Determination of the Activation Energy of the Thermal Isomerization of Oleic Acid with Raman Spectroscopy and Partial Least Squares Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Raman Spectroscopy
2.2. Preparation of Mixed Solutions of Elaidic Acid and Analysis of the Raman Spectra
2.3. Thermal Isomerization of Oleic Acid
3. Results and Discussion
3.1. Prediction of the Fatty Acids Using PLS Regression
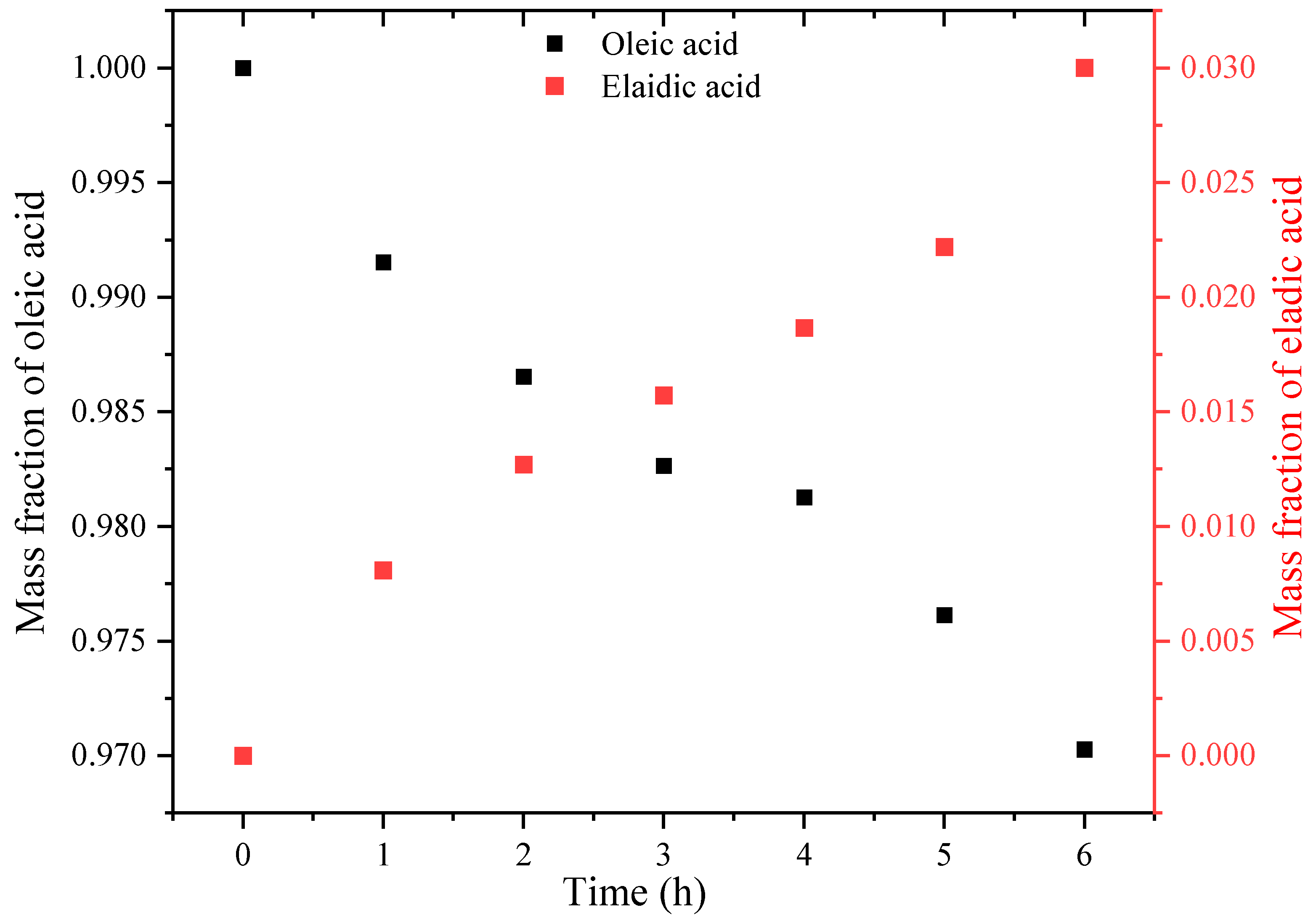
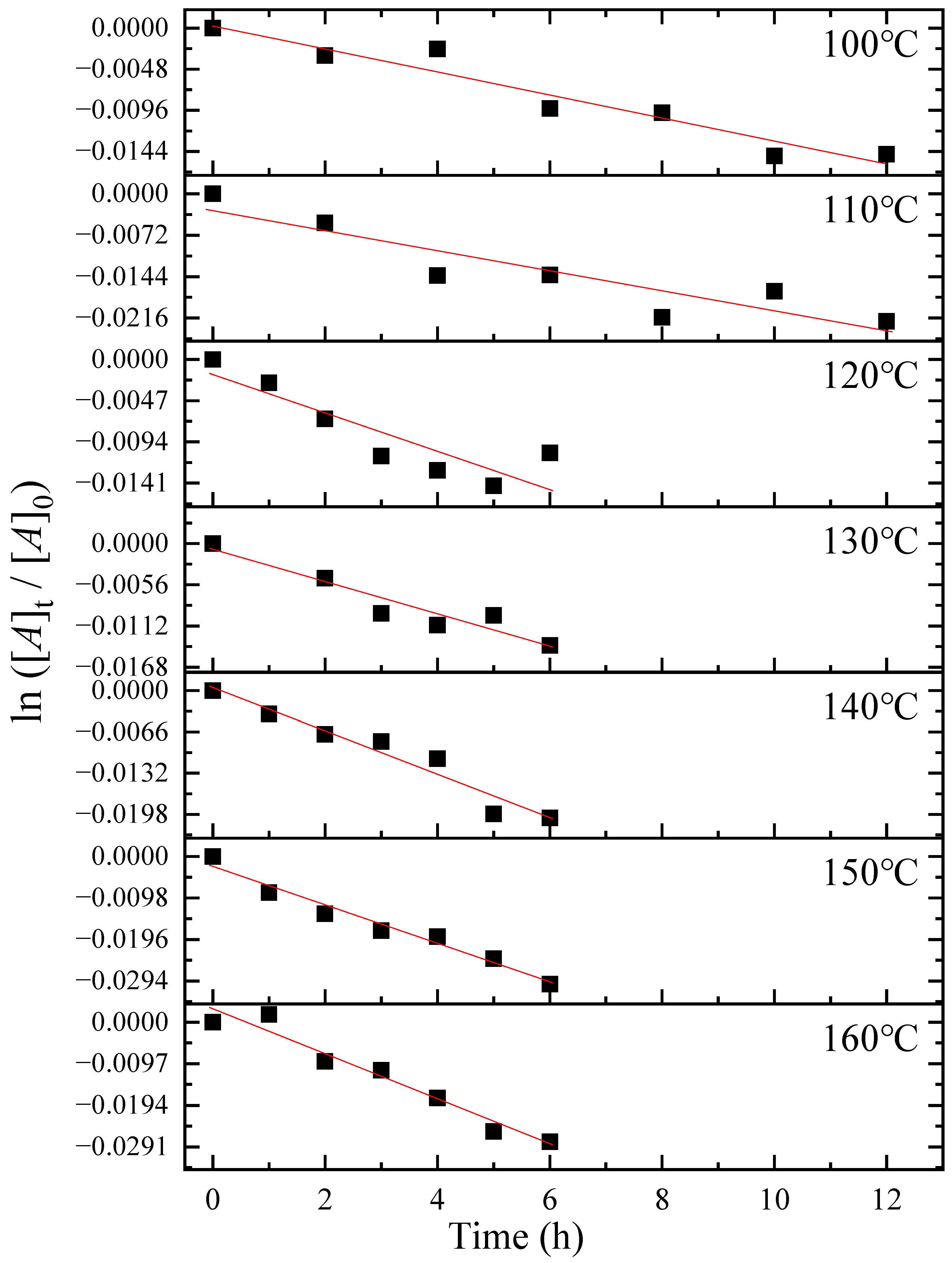
3.2. Thermal Isomerization of Oleic Acid
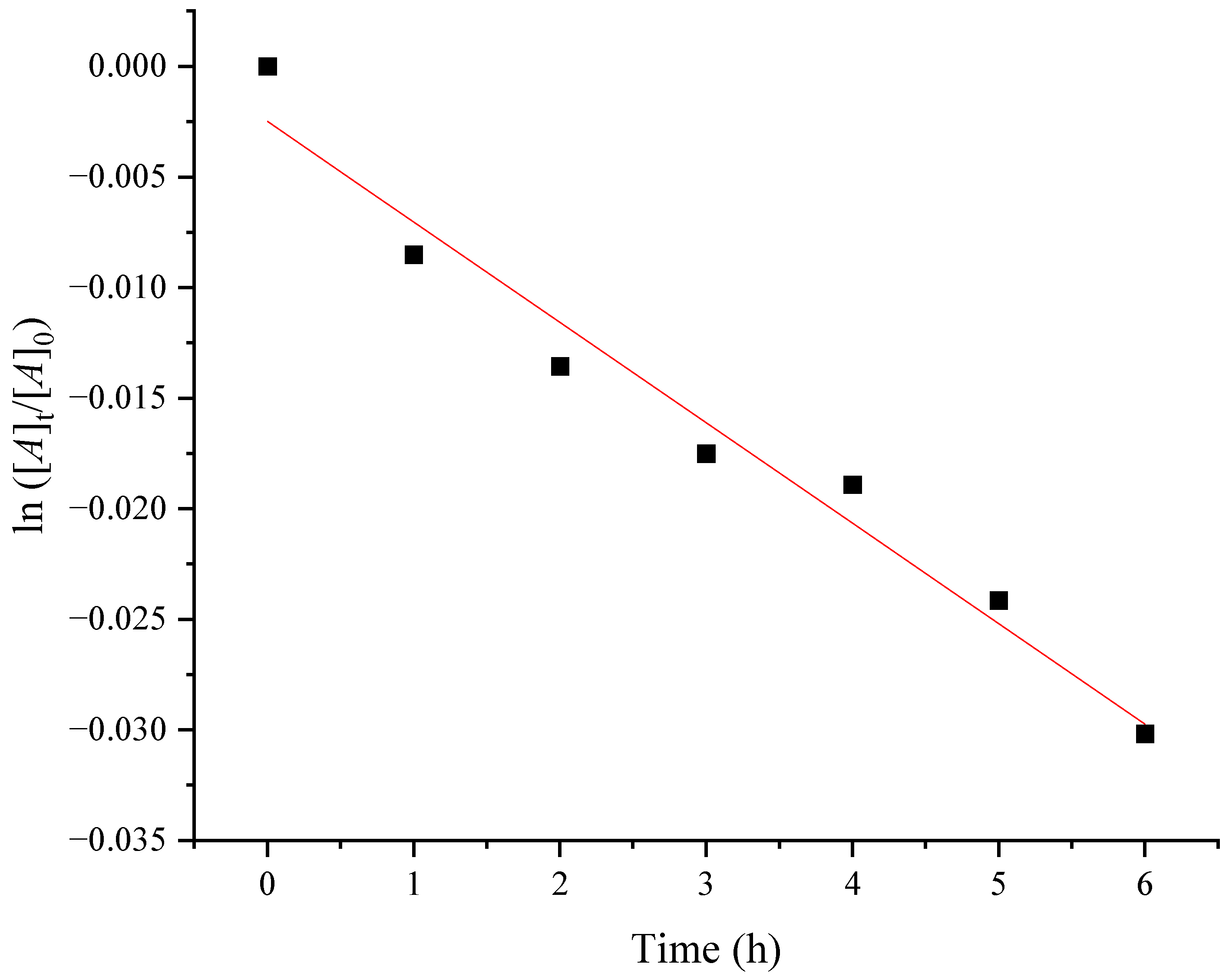
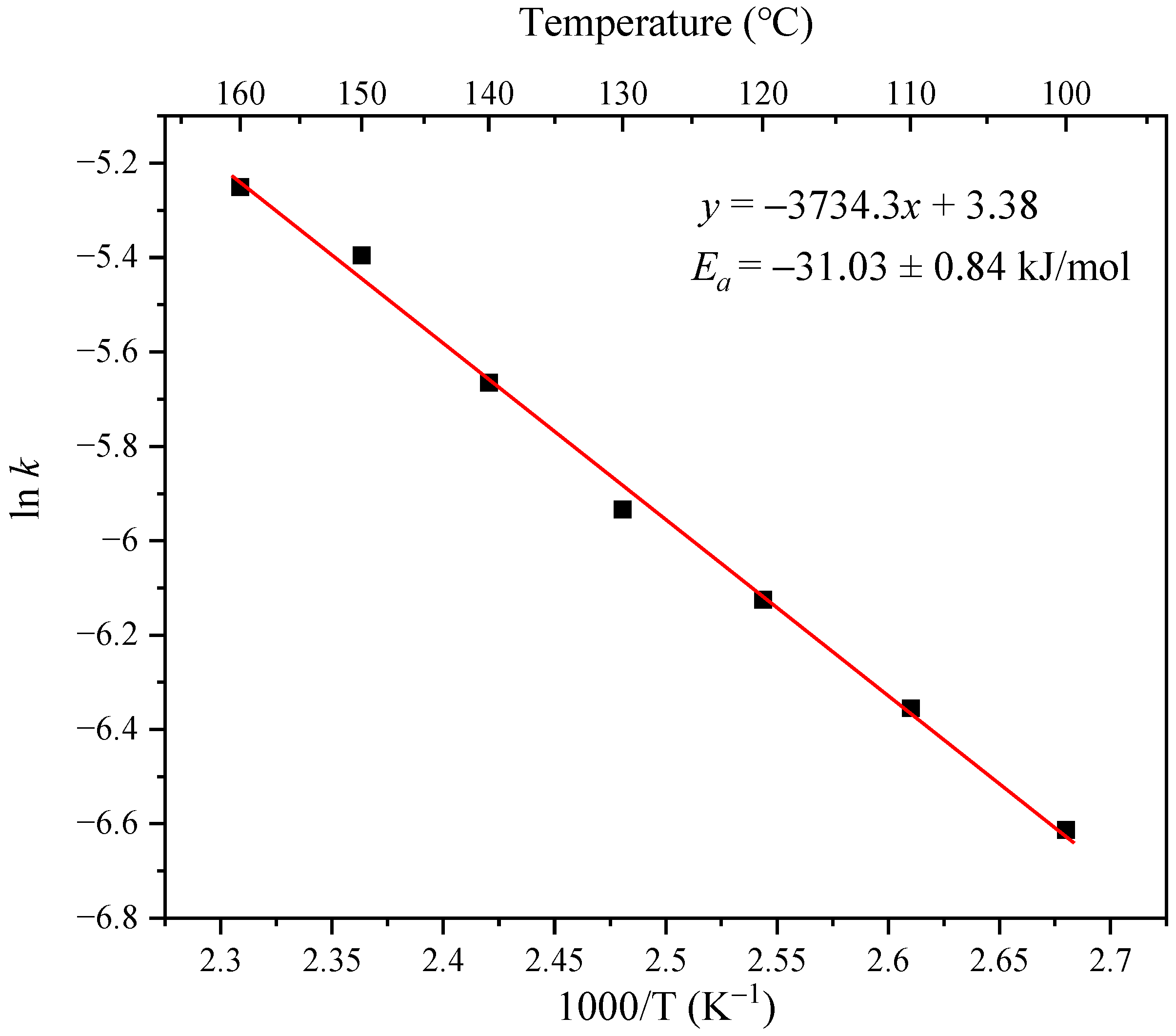
3.3. Activation Energy of the Thermal Isomerization of Oleic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rustan, A.C.; Drevon, C.A. Fatty Acids: Structures and Properties. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Shah, B.; Thadani, U. Trans Fatty Acids Linked to Myocardial Infarction and Stroke: What Is the Evidence? Trends Cardiovasc. Med. 2019, 29, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, G.H. Cis-Trans Isomerization of Unsaturated Fatty Acids in Edible Oils to Prepare Trans Fat. Grasas Aceites 2018, 69, e268. [Google Scholar] [CrossRef]
