The Triangle: Carotenoids–Retinoids–Cytochromes Govern Essential Functions for Development and Progression of Cancer
Abstract
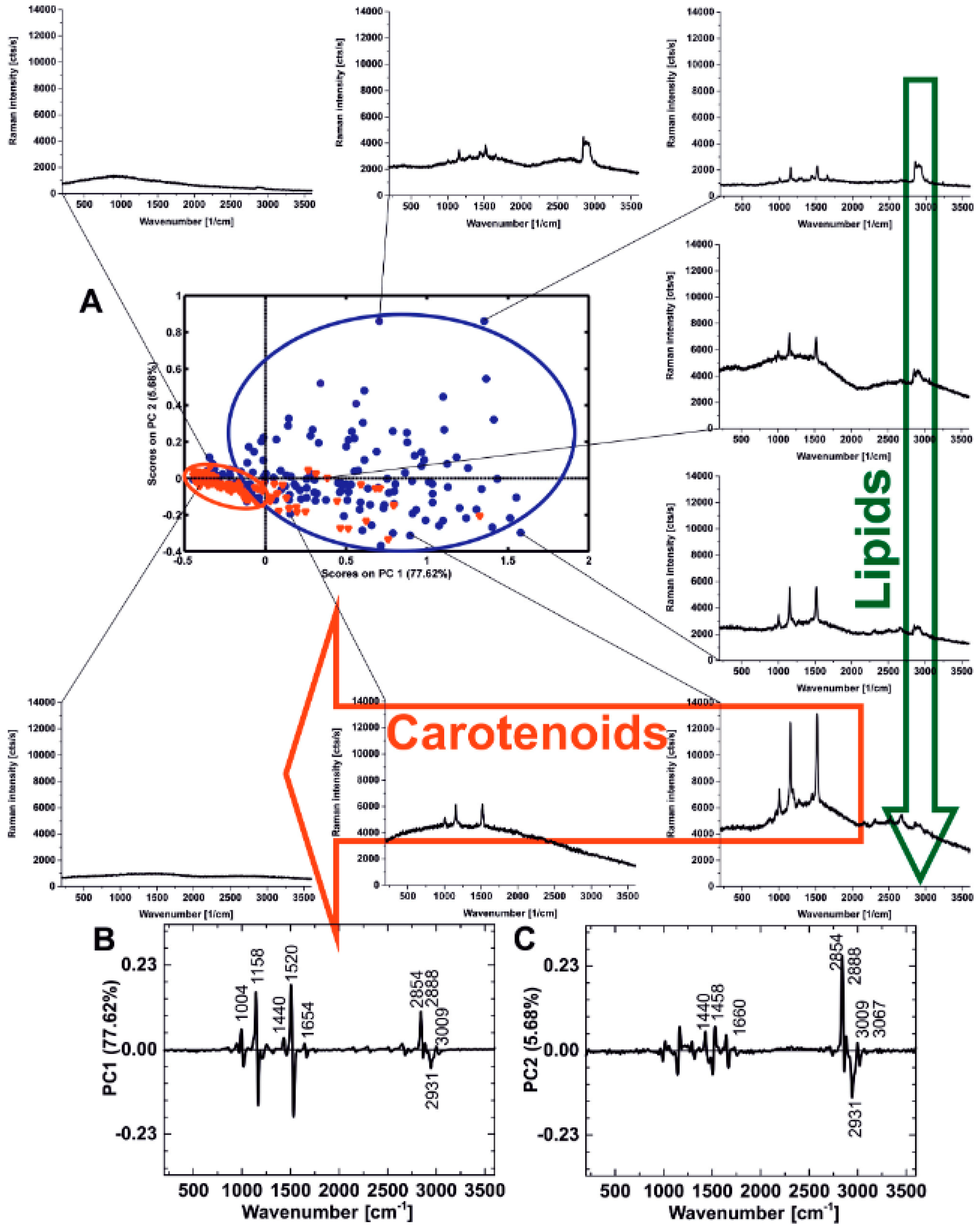
1. The Impact of Carotenoids on Cancers
2. The Impact of Retinoic Acid on Cancers and Immunity
2.1. Retinoid-Binding Proteins and Retinoic Acid Nuclear Receptors
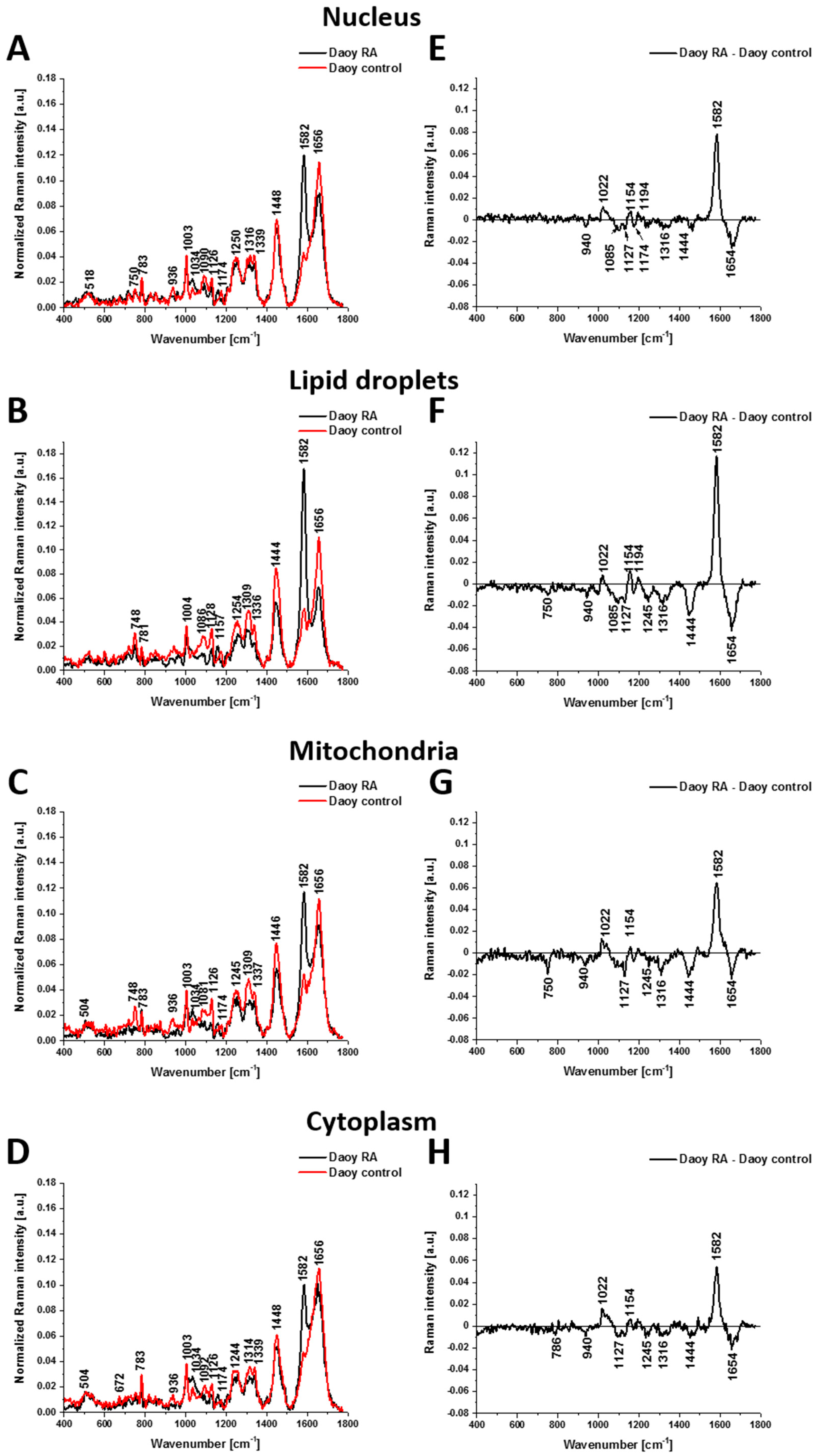
2.2. Retinoids in Cancers Studied by Raman Imaging
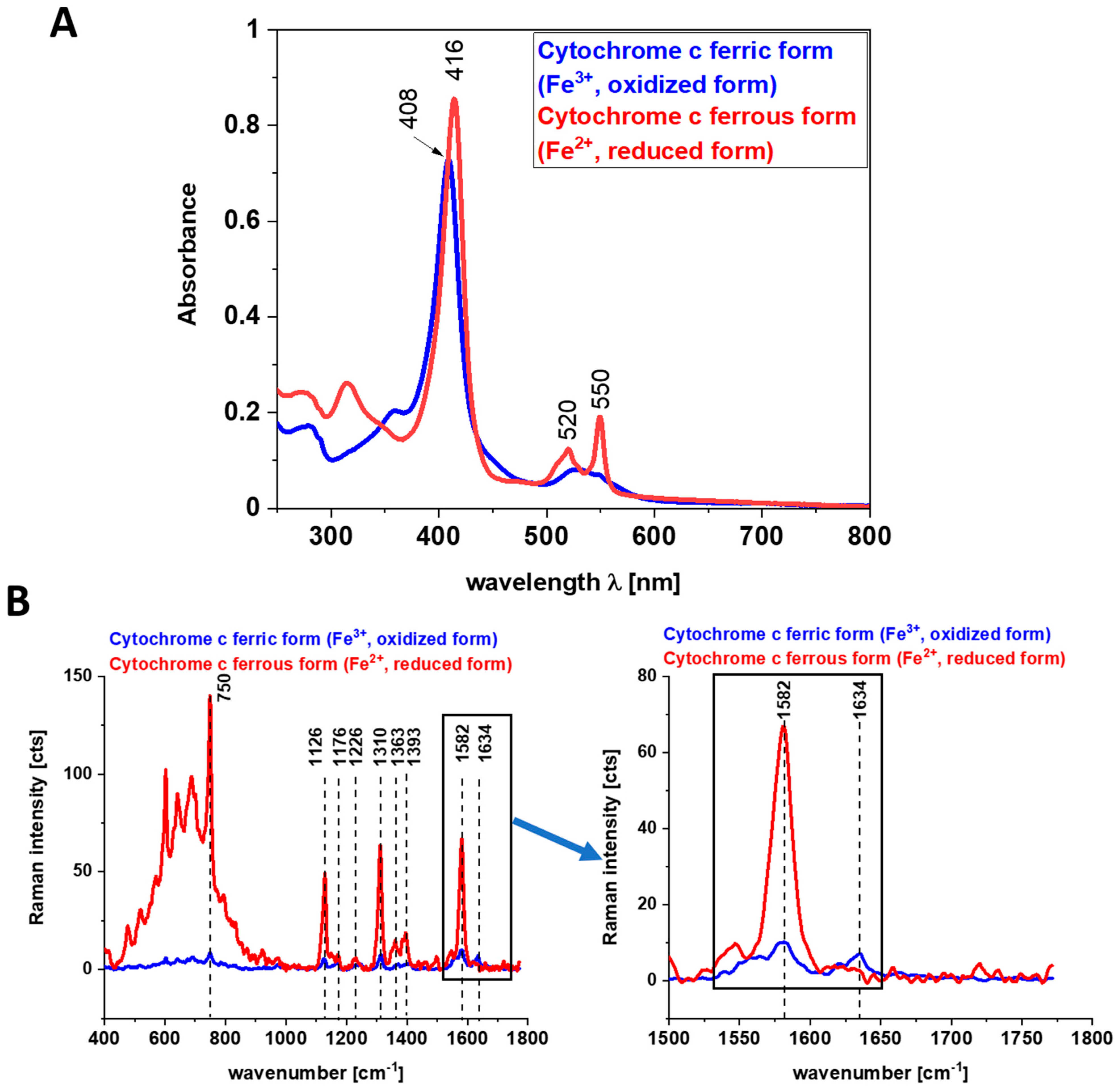
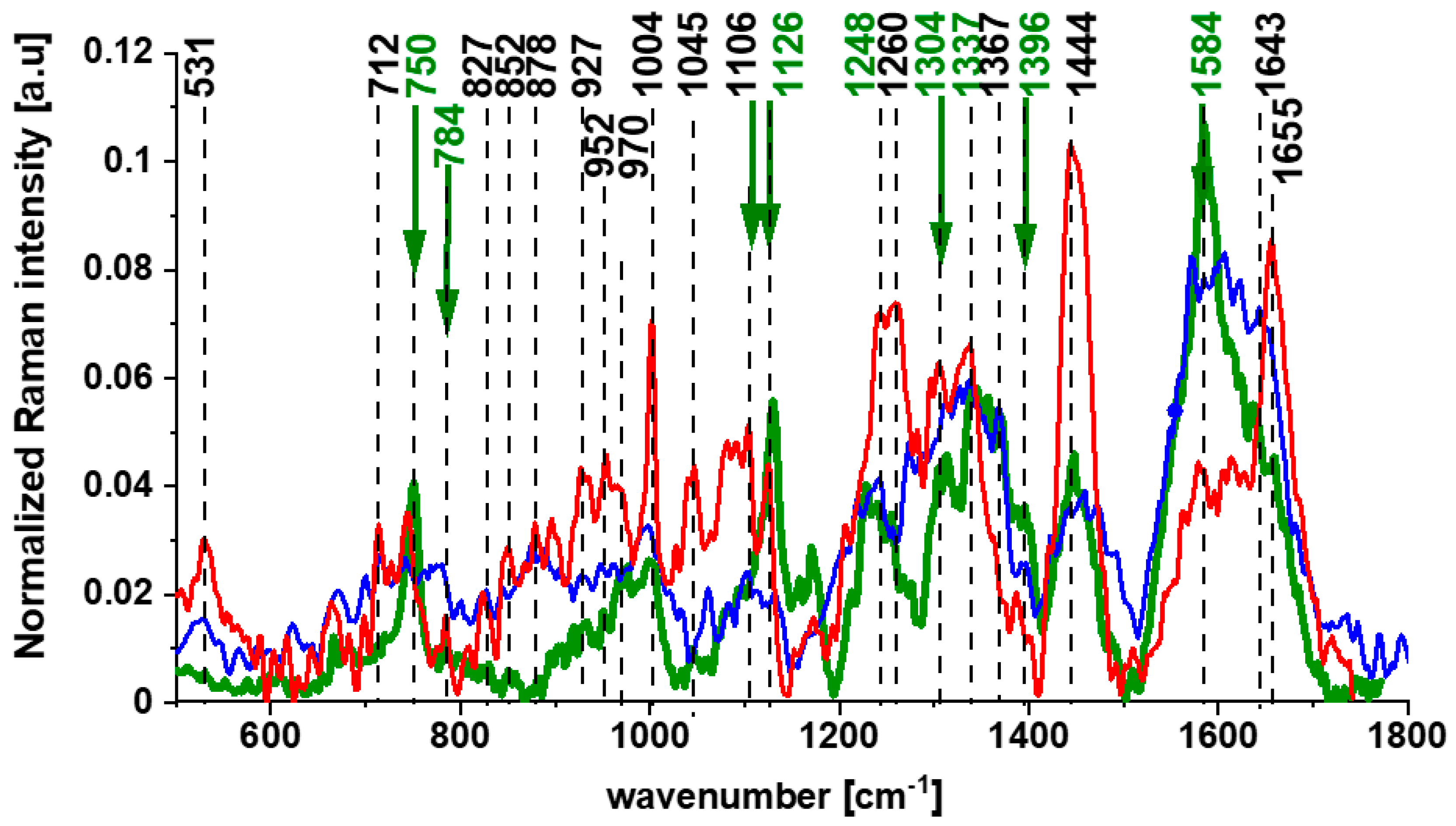
3. The Impact of Cytochrome c on Cancers and Immunity
Impact of Cytochrome c on Immune Cells, Inflammatory Diseases, and Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surmacki, J.; Brozek-Pluska, B.; Kordek, R.; Abramczyk, H. The Lipid-Reactive Oxygen Species Phenotype of Breast Cancer. Raman Spectroscopy and Mapping, PCA and PLSDA for Invasive Ductal Carcinoma and Invasive Lobular Carcinoma. Molecular Tumorigenic Mechanisms beyond Warburg Effect. Analyst 2015, 140, 2121–2133. [Google Scholar] [CrossRef]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Diagnosing Breast Cancer by Using Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 12371–12376. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.; Jablonska-Gajewicz, J.; Kordek, R. Raman “optical Biopsy” of Human Breast Cancer. Prog. Biophys. Mol. Biol. 2012, 108, 74–81. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Musial, J.; Kordek, R.; Bailo, E.; Dieing, T.; Abramczyk, H. Raman Spectroscopy and Imaging: Applications in Human Breast Cancer Diagnosis. Analyst 2012, 137, 3773–3780. [Google Scholar] [CrossRef]
- Surmacki, J.; Musial, J.; Kordek, R.; Abramczyk, H. Raman Imaging at Biological Interfaces: Applications in Breast Cancer Diagnosis. Mol. Cancer 2013, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Brozek-Pluska, B. Raman Imaging in Biochemical and Biomedical Applications. Diagnosis and Treatment of Breast Cancer. Chem. Rev. 2013, 113, 5766–5781. [Google Scholar] [CrossRef]
- Jain, G.K.; Verma, R.; Chougule, A.; Singh, B. Scientific Advances in Cancer Detection Using Raman Spectroscopy. Asian Pac. J. Cancer Prev. 2024, 25, 3977–3986. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Surmacki, J. Effect of COVID-19 mRNA Vaccine on Human Lung Carcinoma Cells In Vitro by Means of Raman Spectroscopy and Imaging. ACS Omega 2023, 8, 42555–42564. [Google Scholar] [CrossRef]
