Interpreting the Microwave Spectra of Diatomic Molecules—Part II: Nuclear Quadrupole Coupling of One Nucleus
Abstract
1. Introduction
2. Materials and Methods
3. Results
Sodium Iodide (NaI)
4. Basic Theory of Nuclear Quadrupolar Coupling
5. Results
5.1. Sodium Iodide (NaI, Reprise)
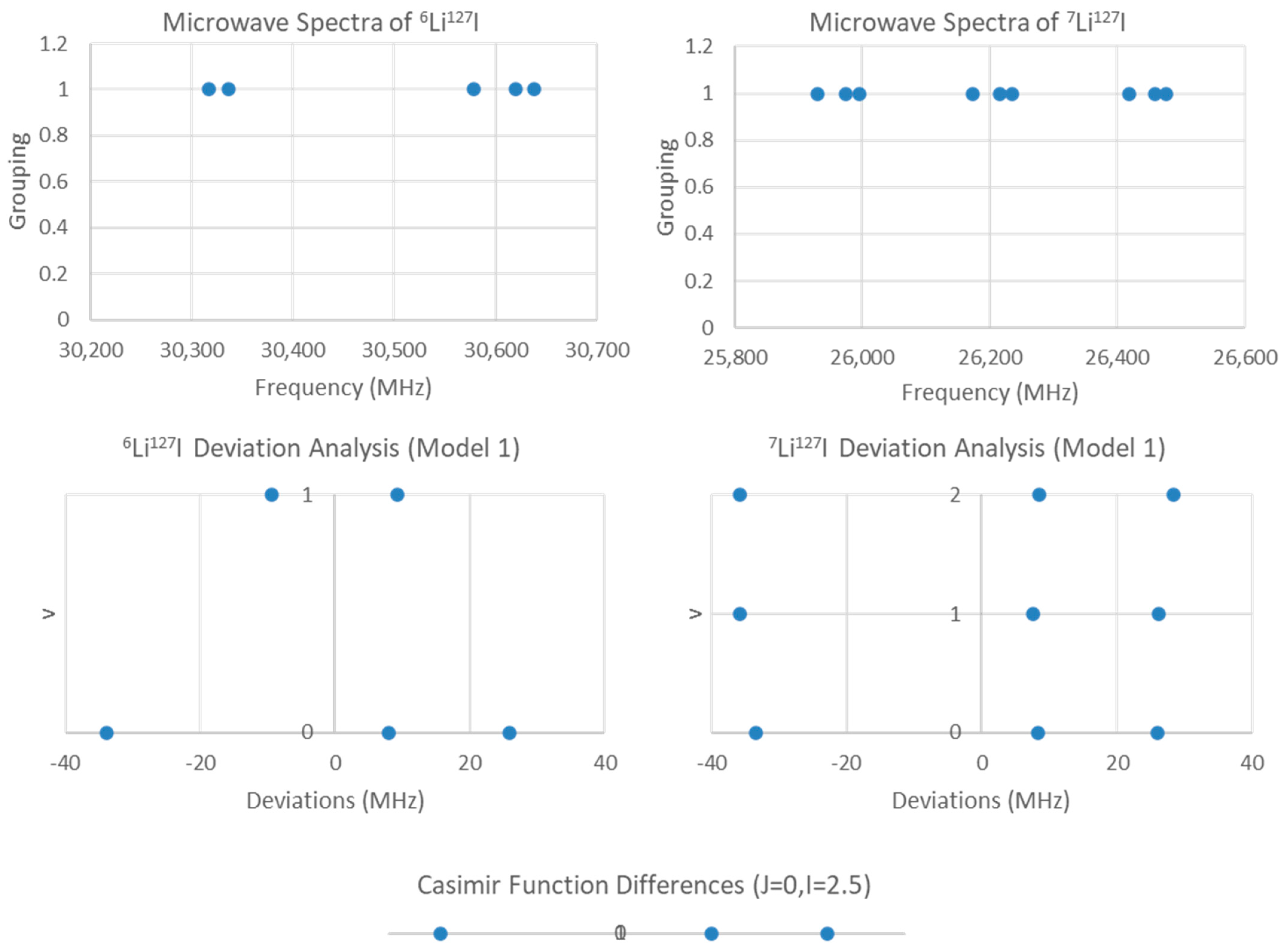
5.2. Lithium Iodide (LiI)
5.3. Interhalogen Diatomics
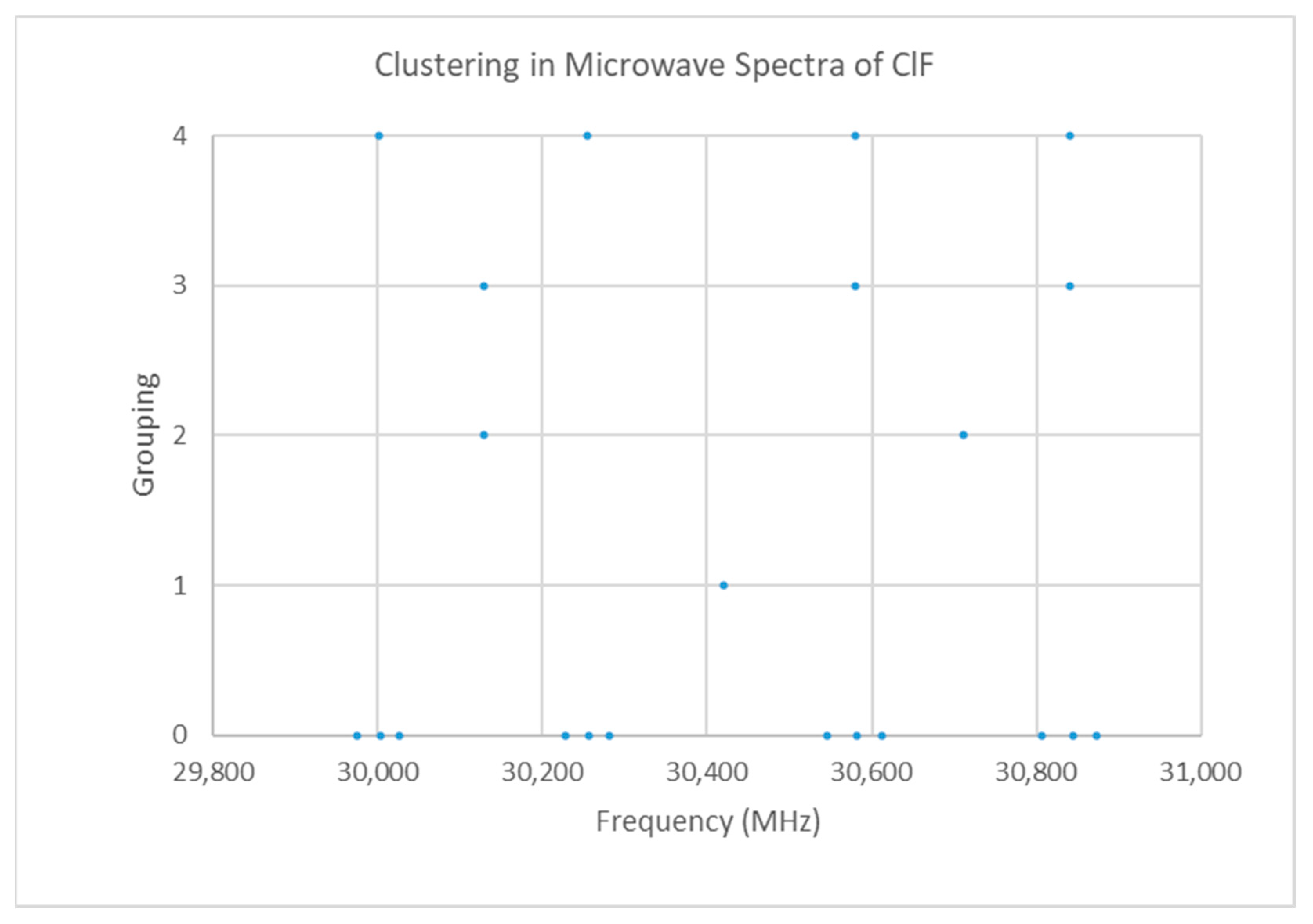
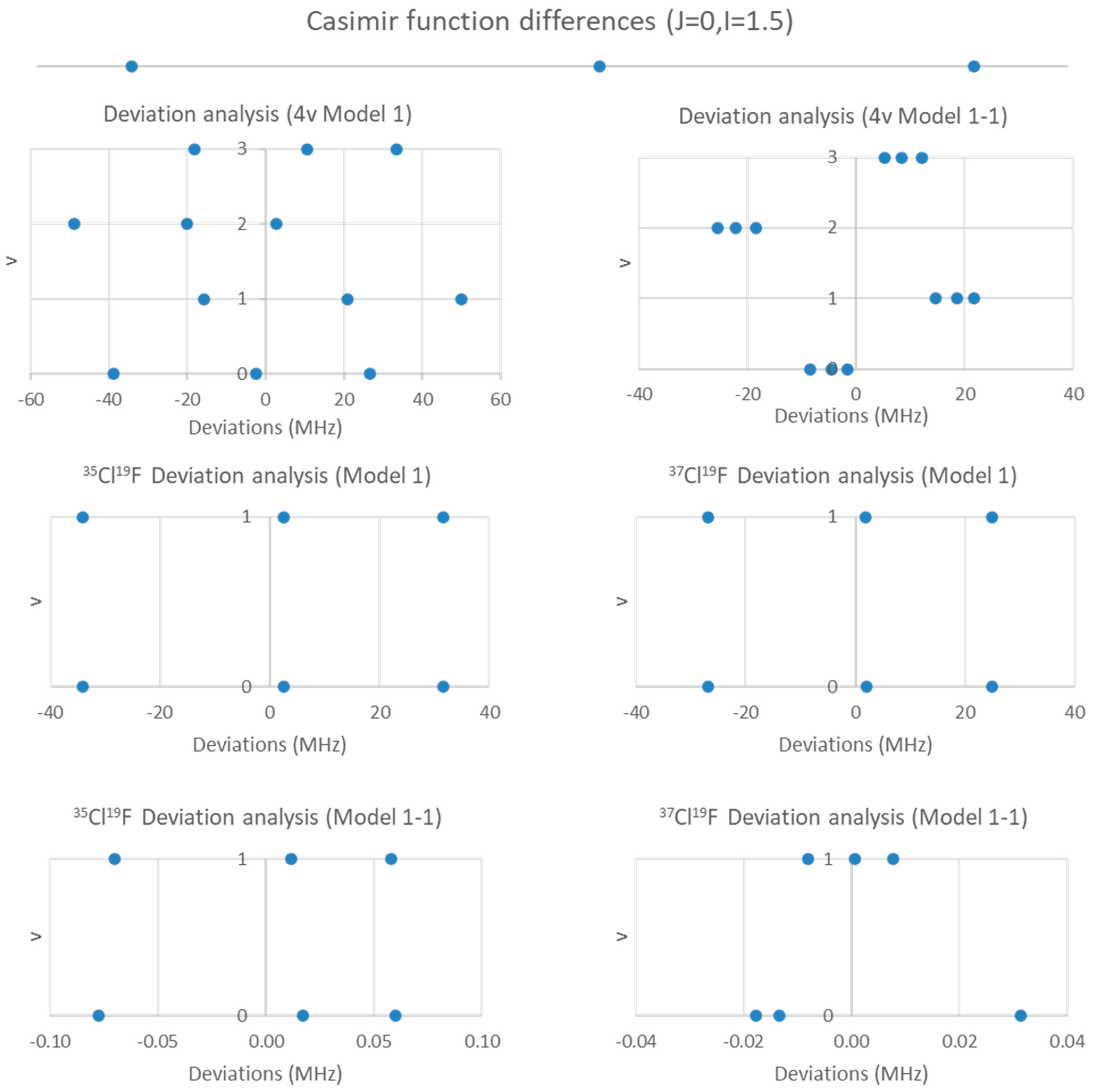
5.4. Chlorine Monofluoride (ClF)
5.5. Bromine Monofluoride (BrF)
5.6. Iodine Monofluoride (IF)
5.7. Deuterium Iodide (DI)
5.8. Deuterium Bromide (DBr)
5.9. Sodium Bromide (NaBr)
5.10. Lithium Bromide (LiBr)
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Analysis of the Microwave Spectrum of Chlorine Monofluoride (ClF)
Appendix B. Analysis of the Microwave Spectrum of Deuterium Iodide (DI)
Appendix C. Analysis of the Microwave Spectrum of Deuterium Iodide (DI)
Appendix D. Analysis of the Microwave Spectrum of Deuterium Bromide (DBr)
