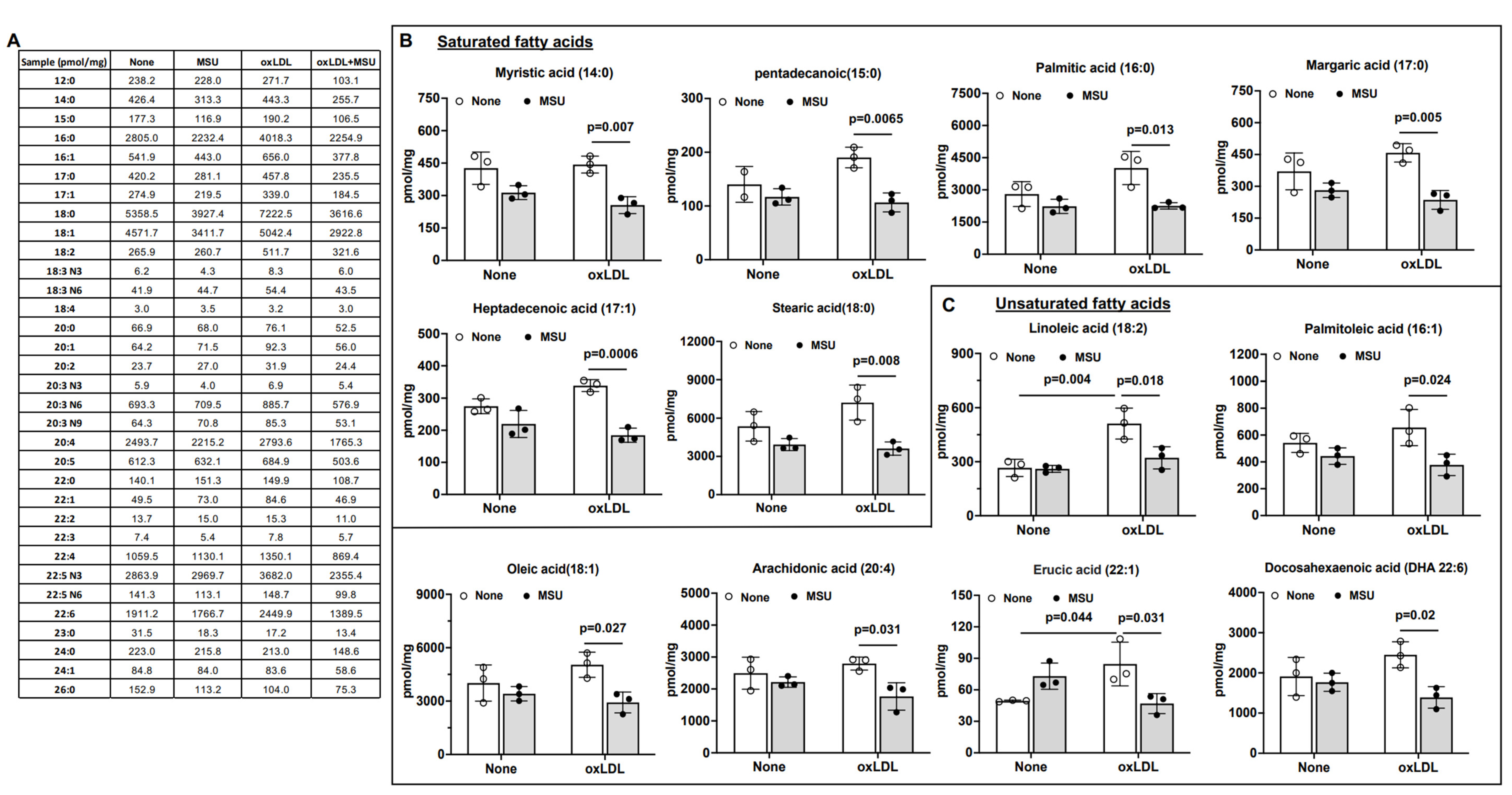
3.5. Impact of MSU Crystals on VSMC Intracellular Free Fatty Acids (FFAs)
3.6. Effects of MSU Crystals on VSMC Eicosanoid Release
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| MSU | monosodium urate |
| VSMCs | vascular smooth muscle cells |
| DEGs | differentially expressed genes |
| ER | endoplasmic reticulum |
| AA | arachidonic acid |
| XO | xanthine oxidase |
| CNS | central nervous system |
| BCP | basic calcium phosphate |
| DECT | dual-energy computed tomography |
| LDs | lipid droplets |
| PDGF | platelet-derived growth factor |
| oxLDL | oxidized low-density lipoprotein |
| PBS | phosphate-buffered saline |
| LPS | lipopolysaccharide |
| LC3B | microtubule-associated protein 1A/1B-light chain 3B |
| LC-MS | liquid chromatography–mass spectrometry |
| FFAs | free fatty acids |
| GC-MS | gas chromatography–mass spectrometry |
| MCFA | medium-chain free fatty acid |
| LCFC | long-chain free fatty acid |
| VLCFC | very-long-chain fatty acid |
| RP-UPLC-MS | reversed-phase ultra-performance liquid chromatography–mass spectrometry |
| ANOVA | analysis of variance |
| MAPKs | mitogen-activated protein kinases |
| JNKs | c-Jun N-terminal kinases |
| MCP | monocyte chemoattractant protein |
| cAMP | cyclic adenosine monophosphate |
| CREB | cAMP response element-binding protein |
| COX | cyclooxygenase |
| LOX | lipoxygenase |
| PG | prostaglandin |
| TX | thromboxane |
| 12-HHTrE | 12(S)-hydroxy-5Z,8E,10E-heptadecatrienoic acid |
| HETE | hydroxyeicosatetraenoic acid |
| HEPE | hydroxyeicosapentaenoic acid |
| HDoHE | hydroxydocoshexaenoic acid |
| dHDPA | dihydro-dipicolinic acid |
| HODE | hydroxyoctadecadienoic acid |
| HETrE | hydroxyeicosatrienoic acid |
| diHOME | dihydroxyoctadec-12-enoic acid |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| AA | arachidonic acid |
| LA | linolenic acid |
| 14-DML | 14-demethyl-lanosterol |
| OHC | hydroxycholesterol |
| DHC | dihydroxycholesterol |
| DHL | dihydrolanosterol |
| T-MAS | testis meiosis-activating sterol |
| FF-MAS | follicular fluid meiosis-activating sterol |
| dOHC | dihydroxycholesterol |
| KC | ketocholesterol |
| GABARAPL1 | GABA type A receptor-associated protein-like 1 |
| LMOD1 | leiomodin 1 |
| SYNPO2 | synaptopodin 2 |
| ATF4 | activating transcription factor 4 |
| CHOP (DDIT3) | C/EBP homologous protein (DNA-damage-inducible transcript 3) |
| NR4A1 | nuclear receptor subfamily 4 group A member 1 |
| NR4A3 | nuclear receptor subfamily 4 group A member 3 |
| FOSB | FosB proto-oncogene, AP-1 transcription factor subunit |
| DUSP | dual-specificity phosphatase |
| CHAC1 | ChaC glutathione-specific gamma-glutamylcyclotransferase 1 |
| TRIB3 | tribbles pseudokinase 3 |
| SLC7A5 | solute carrier family 7 member 5 |