- Conforti, P.M.; Lazzini, G.; Russo, P.; D’Acunto, M. Raman Spectroscopy and AI Applications in Cancer Grading: An Overview. IEEE Access 2024, 12, 54816–54852. [Google Scholar] [CrossRef]
- Aizezi, N.; Ye, Y.; Chen, Z.; Liu, Y. Impact of Soldering Temperatures on Heavy Metal and Dust Emissions: A LIBS-Based Environmental Pollution Analysis. Spectrochim. Acta Part B At. Spectrosc. 2025, 225, 107124. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The Role of Carotenoids in the Prevention of Human Pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.S.; Vine, A.L. Cancer Prevention by Retinoids and Carotenoids: Independent Action on a Common Target. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R. Mechanisms of Genomic and Non-Genomic Actions of Carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 147–154. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Nishino, H. Cancer Prevention by Carotenoids. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 402, 159–163. [Google Scholar] [CrossRef]
- Chedea, V.S.; Jisaka, M. Lipoxygenase and Carotenoids: A Co-Oxidation Story. Afr. J. Biotechnol. 2013, 12, 2786–2791. [Google Scholar]
- Giovannucci, E. Lycopene and Prostate Cancer Risk. Methodological Considerations in the Epidemiologic Literature. Pure Appl. Chem. 2002, 74, 1427–1434. [Google Scholar] [CrossRef]
- Shultz, T.D.; Chew, B.P.; Seaman, W.R.; Luedecke, L.O. Inhibitory Effect of Conjugated Dienoic Derivatives of Linoleic Acid and β-Carotene on the in Vitro Growth of Human Cancer Cells. Cancer Lett. 1992, 63, 125–133. [Google Scholar] [CrossRef]
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Lin, H.; Leffell, D.J.; Welch, E.; Ermakov, I.; Bhosale, P.; Bernstein, P.S.; Gellermann, W. Noninvasive Assessment of Dermal Carotenoids as a Biomarker of Fruit and Vegetable Intake. Am. J. Clin. Nutr. 2010, 92, 794–800. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Antioxidants; Vitamin C, Vitamin E, Seenium and Carotenoids. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.M.; Peng, Y.S.; Lin, Y.; Moon, T.; Roe, D.J.; Ritenbaugh, C. Concentrations and Plasma-Tissue-Diet Relationships of Carotenoids, Retinoids, and Tocopherols in Humans. Nutr. Cancer 1995, 23, 233–246. [Google Scholar] [CrossRef]
- McEligot, A.J.; Rock, C.L.; Flatt, S.W.; Newman, V.; Faerber, S.; Pierce, J.P. Plasma Carotenoids Are Biomarkers of Long-Term High Vegetable Intake in Women with Breast Cancer. J. Nutr. 1999, 129, 2258–2263. [Google Scholar] [CrossRef]