Appendix E. Some Relevant Isotopic Data
| Isotope | Z | m (amu) | Abundance (%) | I | Q (barn) |
|---|---|---|---|---|---|
| 2H | 1 | 2.014101777844 | 0.03 | 1 | +0.0028578 |
| 6Li | 3 | 6.015122795 | 7.59 | 1 | −0.000806 |
| 7Li | 3 | 7.016004550 | 92.41 | 1.5 | −0.0400 |
| 19F | 9 | 18.998403220 | 100.00 | 0.5 | −0.0942 |
| 23Na | 11 | 22.989769281 | 100.00 | 1.5 | +0.104 |
| 35Cl | 17 | 34.968852721 | 75.76 | 2.5 | −0.0817 |
| 37Cl | 17 | 36.965902590 | 24.24 | 2.5 | −0.0644 |
| 79Br | 35 | 78.9183371 | 50.69 | 1.5 | +0.3087 |
| 81Br | 35 | 80.9162906 | 49.31 | 1.5 | +0.2579 |
| 127I | 53 | 126.904473 | 100.00 | 2.5 | −0.688 |
References
- Pye, C.C. Interpreting the Microwave Spectra of Diatomic Molecules. Spectrosc. J. 2023, 1, 3–27. [Google Scholar] [CrossRef]
- Townes, C.H.; Schawlow, A.L. Microwave Spectroscopy; Dover: New York, NY, USA, 1975. [Google Scholar]
- Microsoft Office Professional Plus (Excel); Microsoft Corporation: Redmond, DC, USA, 2016.