| GABARAPL1 | GABAA-receptor-associated protein-like 1 |
| MYOCD | myocardin |
| SRF | serum response factor |
| UPR | unfolded protein response |
| LT | leukotriene |
| LXA4 | lipoxin A4 |
References
- Choi, H.K.; McCormick, N.; Yokose, C. Excess comorbidities in gout: The causal paradigm and pleiotropic approaches to care. Nat. Rev. Rheumatol. 2022, 18, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T.; Richette, P. Impact of comorbidities on gout and hyperuricaemia: An update on prevalence and treatment options. BMC Med. 2017, 15, 123. [Google Scholar] [CrossRef]
- Andrés, M.; Mendieta, L.; Argente-Del-Castillo, E.; Trigueros, M.; Miñano, A.; Pascual, E. Birefringent crystals deposition and inflammasome expression in human atheroma plaques by levels of uricemia. Jt. Bone Spine 2022, 89, 105423. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T.; Nguyen, Q.D.; Tran, K.M.; Le, N.H.; Do, M.D.; Richette, P.; Letavernier, E.; Correas, J.M.; Resche-Rigon, M. A cross-sectional study of 502 patients found a diffuse hyperechoic kidney medulla pattern in patients with severe gout. Kidney Int. 2021, 99, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Gawoski, J.M.; Balogh, K.; Landis, W.J. Aortic valvular tophus: Identification by X-ray diffraction of urate and calcium phosphates. J. Clin. Pathol. 1985, 38, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Scalapino, J.N.; Edwards, W.D.; Steckelberg, J.M.; Wooten, R.S.; Callahan, J.A.; Ginsburg, W.W. Mitral stenosis associated with valvular tophi. Mayo Clin. Proc. 1984, 59, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Patetsios, P.; Rodino, W.; Wisselink, W.; Bryan, D.; Kirwin, J.D.; Panetta, T.F. Identification of uric acid in aortic aneurysms and atherosclerotic artery. Ann. N. Y. Acad. Sci. 1996, 800, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Patetsios, P.; Song, M.; Shutze, W.P.; Pappas, C.; Rodino, W.; Ramirez, J.A.; Panetta, T.F. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am. J. Cardiol. 2001, 88, 188–191, A6. [Google Scholar] [CrossRef]
- Park, J.J.; Roudier, M.P.; Soman, D.; Mokadam, N.A.; Simkin, P.A. Prevalence of birefringent crystals in cardiac and prostatic tissues, an observational study. BMJ Open 2014, 4, e005308. [Google Scholar] [CrossRef]
- Klauser, A.S.; Halpern, E.J.; Strobl, S.; Gruber, J.; Feuchtner, G.; Bellmann-Weiler, R.; Weiss, G.; Stofferin, H.; Jaschke, W. Dual-Energy Computed Tomography Detection of Cardiovascular Monosodium Urate Deposits in Patients with Gout. JAMA Cardiol. 2019, 4, 1019–1028. [Google Scholar] [CrossRef]
- Elsayed, S.; Elsaid, K.A. Protein phosphatase 2A regulates xanthine oxidase-derived ROS production in macrophages and influx of inflammatory monocytes in a murine gout model. Front. Pharmacol. 2022, 13, 1033520. [Google Scholar] [CrossRef] [PubMed]