- Trial, P.P.; Lanza, E.; Schatzkin, A.; Daston, C.; Corle, D.; Freedman, L.; Ballard-barbash, R.; Caan, B.; Lance, P.; Marshall, J.; et al. Implementation of a 4-y, high-fiber, high-fruit-and-vegetable, low-fat dietary intervention: Results of dietary changes in the Polyp Prevention Trial. Am. J. Clin. Nutr. 2001, 74, 387–401. [Google Scholar]
- Marmot, M.; Atinmo, T.; Byers, T. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007; Volume 148, ISBN 9780972252225. [Google Scholar]
- Liu, S.; Manson, J.E.; Lee, I.M.; Cole, S.R.; Hennekens, C.H.; Willett, W.C.; Buring, J.E. Fruit and Vegetable Intake and Risk of Cardiovascular Disease: The Women’s Health Study. Am. J. Clin. Nutr. 2000, 72, 922–928. [Google Scholar] [CrossRef]
- Kristal, A.R.; Peters, U.; Potter, J.D. Is It Time to Abandon the Food Frequency Questionnaire? Cancer Epidemiol. Biomark. Prev. 2005, 14, 2826–2828. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M.O.; Ray, A.L.; Xue, Q.L.; Guralnik, J.M.; Fried, L.P. Low Serum Selenium Is Associated with Anemia among Older Women Living in the Community: The Women’s Health and Aging Studies I and II. Biol. Trace Elem. Res. 2006, 112, 97–107. [Google Scholar] [CrossRef][Green Version]
- van Poppel, G.; van den Berg, H. Vitamins and Cancer. Cancer Lett. 1997, 114, 195–202. [Google Scholar] [CrossRef]
- van Poppel, G. Carotenoids and Cancer: An Update with Emphasis on Human Intervention Studies. Eur. J. Cancer 1993, 29, 1335–1344. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Figueiras, A.; Barros-Dios, J. Diet and Lung Cancer: A New Approach. Eur. J. Cancer Prev. 2000, 9, 395–400. [Google Scholar] [CrossRef]
- DiGiovanna, J.J. Retinoid Chemoprevention in Patients at High Risk for Skin Cancer. Med. Pediatr. Oncol. 2001, 36, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Spiegelman, D.; Hunter, D.J.; Chen, W.Y.; Zhang, S.M.; Colditz, G.A.; Willett, W.C. Premenopausal Intakes of Vitamins A, C, and E, Folate, and Carotenoids, and Risk of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2003, 12, 713–720. [Google Scholar]
- Cook, N.R.; Stampfer, M.J.; Ma, J.; Manson, J.A.E.; Sacks, F.M.; Buring, J.E.; Hennekens, C.H. Β-Carotene Supplementation for Patients With Low Baseline Levels and Decreased Risks of Total and Prostate Carcinoma. Cancer 1999, 86, 1783–1792. [Google Scholar] [CrossRef]