- Solver, Microsoft Office Add-In; Frontline Systems: Incline Village, NV, USA, 2016.
- Available online: http://info.ifpan.edu.pl/~kisiel/prospe.htm (accessed on 4 June 2024).
- Maxwell, L.R.; Hendricks, S.B.; Mosley, V.M. Interatomic Distances of the Alkali Halide Molecules by Electron Diffraction. Phys. Rev. 1937, 52, 968–972. [Google Scholar] [CrossRef]
- Honig, A.; Mandel, M.; Stitch, M.L.; Townes, C.H. Microwave Spectra of the Alkali Halides. Phys. Rev. 1954, 96, 629–642. [Google Scholar] [CrossRef]
- Pye, C.C. One-Dimensional Cluster Analysis and its Application to Chemistry. Chem. Educ. 2020, 25, 50–57. [Google Scholar]
- Casimir, H.B.G. On the Interaction between Atomic Nuclei and Electrons; Springer: Dordrecht, NL, USA, 1936; pp. 201–283. [Google Scholar]
- Bardeen, J.; Townes, C.H. Calculation of Nuclear Quadrupole Effects in Molecules. Phys. Rev. 1948, 73, 97–105. [Google Scholar] [CrossRef]
- Gordy, W.; Cook, R.L. Microwave Molecular Spectra; Wiley: New York, NY, USA, 1984; Ch. 9; pp. 392–449. [Google Scholar]
- Lucken, E.A.C. Nuclear Quadrupole Coupling Constants; Academic Press: London, UK, 1969; Ch. 1-3b; pp. 1–34. [Google Scholar]
- Lo, A.Y.H.; Hanna, J.V.; Schurko, R.W. A Theoretical Study of 51V Electric Field Gradient Tensors in Pyrovanadates and Metavanadates. Appl. Magn. Reson. 