- Martin, C.A.; Milinsk, M.C.; Visentainer, J.V.; Matsushita, M.; de-Souza, N.E. Trans Fatty Acid-Forming Processes in Foods: A Review. An. Acad. Bras. Ciênc. 2007, 79, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Passi, S.J.; Misra, A. Overview of Trans Fatty Acids: Biochemistry and Health Effects. Diabetes Metab. Syndr. Clin. Res. Rev. 2011, 5, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Sugano, M. Industrial Trans Fatty Acid and Serum Cholesterol: The Allowable Dietary Level. J. Lipids 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Brouwer, I.A.; Wanders, A.J.; Katan, M.B. Effect of Animal and Industrial Trans Fatty Acids on HDL and LDL Cholesterol Levels in Humans—A Quantitative Review. PLoS ONE 2010, 5, e9434. [Google Scholar] [CrossRef]
- Li, C.; Cobb, L.K.; Vesper, H.W.; Asma, S. Global Surveillance of Trans-Fatty Acids. Prev. Chronic Dis. 2019, 16, E147. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Stein, P.K.; Imamura, F.; Sattelmair, J.; Lemaitre, R.N.; Siscovick, D.S.; Mota, J.; Mozaffarian, D. Trans-Fatty Acid Consumption and Heart Rate Variability in 2 Separate Cohorts of Older and Younger Adults. Circ. Arrhythmia Electrophysiol. 2012, 5, 728–738. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans Fatty Acids and Lipid Profile: A Serious Risk Factor to Cardiovascular Disease, Cancer and Diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1643–1647. [Google Scholar] [CrossRef]
- Uauy, R.; Aro, A.; Clarke, R.; Ghafoorunissa; L’Abbé, M.R.; Mozaffarian, D.; Skeaff, C.M.; Stender, S.; Tavella, M. WHO Scientific Update on Trans Fatty Acids: Summary and Conclusions. Eur. J. Clin. Nutr. 2009, 63, S68–S75. [Google Scholar] [CrossRef]
- World Health Organization. Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children: WHO Guideline Summary; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/handle/10665/375034 (accessed on 17 July 2023).