- Nardi, V.; Franchi, F.; Prasad, M.; Fatica, E.M.; Alexander, M.P.; Bois, M.C.; Lam, J.; Singh, R.J.; Meyer, F.B.; Lanzino, G.; et al. Uric Acid Expression in Carotid Atherosclerotic Plaque and Serum Uric Acid Are Associated with Cerebrovascular Events. Hypertension 2022, 79, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Ganji, M.; Nardi, V.; Prasad, M.; Jordan, K.L.; Bois, M.C.; Franchi, F.; Zhu, X.Y.; Tang, H.; Young, M.D.; Lerman, L.O.; et al. Carotid Plaques from Symptomatic Patients Are Characterized by Local Increase in Xanthine Oxidase Expression. Stroke 2021, 52, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, K.; Sharma, G.; Singh, K.; Osman, H.; A Gardecki, J.; Tearney, G.J. A novel approach for uric acid crystal detection in human coronary arteries with polarization-sensitive micro-OCT. Eur. Heart J. 2016, 39 (Suppl. S1), 570–571. [Google Scholar]
- Nishimiya, K.; Sharma, G.; Singh, K.; Gardecki, J.A.; Guillermo, T.J. A Novel Approach For Uric Acid Crystal Detection in Human Coronary Plaques Ex-Vivo With Cross-Polarized Micro-OCT. Circulation 2019, 140, A12843. [Google Scholar]
- Becce, F.; Ghoshhajra, B.; Choi, H.K. Identification of Cardiovascular Monosodium Urate Crystal Deposition in Patients With Gout Using Dual-Energy Computed Tomography. JAMA Cardiol. 2020, 5, 486. [Google Scholar] [CrossRef] [PubMed]
- Barazani, S.H.; Chi, W.-W.; Pyzik, R.; Chang, H.; Jacobi, A.; O’donnell, T.; A Fayad, Z.; Ali, Y.; Mani, V. Quantification of uric acid in vasculature of patients with gout using dual-energy computed tomography. World J. Radiol. 2020, 12, 184–194. [Google Scholar] [CrossRef]
- Pascart, T.; Carpentier, P.; Choi, H.K.; Norberciak, L.; Ducoulombier, V.; Luraschi, H.; Houvenagel, E.; Legrand, J.; Verclytte, S.; Becce, F.; et al. Identification and characterization of peripheral vascular color-coded DECT lesions in gout and non-gout patients: The VASCURATE study. Semin. Arthritis Rheum. 2021, 51, 895–902. [Google Scholar]
- Dalbeth, N.; Alhilali, M.; Riordan, P.; Narang, R.; Chhana, A.; McGlashan, S.; Doyle, A.; Andres, M. Vascular deposition of monosodium urate crystals in gout: Analysis of cadaveric tissue by dual-energy computed tomography and compensated polarizing light microscopy. Arthritis Rheumatol. 2022, 74, 1295–1296. [Google Scholar]
- Ban, X.; Li, Z.; Duan, Y.; Xu, K.; Xiong, J.; Tu, Y. Advanced imaging modalities provide new insights into coronary artery calcification. Eur. J. Radiol. 2022, 157, 110601. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- So, A.K.; Martinon, F. Inflammation in gout: Mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Han, L.; Ouyang, X.; Kahn, A.M.; Kanellis, J.; Li, P.; Feng, L.; Nakagawa, T.; Watanabe, S.; Hosoyamada, M.; et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am. J. Nephrol. 