2007, 32, 691–708. [Google Scholar] [CrossRef]
- Boese, R.; Boese, A.D.; Blaser, D.; Antipin, M.Y.; Ellern, A.; Seppelt, K. The Surprising Crystal Packing of Chlorinefluoride. Angew. Chem. Int. Ed. 1997, 36, 1489–1492. [Google Scholar] [CrossRef]
- Drews, T.; Seppelt, K. Bromine Monofluoride. Z. Anorg. Allg. Chem. 2012, 638, 2106–2110. [Google Scholar] [CrossRef]
- Boswijk, K.H.; van der Heide, J.; Vos, A.; Wiebenga, E.H. The Crystal Structure of α-ICl. Acta Cryst. 1956, 9, 274–277. [Google Scholar]
- Carpenter, G.B.; Richards, S.M. The Crystal Structure of β-Iodine Monochloride. Acta Cryst. 1962, 15, 360–364. [Google Scholar] [CrossRef]
- Swink, L.N.; Carpenter, G.B. The Crystal Structure of Iodine Monobromide. Acta Cryst. B 1968, 24, 429–433. [Google Scholar] [CrossRef]
- Gilbert, D.A.; Roberts, A.; Griswold, P.A. Nuclear and Molecular Information from the Microwave Spectrum of FCl. Phys. Rev. 1949, 76, 1723. [Google Scholar] [CrossRef]
- Smith, D.F.; Tidwell, M.; Williams, D.V.P. The Microwave Spectrum of Bromine Monofluoride. Phys. Rev. 1950, 77, 420–421. [Google Scholar] [CrossRef]
- Tiemann, E.; Hoeft, J.; Torring, T. Das Rotationsspektrum des JF. Z. Naturforsch. A 1973, 28, 1405–1407. [Google Scholar] [CrossRef][Green Version]