- Ritenbaugh, C. Carotenoids and Cancer. Nutr. Today 1987, 21, 14–19. [Google Scholar] [CrossRef]
- Ziegler, R.G. Vegetables, Fruits, and Carotenoids and the Risk of Cancer. Am. J. Clin. Nutr. 1991, 53, 251S–259S. [Google Scholar] [CrossRef]
- Gallicchio, L.; Boyd, K.; Matanoski, G.; Tao, X.; Chen, L.; Lam, T.K.; Shiels, M.; Hammond, E.; Robinson, K.A.; Caulfield, L.E.; et al. Carotenoids and the Risk of Developing Lung Cancer: A Systematic Review. Am. J. Clin. Nutr. 2008, 88, 372–383. [Google Scholar] [CrossRef]
- Sharoni, Y.; Danilenko, M.; Dubi, N.; Ben-Dor, A.; Levy, J. Carotenoids and Transcription. Arch. Biochem. Biophys. 2004, 430, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a Combination of Beta Carotene and Vitamin A on Lung Cancer and Cardiovascular Disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Connor, E.M.; Sperling, R.S.; Gelber, R.; Kiselev, P.; Scott, G.O.M. The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers. N. Engl. J. Med. 1994, 331, 1173–1180. [Google Scholar] [CrossRef]
- Albanes, D.; Heinonen, O.P.; Taylor, P.R.; Virtamo, J.; Edwards, B.K.; Rautalahti, M.; Hartman, A.M.; Palmgren, J.; Freedman, L.S.; Haapakoski, J.; et al. α-Tocopherol and β-Carotene Supplements and Lung Cancer Incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: Effects of Base-Line Characteristics and Study Compliance. J. Natl. Cancer Inst. 1996, 88, 1560–1570. [Google Scholar] [CrossRef]
- Mayne, S.T.; Taylor, S. Beta-Carotene, Carotenoids, and Disease Prevention in Humans. Carotenoids Dis. Hum. 1996, 5, 690–701. [Google Scholar] [CrossRef]
- Wang, X.-D.; Russell, R.M. Procarcinogenic and Anticarcinogenic Effects of B-Carotene. Nutr. Rev 1999, 57, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Structure and Properties of Carotenoids in Relation to Function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Siems, W.; Sommerburg, O.; Schild, L.; Augustin, W.; Langhans, C.D.; Wiswedel, I. B-Carotene Cleavage Products Induce Oxidative Stress in Vitro by Impairing Mitochondrial Respiration. FASEB J. 2002, 16, 1805–1807. [Google Scholar] [CrossRef]
- Maoka, T. Recent Progress in Structural Studies of Carotenoids in Animals and Plants. Arch. Biochem. Biophys. 2009, 483, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Brozek-Pluska, B.; Jablonska-Gajewicz, J.; Kordek, R.; Abramczyk, H. Phase Transitions in Oleic Acid and in Human Breast Tissue as Studied by Raman Spectroscopy and Raman Imaging. J. Med. Chem. 2011, 54, 3386–3392. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.; Jablonska, J.; Kordek, R. The Label-Free Raman Imaging of Human Breast Cancer. J. Mol. Liq. 2011, 164, 123–131. [Google Scholar] [CrossRef]