2005, 25, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Scott, P.; Sydlaske, A.; Rose, D.M.; Terkeltaub, R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005, 52, 2936–2946. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid. Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.R.; Yamamura, S.; Mureebe, L.; Itoh, H.; Kent, K. Smooth muscle cell migration and proliferation are mediated by distinct phases of activation of the intracellular messenger mitogen-activated protein kinase. J. Vasc. Surg. 1998, 27, 117–125. [Google Scholar] [CrossRef]
- Dubland, J.A.; Francis, G.A. So Much Cholesterol: The unrecognized importance of smooth muscle cells in atherosclerotic foam cell formation. Curr. Opin. Lipidol. 2016, 27, 155–161. [Google Scholar] [CrossRef]
- Rong, J.X.; Shapiro, M.; Trogan, E.; Fisher, E.A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl. Acad. Sci. USA 2003, 100, 13531–13536. [Google Scholar] [CrossRef]
- Huff, M.W.; Pickering, J.G. Can a vascular smooth muscle-derived foam-cell really change its spots? Arter. Thromb. Vasc. Biol. 2015, 35, 492–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giannotti, K.C.; Weinert, S.; Viana, M.N.; Leiguez, E.; Araujo, T.L.S.; Laurindo, F.R.M.; Lomonte, B.; Braun-Dullaeus, R.; Teixeira, C. A Secreted Phospholipase A2 Induces Formation of Smooth Muscle Foam Cells Which Transdifferentiate to Macrophage-Like State. Molecules 2019, 24, 3244. [Google Scholar] [CrossRef] [PubMed]
- Salabei, J.K.; Hill, B.G. Implications of autophagy for vascular smooth muscle cell function and plasticity. Free. Radic. Biol. Med. 2013, 65, 693–703. [Google Scholar] [CrossRef]
- Salabei, J.K.; Hill, B.G. Autophagic regulation of smooth muscle cell biology. Redox Biol. 2015, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Mauvezin, C.; Neufeld, T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 2015, 11, 1437–1438. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, J.; Cañes, L.; Alonso, J.; Ballester-Servera, C.; Rodríguez-Sinovas, A.; Corrales, I.; Rodríguez, C. NR4A3: A Key Nuclear Receptor in Vascular Biology, Cardiovascular Remodeling, and Beyond. Int. J. Mol. Sci. 2021, 22, 11371. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Curtiss, L.K.; Tenner, A.J.; Ginsberg, M.H. Lipoproteins containing apoprotein B are a major regulator of neutrophil responses to monosodium urate crystals. J. Clin. Investig. 1984, 73, 1719–1730. [Google Scholar] [CrossRef]
- Choi, S.-H.; Sviridov, D.; Miller, Y.I. Oxidized cholesteryl esters and inflammation. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2017, 1862, 393–397. [Google Scholar] [CrossRef]