- Eder, K. Gas Chromatographic Analysis of Fatty Acid Methyl Esters. J. Chromatogr. B Biomed. Sci. Appl. 1995, 671, 113–131. [Google Scholar] [CrossRef]
- Seppänen-Laakso, T.; Laakso, I.; Hiltunen, R. Analysis of Fatty Acids by Gas Chromatography, and Its Relevance to Research on Health and Nutrition. Anal. Chim. Acta 2002, 465, 39–62. [Google Scholar] [CrossRef]
- Chiu, H.-H.; Kuo, C.-H. Gas Chromatography-Mass Spectrometry-Based Analytical Strategies for Fatty Acid Analysis in Biological Samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef]
- May, W.E.; Cavanagh, R.R.; Turk, G.C.; Winchester, M.; Travis, J.; Smith, M.V.; DeRose, P.; Choquette, S.J.; Kramer, G.W.; Sieber, J.R.; et al. Analytical Chemistry. In Springer Handbook of Metrology and Testing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; pp. 145–203. [Google Scholar] [CrossRef]
- Hasegawa, T. Infrared Spectroscopy as a Vibrational Spectroscopy. In Quantitative Infrared Spectroscopy for Understanding of a Condensed Matter; Springer: Tokyo, Japan, 2017; pp. 1–36. [Google Scholar] [CrossRef]
- Othman, N. IR Spectroscopy in Qualitative and Quantitative Analysis. In Infrared Spectroscopy—Perspectives and Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Sinclair, R.G.; McKay, A.F.; Myers, G.S.; Jones, R.N. The Infrared Absorption Spectra of Unsaturated Fatty Acids and Esters1. J. Am. Chem. Soc. 1952, 74, 2578–2585. [Google Scholar] [CrossRef]
- Christy, A.A.; Egeberg, P.K. Quantitative Determination of Saturated and Unsaturated Fatty Acids in Edible Oils by Infrared Spectroscopy and Chemometrics. Chemom. Intell. Lab. Syst. 2006, 82, 130–136. [Google Scholar] [CrossRef]
- Mossoba, M.M.; Azizian, H.; Kramer, J.K.G. Application of infrared spectroscopy to the rapid determination of total saturated, trans, monounsaturated, and polyunsaturated fatty acids In AOCS Lipid Library, Oils and Fats; AOCS: Urbana, IL, USA, 2019; Available online: https://www.aocs.org/resource/application-of-infrared-spectroscopy-to-the-rapid-determination-of-total-saturated-trans-monounsaturated-and-polyunsaturated-fatty-acids/ (accessed on 17 July 2023).