- Abramczyk, H.; Surmacki, J.; Brozek-Płuska, B.; Morawiec, Z.; Tazbir, M. The Hallmarks of Breast Cancer by Raman Spectroscopy. J. Mol. Struct. 2009, 924–926, 175–182. [Google Scholar] [CrossRef]
- Kopec, M.; Błaszczyk, M.; Radek, M.; Abramczyk, H. Raman Imaging and Statistical Methods for Analysis Various Type of Human Brain Tumors and Breast Cancers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120091. [Google Scholar] [CrossRef]
- Abramczyk, H.; Surmacki, J.M.; Brozek-Pluska, B.K.M. Revision of Commonly Accepted Warburg Mechanism of Cancer Development: Redox-Sensitive Mitochondrial Cytochromes in Breast and Brain Cancers by Raman Imaging. Cancers 2021, 13, 2599. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Dziki, A.; Abramczyk, H. Virtual Spectral Histopathology of Colon Cancer—Biomedical Applications of Raman Spectroscopy and Imaging. J. Mol. Liq. 2020, 303, 112676. [Google Scholar] [CrossRef]
- Brozek-Pluska, B. Statistics Assisted Analysis of Raman Spectra and Imaging of Human Colon Cell Lines–Label Free, Spectroscopic Diagnostics of Colorectal Cancer. J. Mol. Struct. 2020, 1218, 128524. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Kopec, M.; Niedzwiecka, I.; Morawiec-Sztandera, A. Label-Free Determination of Lipid Composition and Secondary Protein Structure of Human Salivary Noncancerous and Cancerous Tissues by Raman Microspectroscopy. Analyst 2015, 140, 2107–2113. [Google Scholar] [CrossRef]
- Surmacki, J.M.; Abramczyk, H. Confocal Raman Imaging Reveals the Impact of Retinoids on Human Breast Cancer via Monitoring the Redox Status of Cytochrome c. Sci. Rep. 2023, 13, 15049. [Google Scholar] [CrossRef]
- Kowatz, T.; Babino, D.; Kiser, P.; Palczewski, K.; Von Lintig, J. Characterization of Human β,β-Carotene-15,15′-Monooxygenase (BCMO1) as a Soluble Monomeric Enzyme. Arch. Biochem. Biophys. 2013, 539, 214–222. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Smith, M.K.; Piper, J.B.; Smith, J.C. β-Carotene 15,15′-Dioxygenase Activity in Human Tissues and Cells: Evidence of an Iron Dependency. J. Nutr. Biochem. 2001, 12, 640–647. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Nagao, A.; Terao, J. B-Carotene 15,15*-Dioxygenase Activity and Cellular Retinol-Binding Protein Type II Level Are Enhanced by Dietary Unsaturated Triacylglycerols in Rat Intestines. Biochem. Mol. Roles Nutr. 1998, 128, 1614–1619. [Google Scholar] [CrossRef]