- Zhang, X.; McDonald, J.G.; Aryal, B.; Canfrán-Duque, A.; Goldberg, E.L.; Araldi, E.; Ding, W.; Fan, Y.; Thompson, B.M.; Singh, A.K.; et al. Desmosterol suppresses macrophage inflammasome activation and protects against vascular inflammation and atherosclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2107682118. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Lee, G.-L.; Yeh, C.-C.; Wu, J.-Y.; Lin, H.-C.; Wang, Y.-F.; Kuo, Y.-Y.; Hsieh, Y.-T.; Hsu, Y.-J.; Kuo, C.-C. TLR2 Promotes Vascular Smooth Muscle Cell Chondrogenic Differentiation and Consequent Calcification via the Concerted Actions of Osteoprotegerin Suppression and IL-6-Mediated RANKL Induction. Arter. Thromb. Vasc. Biol. 2019, 39, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, M.; Li, X.; Zhao, R.; Zhao, Y.; Liu, L.; Wang, S. Role of Endoplasmic Reticulum Stress in Atherosclerosis and Its Potential as a Therapeutic Target. Oxidative Med. Cell. Longev. 2020, 2020, 9270107. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, Y.; Zhao, C. CHOP Increases TRIB3-Dependent miR-208 Expression to Potentiate Vascular Smooth Muscle Cell Proliferation and Migration by Downregulating TIMP3 in Atherosclerosis. Cardiovasc. Drugs Ther. 2022, 36, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, J.; Hou, H.; Chen, D. The relationship with the stability between GRP78, CHOP and human carotid atherosclerotic plaque. Clin. Neurol. Neurosurg. 2022, 212, 107067. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Miyazaki-Anzai, S.; Keenan, A.L.; Shiozaki, Y.; Okamura, K.; Chick, W.S.; Williams, K.; Zhao, X.; Rahman, S.M.; Tintut, Y.; et al. Activating transcription factor-4 promotes mineralization in vascular smooth muscle cells. JCI Insight. 2016, 1, e88646. [Google Scholar] [CrossRef]
- Schauer, I.E.; Knaub, L.A.; Lloyd, M.; Watson, P.A.; Gliwa, C.; Lewis, K.E.; Chait, A.; Klemm, D.J.; Gunter, J.M.; Bouchard, R.; et al. CREB downregulation in vascular disease: A common response to cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 733–741. [Google Scholar] [CrossRef]
- Boyer-Guittaut, M.; Poillet, L.; Liang, Q.; Bôle-Richard, E.; Ouyang, X.; Benavides, G.A.; Chakrama, F.Z.; Fraichard, A.; Darley-Usmar, V.M.; Despouy, G.; et al. The role of GABARAPL1/GEC1 in autophagic flux and mitochondrial quality control in MDA-MB-436 breast cancer cells. Autophagy 2014, 10, 986–1003. [Google Scholar] [CrossRef]
- Allaeys, I.; Marceau, F.; E Poubelle, P. NLRP3 promotes autophagy of urate crystals phagocytized by human osteoblasts. Arthritis Res. Ther. 2013, 15, R176. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, S.; Xu, W.; Sun, E. Downregulation of Sox8 mediates monosodium urate crystal-induced autophagic impairment of cartilage in gout arthritis. Cell Death Discov. 2023, 9, 95. [Google Scholar] [CrossRef]