- Sherazi, S.T.H.; Kandhro, A.; Mahesar, S.A.; Bhanger, M.I.; Talpur, M.Y.; Arain, S. Application of Transmission FT-IR Spectroscopy for the Trans Fat Determination in the Industrially Processed Edible Oils. Food Chem. 2009, 114, 323–327. [Google Scholar] [CrossRef]
- Rensonnet, A.; Tipping, W.J.; Malherbe, C.; Faulds, K.; Eppe, G.; Graham, D. Spectral Fingerprinting of Cellular Lipid Droplets Using Stimulated Raman Scattering Microscopy and Chemometric Analysis. Analyst 2024, 149, 553–562. [Google Scholar] [CrossRef]
- Baranov, D.; Lynch, M.J.; Curtis, A.C.; Carollo, A.R.; Douglass, C.R.; Mateo-Tejada, A.M.; Jonas, D.M. Purification of Oleylamine for Materials Synthesis and Spectroscopic Diagnostics for Trans Isomers. Chem. Mater. 2019, 31, 1223–1230. [Google Scholar] [CrossRef]
- Numata, Y.; Kobayashi, H.; Oonami, N.; Kasai, Y.; Tanaka, H. Simultaneous Determination of Oleic and Elaidic Acids in Their Mixed Solution by Raman Spectroscopy. J. Mol. Struct. 2019, 1185, 200–204. [Google Scholar] [CrossRef]
- Rahmania, H.; Indrayanto, G.; Windarsih, A.; Fernando, D.; Bakar, N.K.A.; Rohman, A. Standard Review of the Application of Molecular Spectroscopic and Chromatographic-Based Methods for Determination of Trans Fatty Acids in Food Products. Appl. Food Res. 2025, 5, 100657. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman Spectroscopy of Lipids: A Review. J. Raman Spectrosc. 2014, 46, 4–20. [Google Scholar] [CrossRef]