- Farias, E.F.; Ong, D.E.; Ghyselinck, N.B.; Nakajo, S.; Kuppumbatti, Y.S.; Lopez, R.M. Cellular Retinol-Binding Protein I, a Regulator of Breast Epithelial Retinoic Acid Receptor Activity, Cell Differentiation, and Tumorigenicity. J. Natl. Cancer Inst. 2005, 97, 21–29. [Google Scholar] [CrossRef]
- Doldo, E.; Costanza, G.; Agostinelli, S.; Tarquini, C.; Ferlosio, A.; Arcuri, G.; Passeri, D.; Scioli, M.G.; Orlandi, A. Vitamin A, Cancer Treatment and Prevention: The New Role of Cellular Retinol Binding Proteins. BioMed. Res. Int. 2015, 2015, 624627. [Google Scholar] [CrossRef]
- Mawson, A.R. Retinoids in the Treatment of Glioma: A New Perspective. Cancer Manag. Res. 2012, 4, 233–241. [Google Scholar] [CrossRef]
- Napoli, J.L. Retinoic Acid: Its Biosynthesis and Metabolism. Prog. Nucleic Acid Res. Mol. Biol. 1999, 63, 139–188. [Google Scholar] [PubMed]
- Travis, G.H.; Golczak, M.; Moise, A.R.; Palczewski, K. Diseases Caused by Defects in the Visual Cycle: Retinoids as Potential Therapeutic Agents. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 469–512. [Google Scholar] [CrossRef] [PubMed]
- Malkovsky, M.; Dore, C.; Hunt, R.; Palmer, L.; Chandler, P.; Medawar, P.B. Enhancement of Specific Antitumor Immunity in Mice Fed a Diet Enriched in Vitamin A Acetate. Proc. Natl. Acad. Sci. USA 1983, 80, 6322–6326. [Google Scholar] [CrossRef]
- Malkovský, M.; Edwards, A.J.; Hunt, R.; Palmer, L.; Medawar, P.B. T-Cell-Mediated Enhancement of Host-versus-Graft Reactivity in Mice Fed a Diet Enriched in Vitamin A Acetate. Nature 1983, 302, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L. Cellular Retinoid Binding-Proteins, CRBP, CRABP, FABP5: Effects on Retinoid Metabolism, Function and Related Diseases. Pharmacol. Ther. 2017, 173, 19–33. [Google Scholar] [CrossRef]
- Kwong, J.; Lo, K.W.; Chow, L.S.N.; To, K.F.; Choy, K.W.; Chan, F.L.; Mok, S.C.; Huang, D.P. Epigenetic Silencing of Cellular Retinol-Binding Proteins in Nasopharyngeal Carcinoma. Neoplasia 2005, 7, 67–74. [Google Scholar] [CrossRef][Green Version]
- Shete, V.; Quadro, L. Mammalian Metabolism of β-Carotene: Gaps in Knowledge. Nutrients 2013, 5, 4849–4868. [Google Scholar] [CrossRef]