- Perisic Matic, L.; Rykaczewska, U.; Razuvaev, A.; Sabater-Lleal, M.; Lengquist, M.; Miller, C.L.; Ericsson, I.; Röhl, S.; Kronqvist, M.; Aldi, S.; et al. Phenotypic Modulation of Smooth Muscle Cells in Atherosclerosis Is Associated With Downregulation of LMOD1, SYNPO2, PDLIM7, PLN, and SYNM. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1947–1961. [Google Scholar] [CrossRef]
- Turczyńska, K.M.; Swärd, K.; Hien, T.T.; Wohlfahrt, J.; Mattisson, I.Y.; Ekman, M.; Nilsson, J.; Sjögren, J.; Murugesan, V.; Hultgårdh-Nilsson, A.; et al. Regulation of smooth muscle dystrophin and synaptopodin 2 expression by actin polymerization and vascular injury. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Umetani, M.; Ghosh, P.; Ishikawa, T.; Umetani, J.; Ahmed, M.; Mineo, C.; Shaul, P.W. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2019, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wachsmann-Maga, A.; Kaszuba, M.; Maga, M.; Włodarczyk, A.; Krężel, J.; Kaczmarczyk, P.; Bogucka, K.; Maga, P. Leukotrienes in the atherosclerotic cardiovascular diseases—A systematic review. Acta Angiol. 2022, 28, 147–153. [Google Scholar] [CrossRef]
- Piper, K.; Garelnabi, M. Eicosanoids: Atherosclerosis and cardiometabolic health. J. Clin. Transl. Endocrinol. 2020, 19, 100216. [Google Scholar] [CrossRef] [PubMed]
- Beccacece, L.; Abondio, P.; Bini, C.; Pelotti, S.; Luiselli, D. The Link between Prostanoids and Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 4193. [Google Scholar] [CrossRef] [PubMed]
- Maga, P.; Sanak, M.; Rewerska, B.; Maga, M.; Jawien, J.; Wachsmann, A.; Rewerski, P.; Szczeklik, W.; Celejewska-Wójcik, N. Urinary cysteinyl leukotrienes in one-year follow-up of percutaneous transluminal angioplasty for peripheral arterial occlusive disease. Atherosclerosis 2016, 249, 174–180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu-Bryan, R.; Guo, T.; Lee, J.; Terkeltaub, R. Atherogenic Activation of Human Vascular Smooth Muscle Cells by Monosodium Urate Crystals. Gout Urate Cryst. Depos. Dis. 2023, 1, 192-207. https://doi.org/10.3390/gucdd1030016
Liu-Bryan R, Guo T, Lee J, Terkeltaub R. Atherogenic Activation of Human Vascular Smooth Muscle Cells by Monosodium Urate Crystals. Gout, Urate, and Crystal Deposition Disease. 2023; 1(3):192-207. https://doi.org/10.3390/gucdd1030016
Chicago/Turabian StyleLiu-Bryan, Ru, Tracy Guo, Jennifer Lee, and Robert Terkeltaub. 2023. "Atherogenic Activation of Human Vascular Smooth Muscle Cells by Monosodium Urate Crystals" Gout, Urate, and Crystal Deposition Disease 1, no. 3: 192-207. https://doi.org/10.3390/gucdd1030016
APA StyleLiu-Bryan, R., Guo, T., Lee, J., & Terkeltaub, R. (2023). Atherogenic Activation of Human Vascular Smooth Muscle Cells by Monosodium Urate Crystals. Gout, Urate, and Crystal Deposition Disease, 1(3), 192-207. https://doi.org/10.3390/gucdd1030016