- He, C.; Qin, L.; Yang, J.; Zhu, Y. Revealing the Heat-Induced Cis-Trans Isomerization of Unsaturated Fatty Acids in Camellia Oil. LWT 2023, 173, 114293. [Google Scholar] [CrossRef]
- Cheng, N.; Zhang, J.; Yin, J.; Li, S. Computational and Experimental Research on Mechanism of Cis/Trans Isomerization of Oleic Acid. Heliyon 2018, 4, e00768. [Google Scholar] [CrossRef] [PubMed]
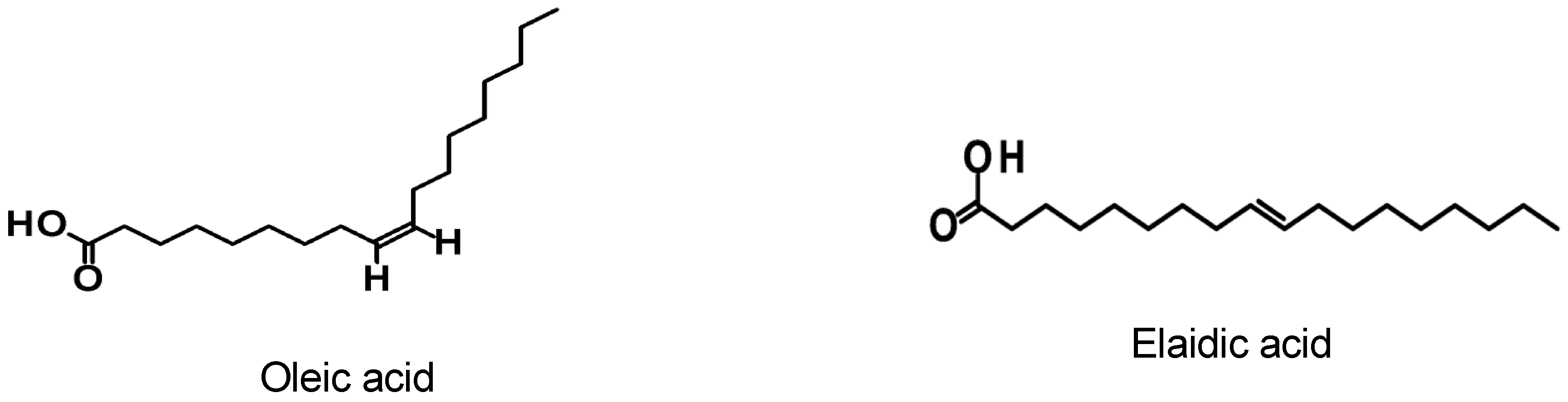
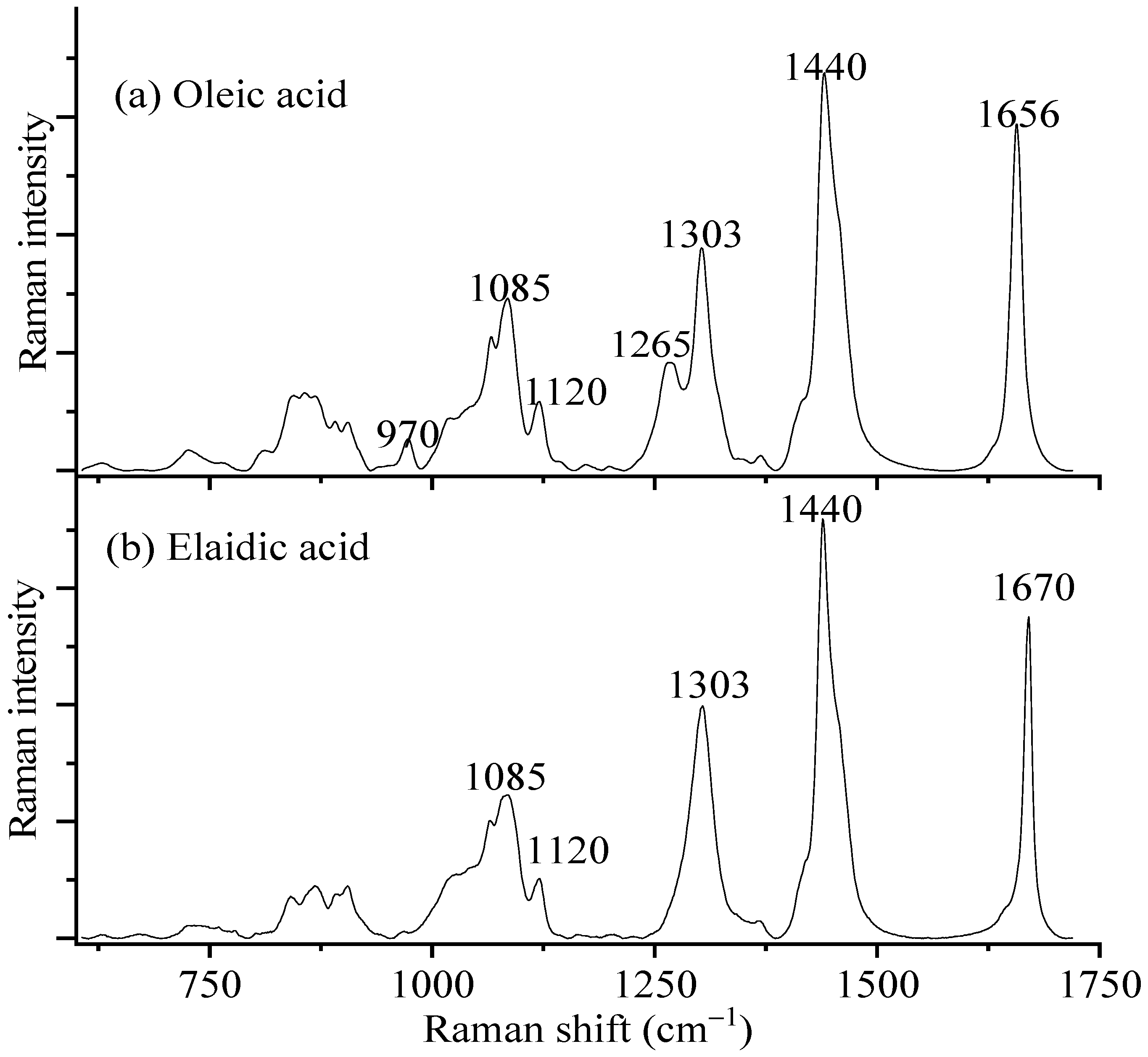


| Raman Shift (cm−1) | Assignments [27] | |
|---|---|---|
| Oleic Acid | Elaidic Acid | |
| 970 | – | C-H out-of-plane bending |
| 1065 | 1065 | C-C antisym. stretching |
| 1085 | 1085 | C-C symmetry stretching (COOH side) |
| 1120 | 1120 | C-C symmetry stretching (CH3 side) |
| 1265 | – | C-H in-plane stretching |
| 1303 | 1303 | CH3 twisting |
| 1440 | 1440 | CH2 stretching |
| 1656 | 1670 | C=C stretching |
| Oleic Acid | Elaidic Acid | ||
|---|---|---|---|
| Actual Mass Fraction | Estimated Mass Fraction | Actual Mass Fraction | Estimated Mass Fraction |
| 1.0000 | 1.0000 ± 0.0011 | 0.0000 | 0.0001 ± 0.0011 |
| 0.9000 | 0.9007 ± 0.0098 | 0.1000 | 0.1003 ± 0.0010 |
| 0.7999 | 0.8032 ± 0.0010 | 0.2001 | 0.2030 ± 0.0010 |
| 0.7002 | 0.7019 ± 0.0012 | 0.2998 | 0.3013 ± 0.0012 |
| 0.5998 | 0.6023 ± 0.0011 | 0.4002 | 0.4022 ± 0.0011 |