- Pino-Lagos, K.; Guo, Y.; Noelle, R.J. Retinoic Acid: A Key Player in Immunity. BioFactors 2010, 36, 430–436. [Google Scholar] [CrossRef]
- Yamada, T.; Sato, S.; Sotoyama, Y.; Orba, Y.; Sawa, H.; Yamauchi, H.; Sasaki, M.; Takaoka, A. RIG-I Triggers a Signaling-Abortive Anti-SARS-CoV-2 Defense in Human Lung Cells. Nat. Immunol. 2021, 22, 820–828. [Google Scholar] [CrossRef]
- Abramczyk, H.; Imiela, A.; Surmacki, J. Novel Strategies of Raman Imaging for Monitoring Intracellular Retinoid Metabolism in Cancer Cells. J. Mol. Liq. 2021, 334, 116033. [Google Scholar] [CrossRef]
- Noy, N. Vitamin a Transport and Cell Signaling by the Retinol-Binding Protein Receptor STRA6. In Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2016; Volume 81, pp. 77–93. [Google Scholar] [CrossRef]
- Berry, D.C.; O’Byrne, S.M.; Vreeland, A.C.; Blaner, W.S.; Noy, N. Cross Talk between Signaling and Vitamin A Transport by the Retinol-Binding Protein Receptor STRA6. Mol. Cell. Biol. 2012, 32, 3164–3175. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S. STRA6, a Cell-Surface Receptor for Retinol-Binding Protein: The Plot Thickens. Cell Metab. 2007, 5, 164–166. [Google Scholar] [CrossRef]
- Goswami, R.; Kaplan, M.H. STAT Transcription Factors in T Cell Control of Health and Disease, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 331. [Google Scholar]
- Mora, J.R.; Iwata, M.; Eksteen, B.; Song, S.Y.; Junt, T.; Senman, B.; Otipoby, K.L.; Yokota, A.; Takeuchi, H.; Ricciardi-Castagnoli, P.; et al. Generation of Gut-Homing IgA-Secreting B Cells by Intestinal Dendritic Cells. Science 2006, 314, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Surmacki, J.M.; Brozek-Pluska, B. Redox State Changes of Mitochondrial Cytochromes in Brain and Breast Cancers by Raman Spectroscopy and Imaging. J. Mol. Struct. 2022, 1252, 132134. [Google Scholar] [CrossRef]
- Taimi, M.; Helvig, C.; Wisniewski, J.; Ramshaw, H.; White, J.; Amad, M.; Korczak, B.; Petkovich, M. A Novel Human Cytochrome P450, CYP26C1, Involved in Metabolism of 9-Cis and All-Trans Isomers of Retinoic Acid. J. Biol. Chem. 2004, 279, 77–85. [Google Scholar] [CrossRef]
- Abu-Abed, S.S.; Beckett, B.R.; Chiba, H.; Chithalen, J.V.; Jones, G.; Metzger, D.; Chambon, P.; Petkovich, M. Mouse P450RAI (CYP26) Expression and Retinoic Acid-Inducible Retinoic Acid Metabolism in F9 Cells Are Regulated by Retinoic Acid Receptor γ and Retinoid X Receptor α. J. Biol. Chem. 1998, 273, 2409–2415. [Google Scholar] [CrossRef]
- Ribes, V.; Otto, D.M.E.; Dickmann, L.; Schmidt, K.; Schuhbaur, B.; Henderson, C.; Blomhoff, R.; Wolf, C.R.; Tickle, C.; Dollé, P. Rescue of Cytochrome P450 Oxidoreductase (Por) Mouse Mutants Reveals Functions in Vasculogenesis, Brain and Limb Patterning Linked to Retinoic Acid Homeostasis. Dev. Biol. 2007, 303, 66–81. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. Cytochrome c as a Potentially Clinical Useful Marker of Mitochondrial and Cellular Damage. Front. Immunol. 2016, 7, 279. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Kopeć, M. Double Face of Cytochrome c in Cancers by Raman Imaging. Sci. Rep. 2022, 12, 2120. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Kopec, M.; Jakub, S.; Błaszczyk, M.; Radek, M. Redox Imbalance and Biochemical Changes in Cancer by Probing Redox-Sensitive Mitochondrial Cytochromes in Label-Free Visible Resonance Raman Imaging. Cancers 2021, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Ow, Y.L.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond Respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542. [Google Scholar] [CrossRef] [PubMed]



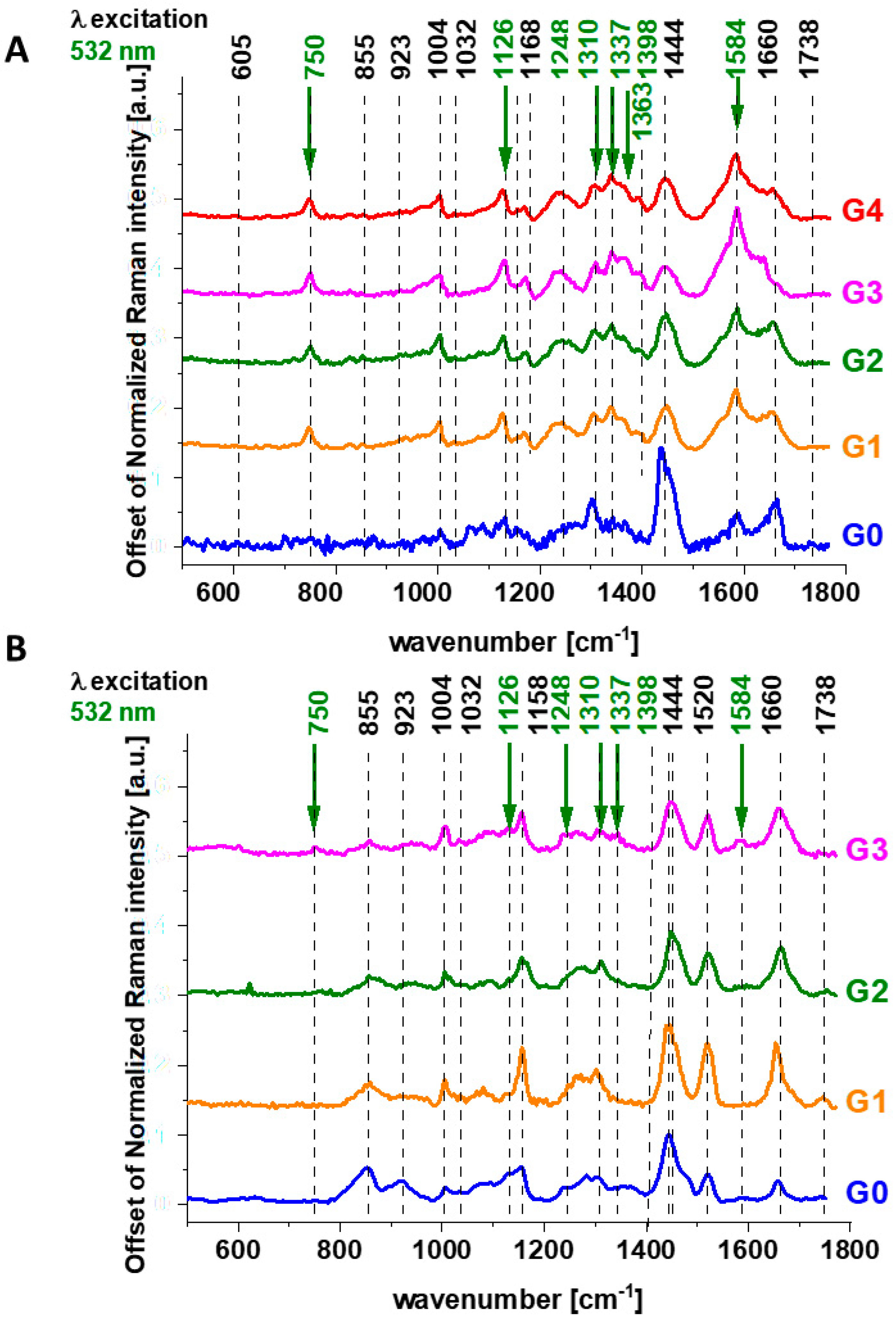
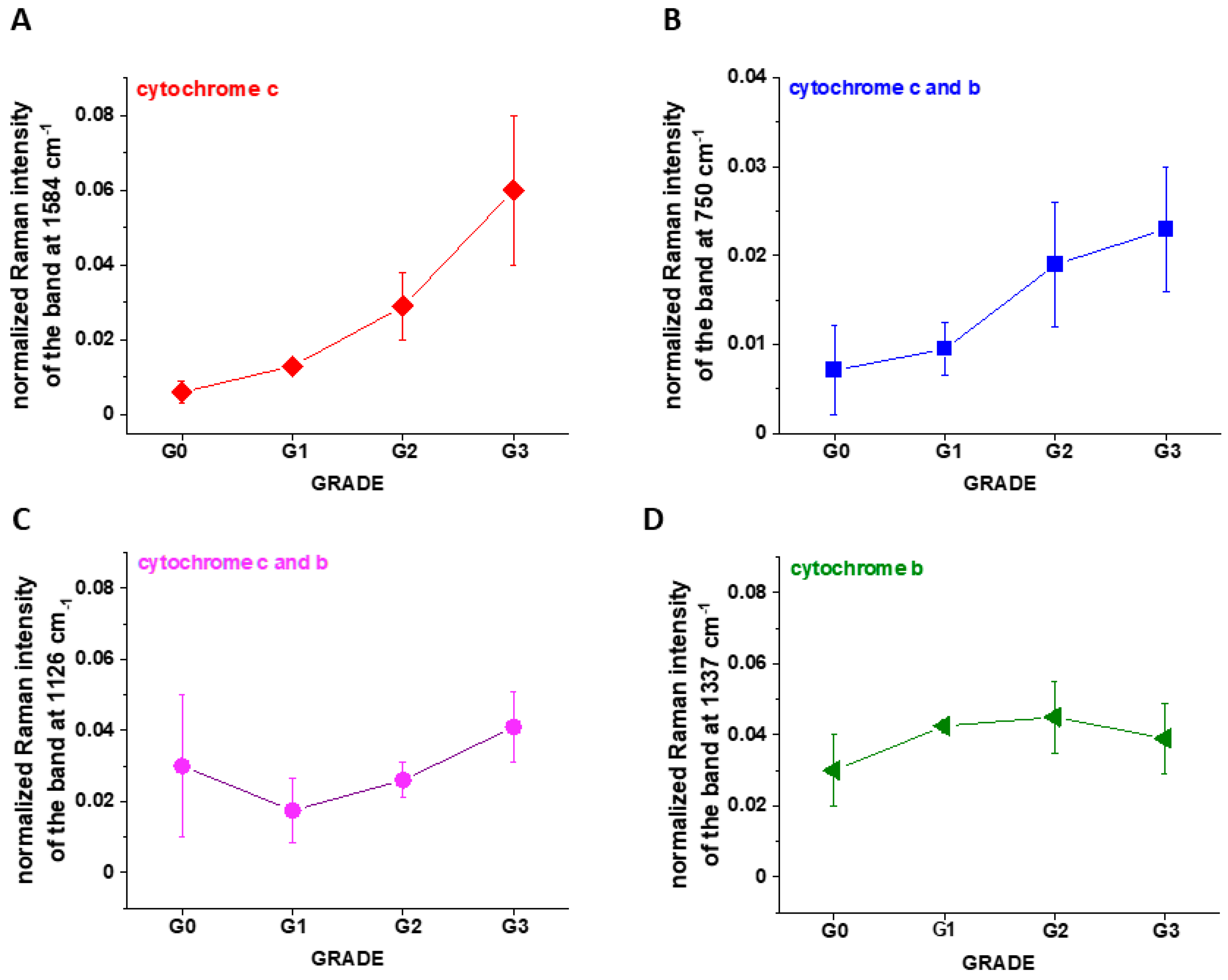

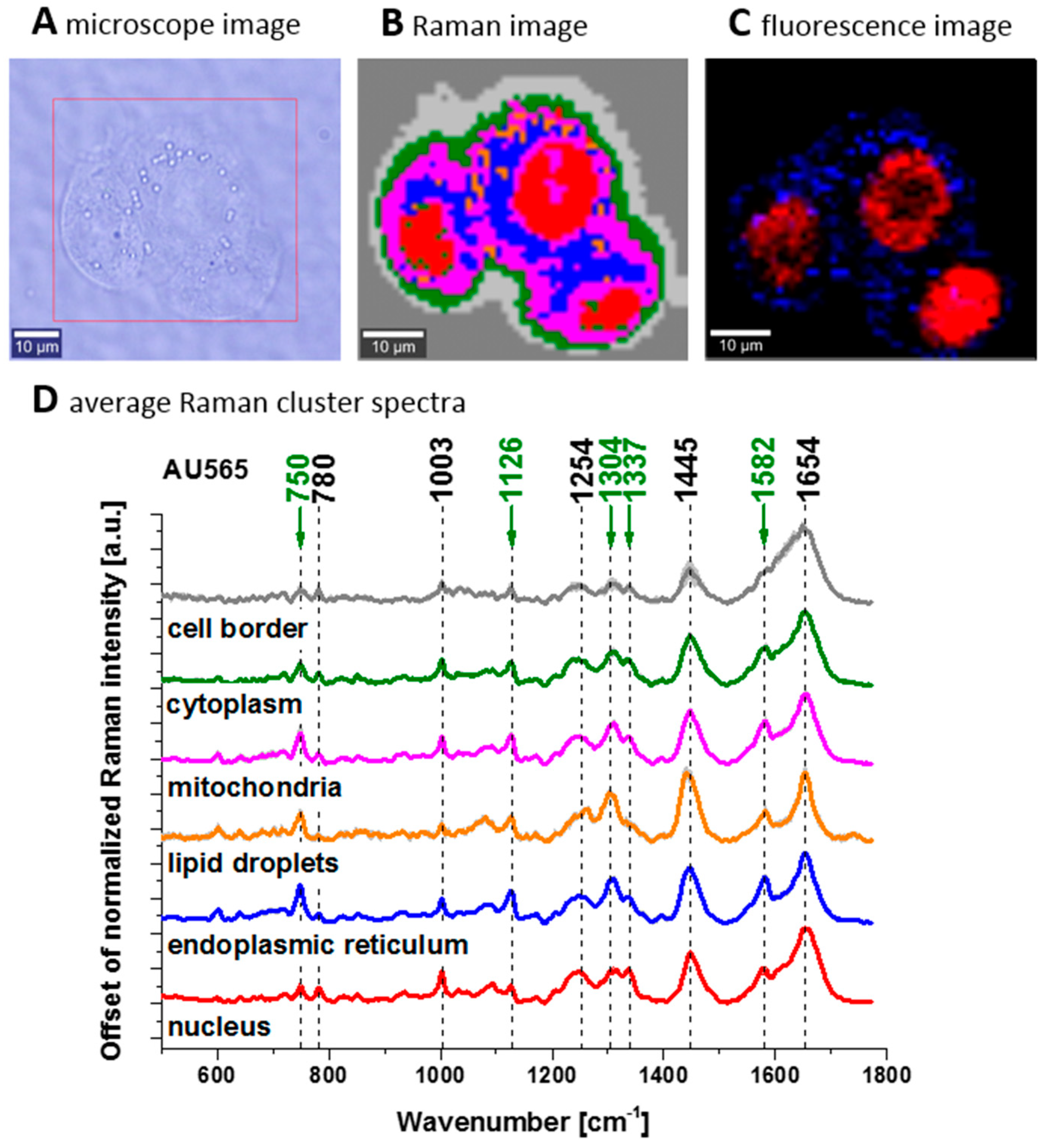

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramczyk, H.; Kopeć, M.; Surmacki, J. The Triangle: Carotenoids–Retinoids–Cytochromes Govern Essential Functions for Development and Progression of Cancer. Spectrosc. J. 2025, 3, 9. https://doi.org/10.3390/spectroscj3010009
Abramczyk H, Kopeć M, Surmacki J. The Triangle: Carotenoids–Retinoids–Cytochromes Govern Essential Functions for Development and Progression of Cancer. Spectroscopy Journal. 2025; 3(1):9. https://doi.org/10.3390/spectroscj3010009
Chicago/Turabian StyleAbramczyk, Halina, Monika Kopeć, and Jakub Surmacki. 2025. "The Triangle: Carotenoids–Retinoids–Cytochromes Govern Essential Functions for Development and Progression of Cancer" Spectroscopy Journal 3, no. 1: 9. https://doi.org/10.3390/spectroscj3010009
APA StyleAbramczyk, H., Kopeć, M., & Surmacki, J. (2025). The Triangle: Carotenoids–Retinoids–Cytochromes Govern Essential Functions for Development and Progression of Cancer. Spectroscopy Journal, 3(1), 9. https://doi.org/10.3390/spectroscj3010009