| 0.5001 | 0.5012 ± 0.0010 | 0.4999 | 0.5010 ± 0.0010 |
| RMSEP | 0.0045 | RMSEP | 0.0041 |
| Temperature (°C) | Rate Constant (h−1) × 103 |
|---|---|
| 100 | 1.34 ± 0.16 |
| 110 | 1.74 ± 0.34 |
| 120 | 2.19 ± 0.52 |
| 130 | 2.65 ± 0.35 |
| 140 | 3.47 ± 0.37 |
| 150 | 4.54 ± 0.36 |
| 160 | 5.25 ± 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, A.; Numata, Y. Determination of the Activation Energy of the Thermal Isomerization of Oleic Acid with Raman Spectroscopy and Partial Least Squares Regression. Spectrosc. J. 2025, 3, 17. https://doi.org/10.3390/spectroscj3020017
Watanabe A, Numata Y. Determination of the Activation Energy of the Thermal Isomerization of Oleic Acid with Raman Spectroscopy and Partial Least Squares Regression. Spectroscopy Journal. 2025; 3(2):17. https://doi.org/10.3390/spectroscj3020017
Chicago/Turabian StyleWatanabe, Akihiro, and Yasushi Numata. 2025. "Determination of the Activation Energy of the Thermal Isomerization of Oleic Acid with Raman Spectroscopy and Partial Least Squares Regression" Spectroscopy Journal 3, no. 2: 17. https://doi.org/10.3390/spectroscj3020017
APA StyleWatanabe, A., & Numata, Y. (2025). Determination of the Activation Energy of the Thermal Isomerization of Oleic Acid with Raman Spectroscopy and Partial Least Squares Regression. Spectroscopy Journal, 3(2), 17. https://doi.org/10.3390/spectroscj3020017