- Klein, J.A.; Nethercot, A.H., Jr. Microwave Spectrum of DI at 1.5-mm Wavelength. Phys. Rev. 1953, 91, 1018. [Google Scholar] [CrossRef]
- Andreani, C.; Nardone, M.; Ricci, F.P.; Soper, A.K. Neutron-diffraction study of liquid hydrogen iodide. Phys. Rev. A 1992, 46, 4709–4716. [Google Scholar] [CrossRef]
- Burrus, C.A.; Gordy, W. One-to-Two Millimeter Wave Spectroscopy. III. NO and DI. Phys. Rev. 1953, 92, 1437–1439. [Google Scholar] [CrossRef]
- Gordy, W.; Burrus, C.A. Spectrum of DBr in the One-Millimeter Wave Region. Phys. Rev. 1954, 93, 419–420. [Google Scholar] [CrossRef]
- Andreani, C.; Menzinger, F.; Ricci, M.A.; Soper, A.K.; Dreyer, J. Neutron-diffraction from liquid hydrogen bromide: Study of the orientational correlations. Phys. Rev. B 1994, 49, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
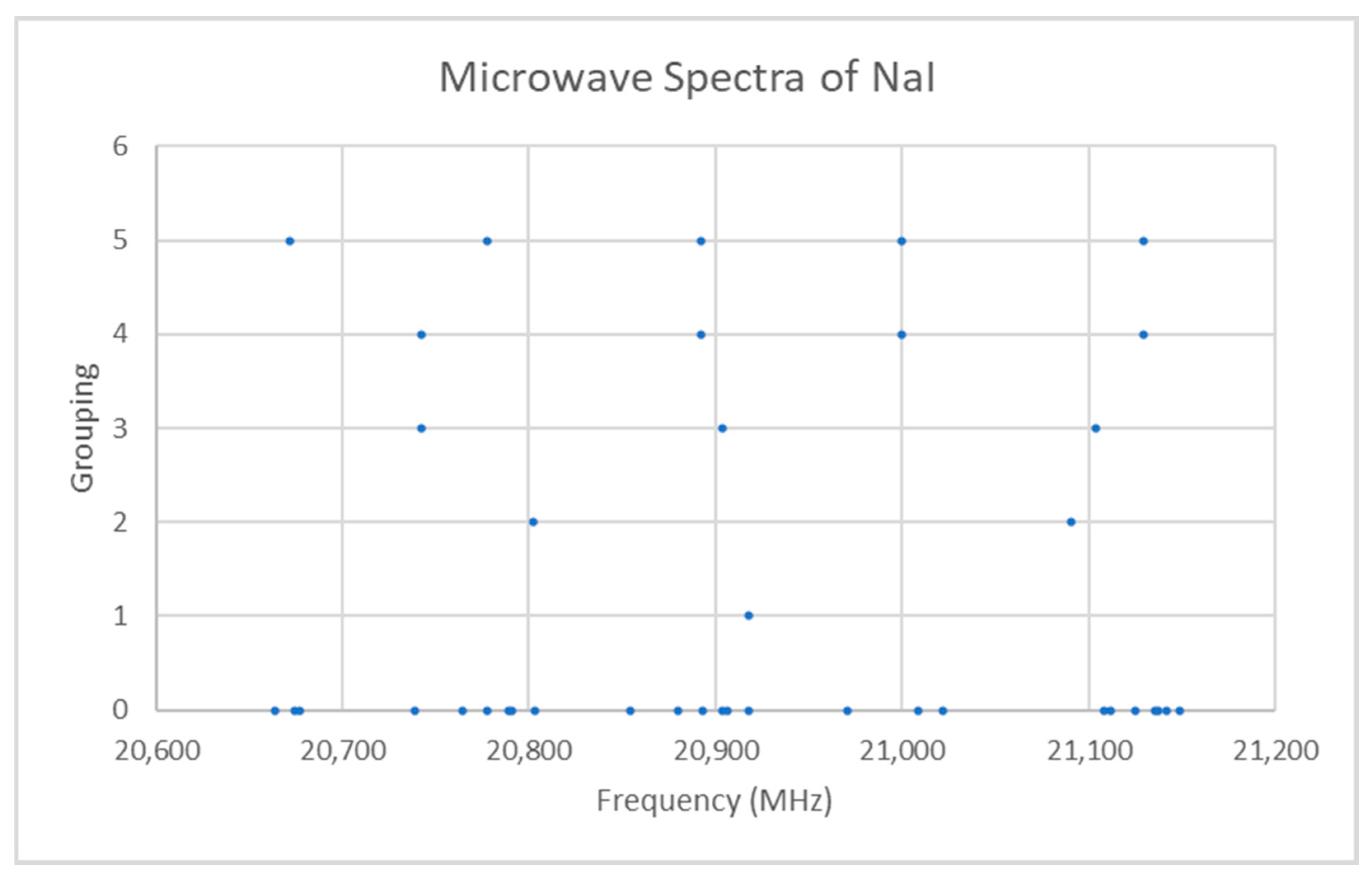
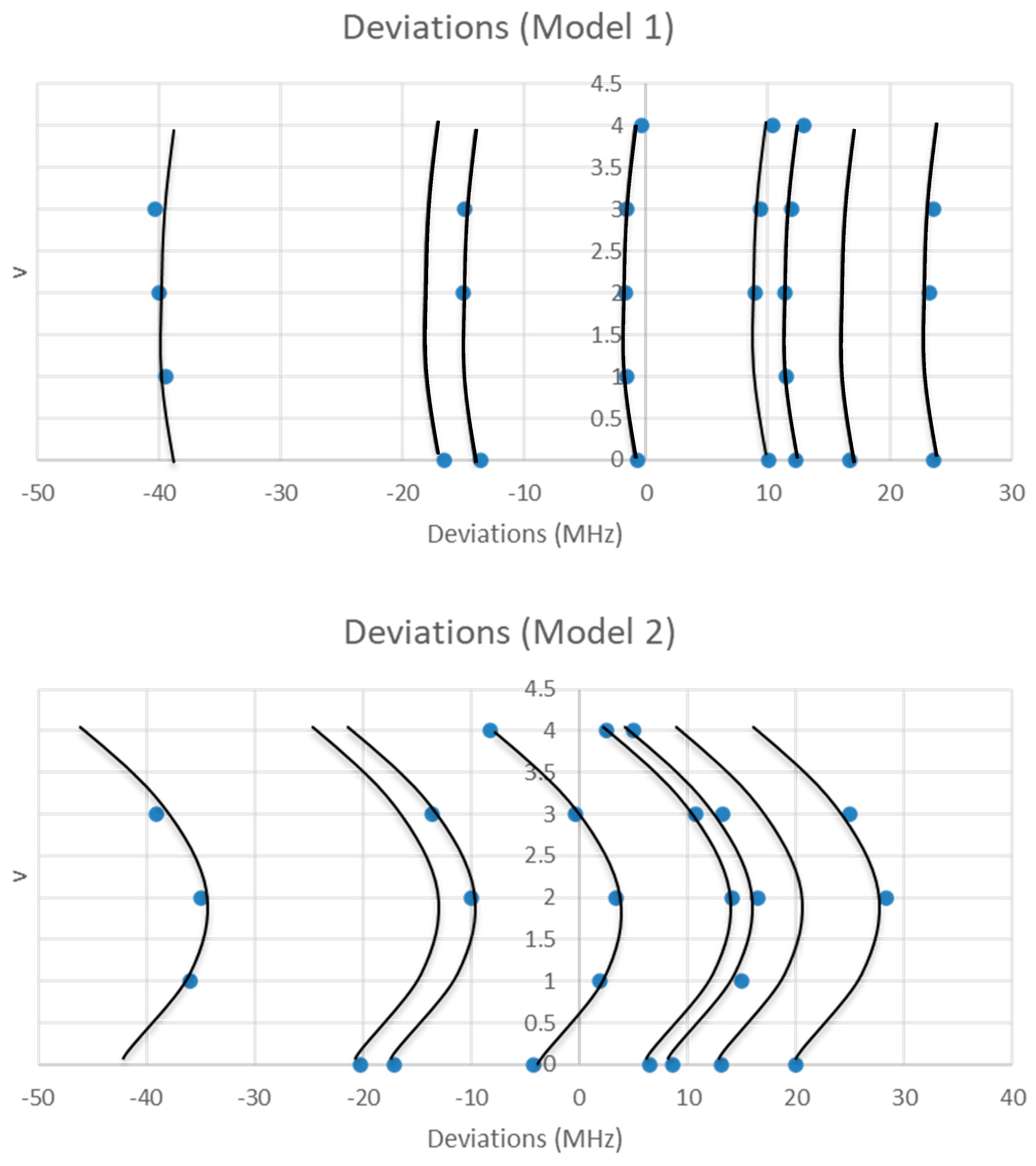
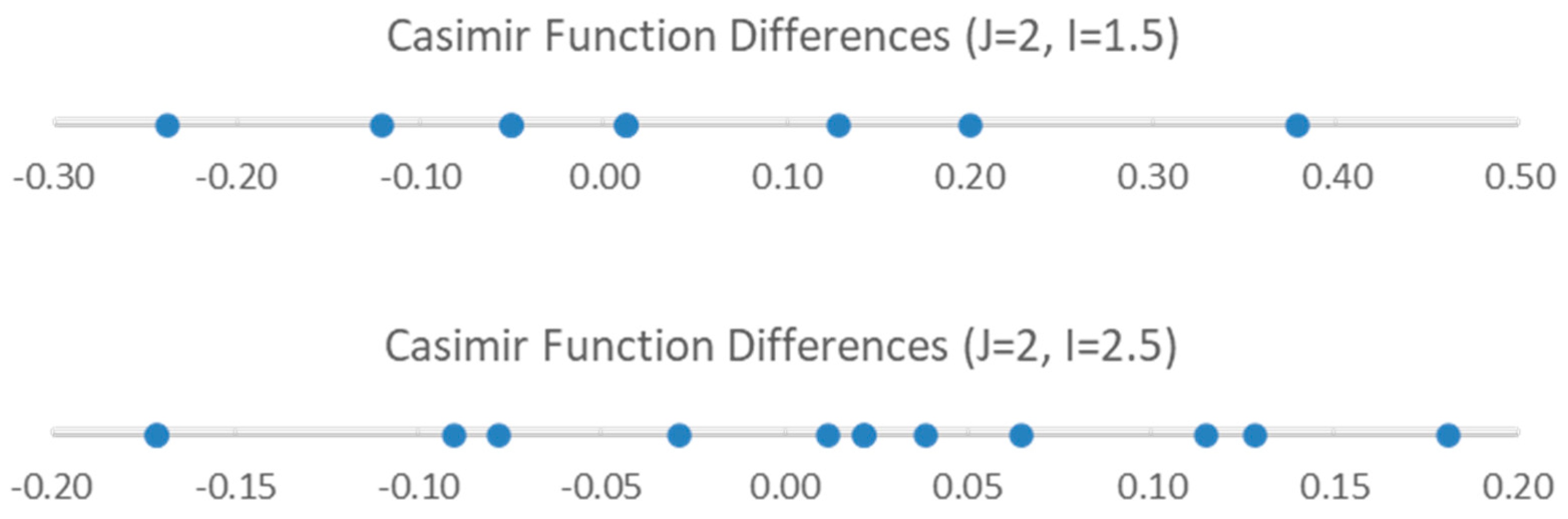

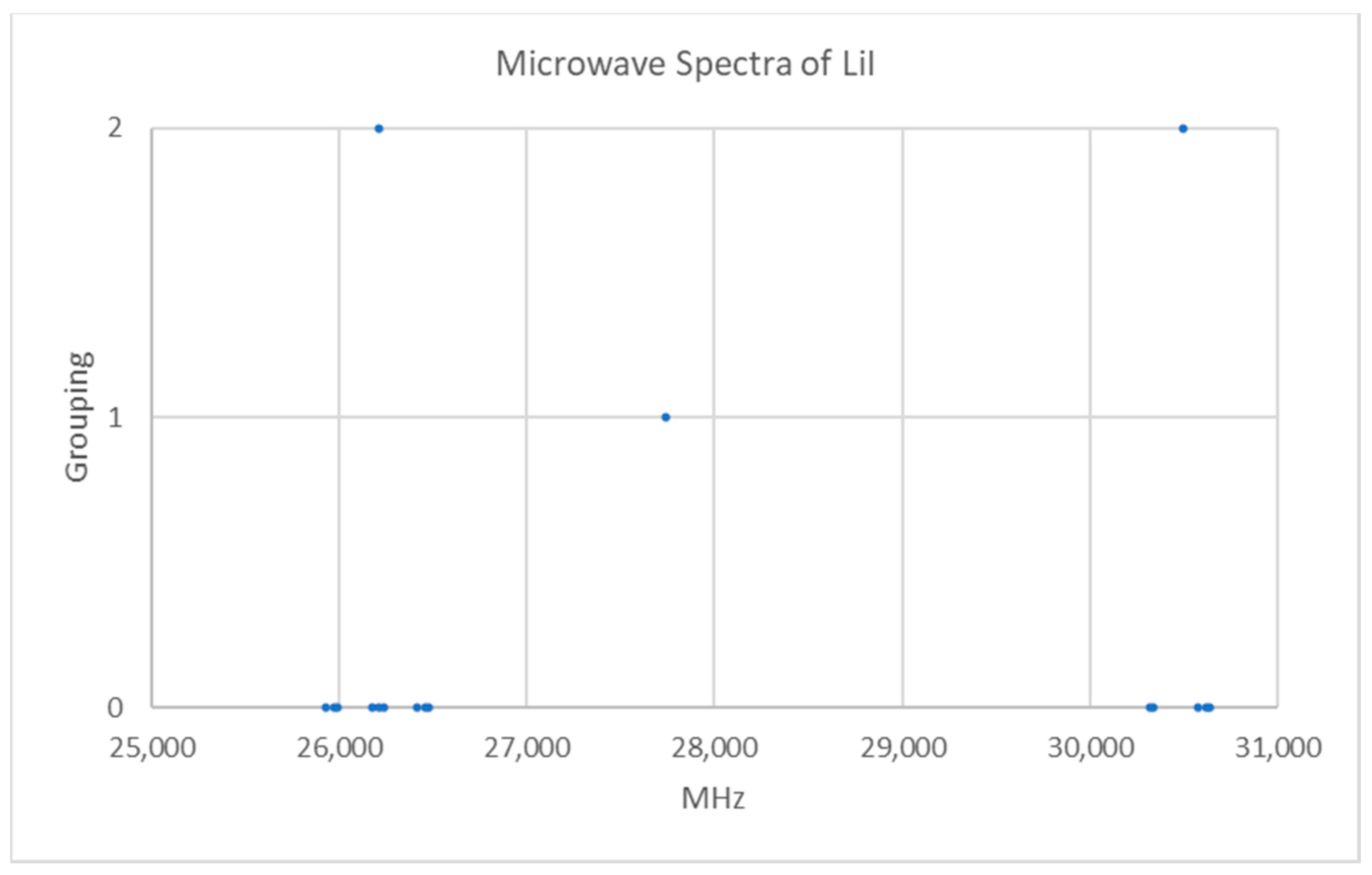
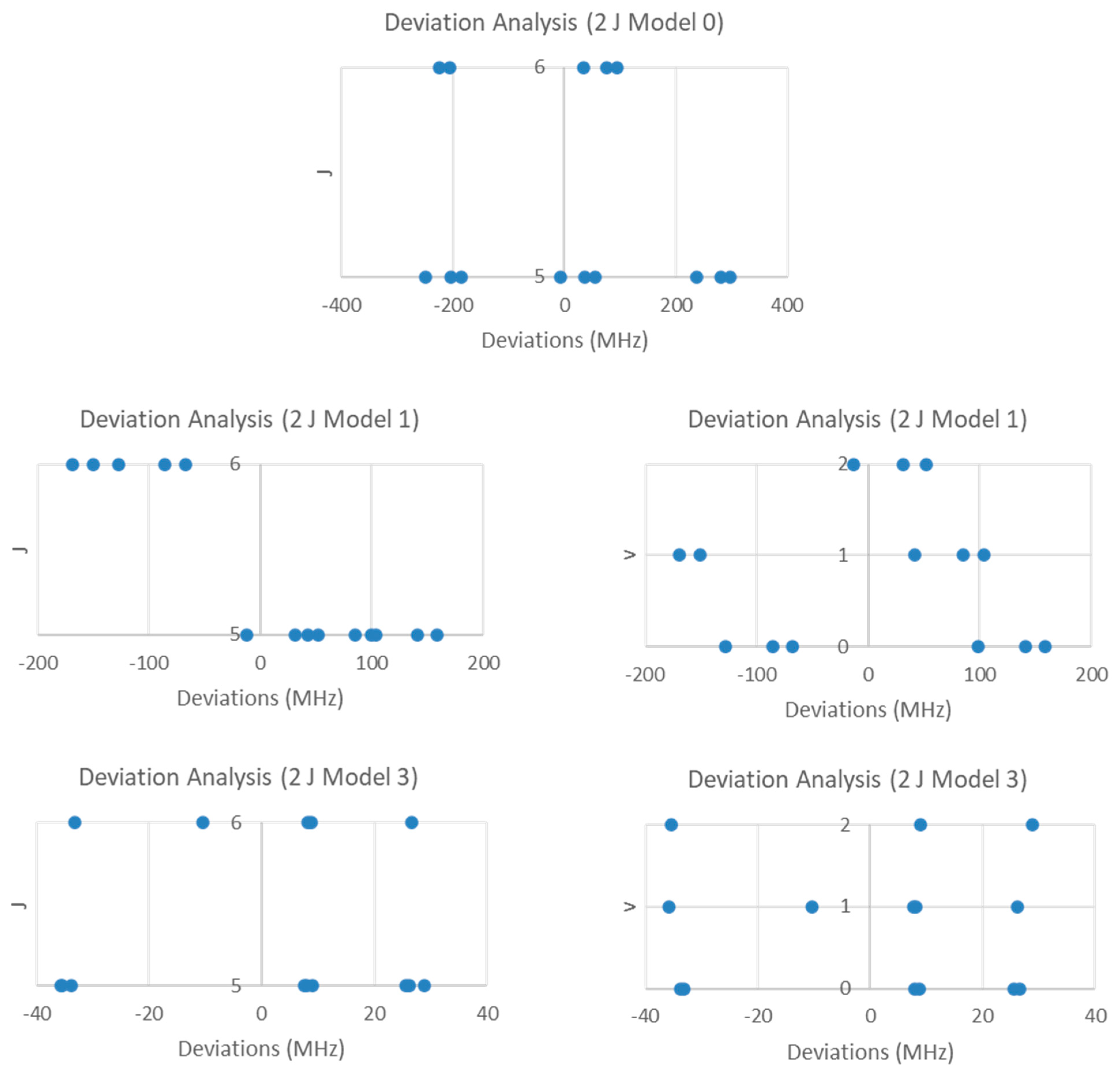
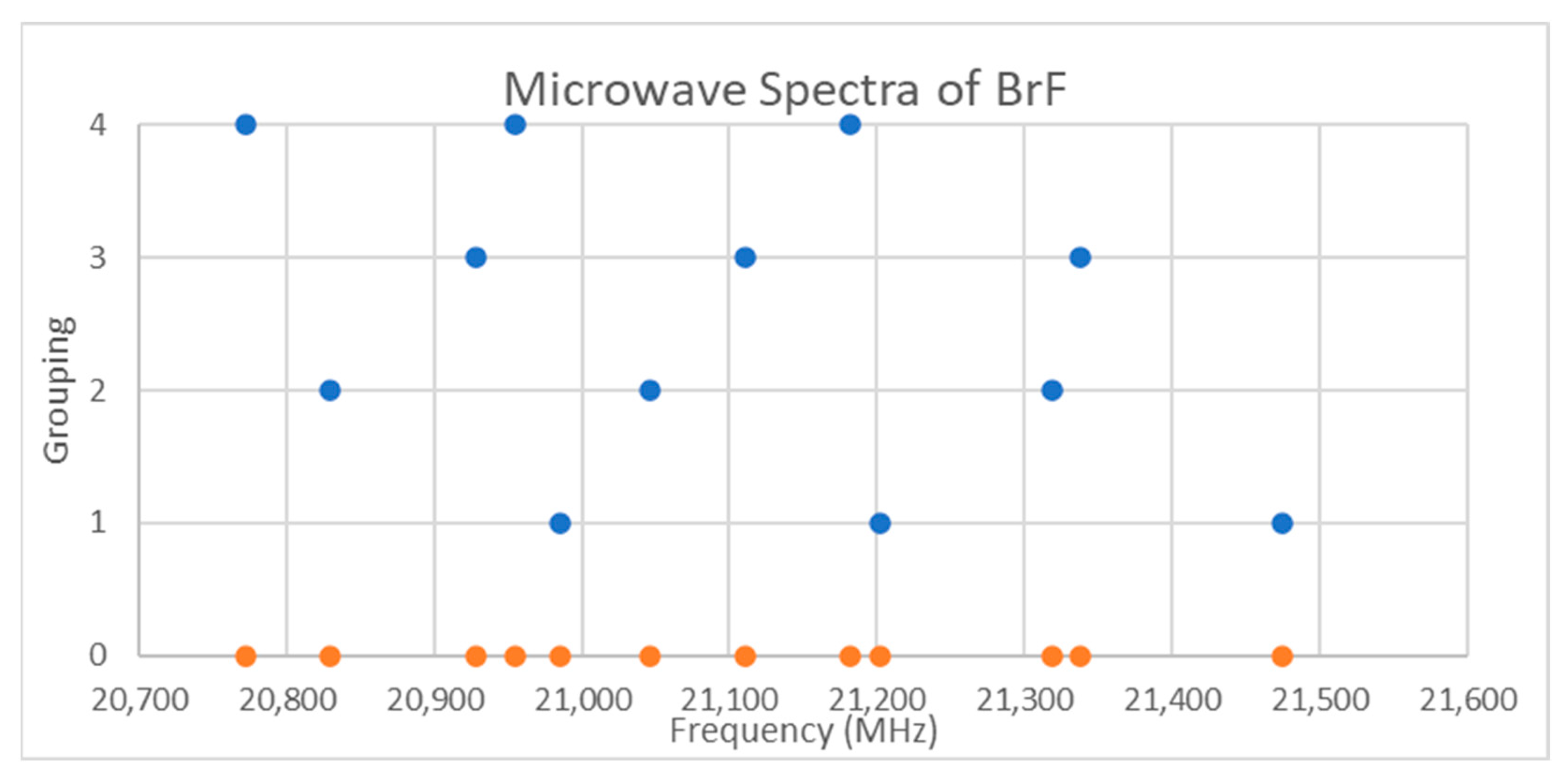
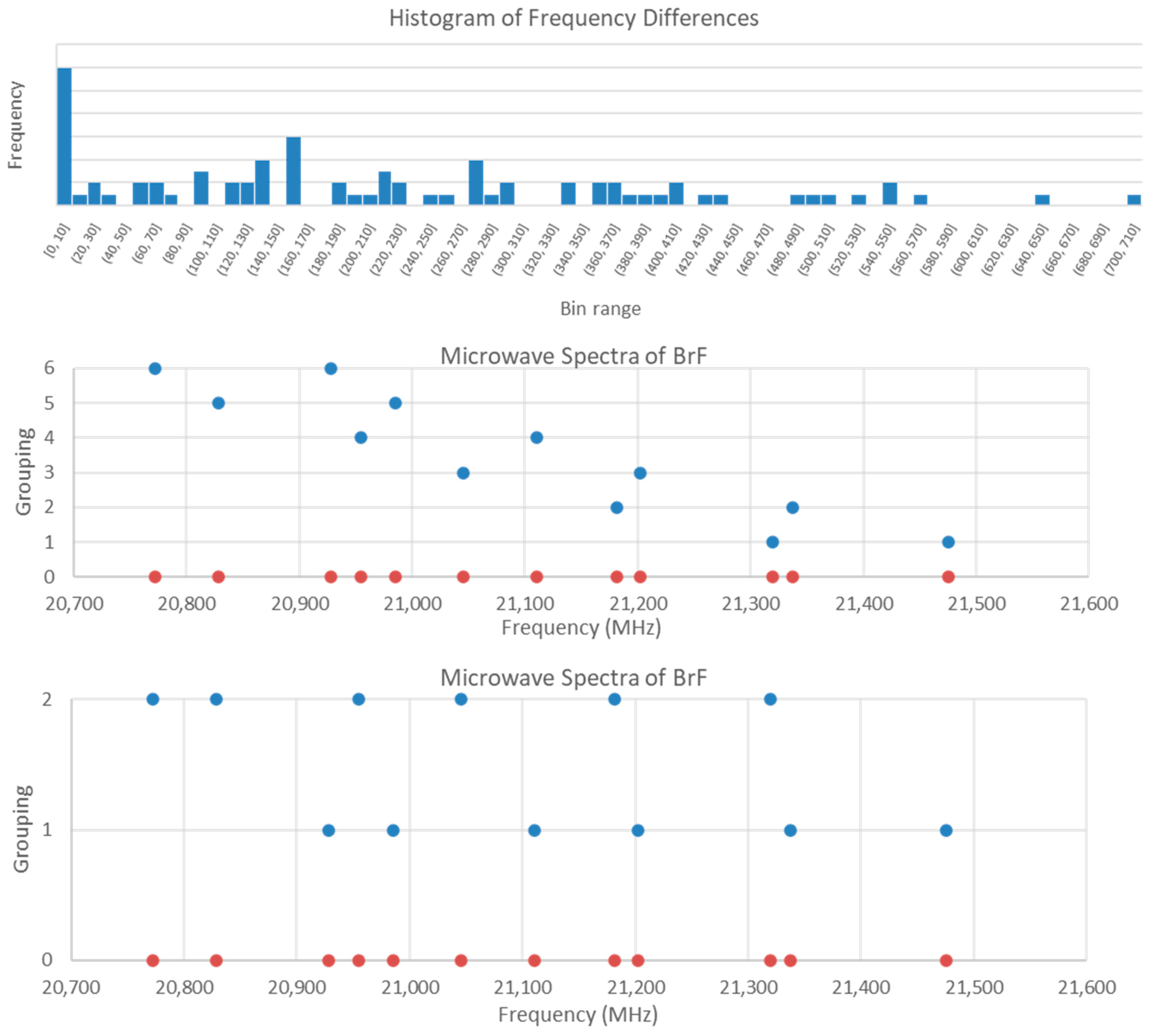


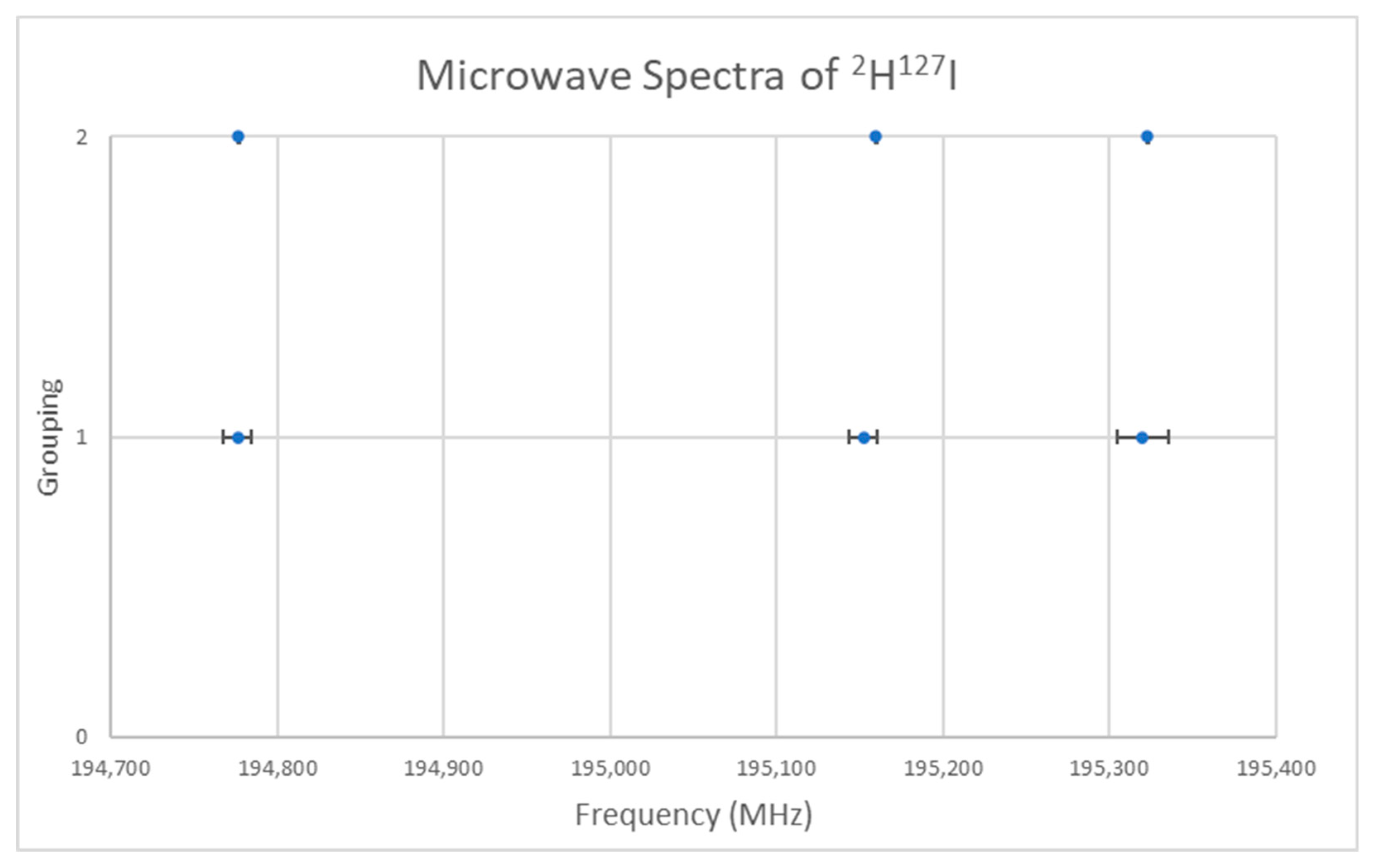
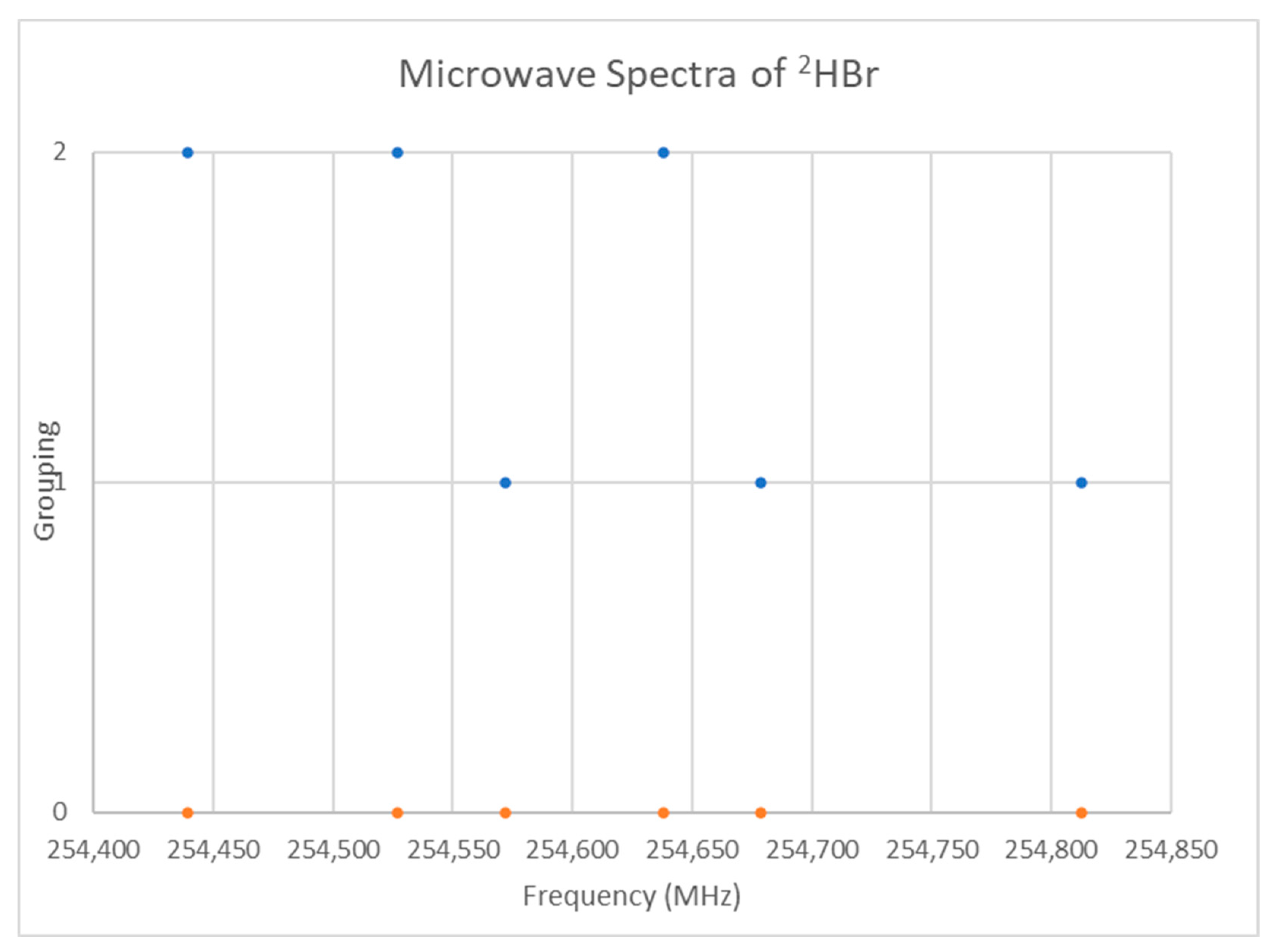
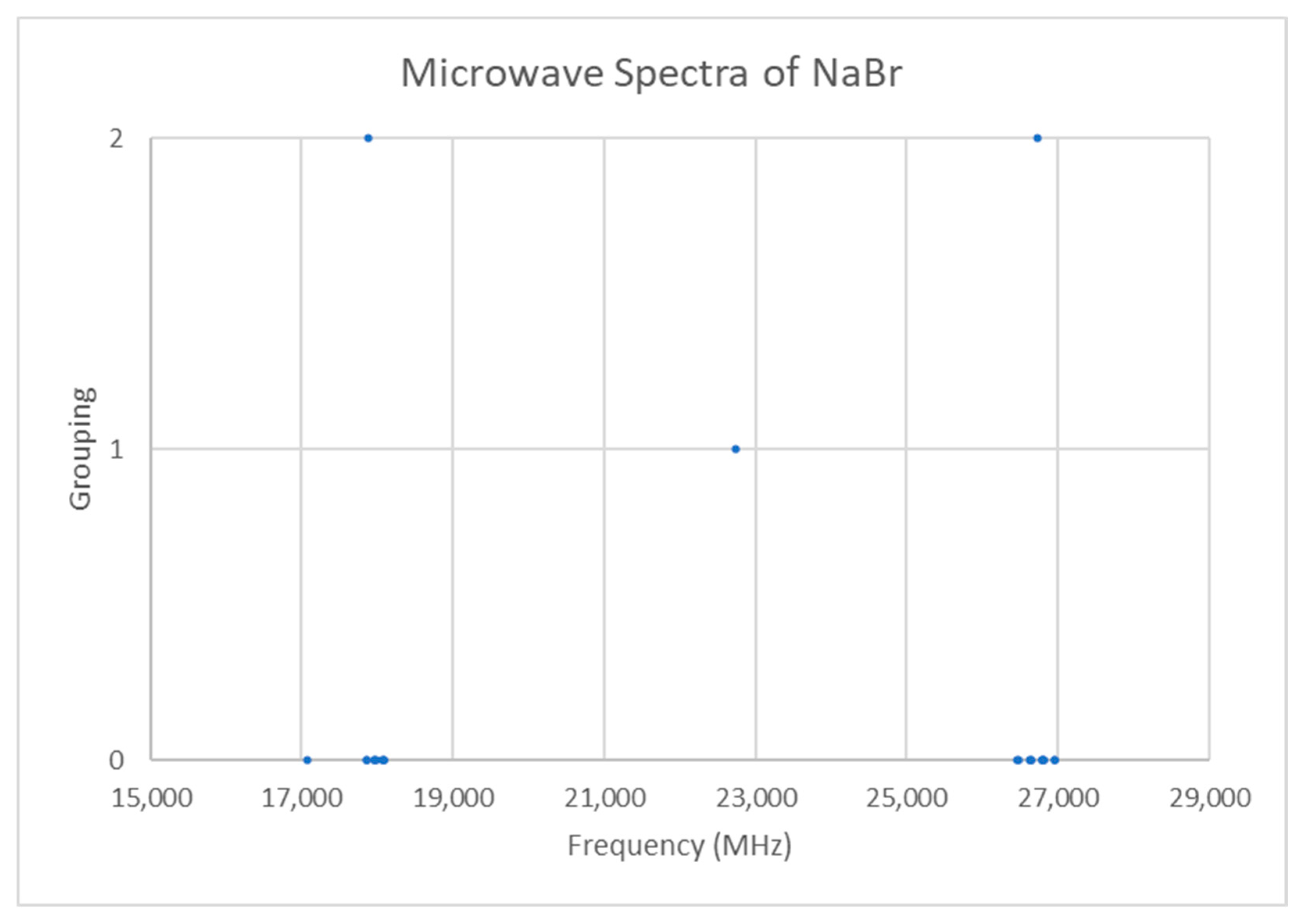

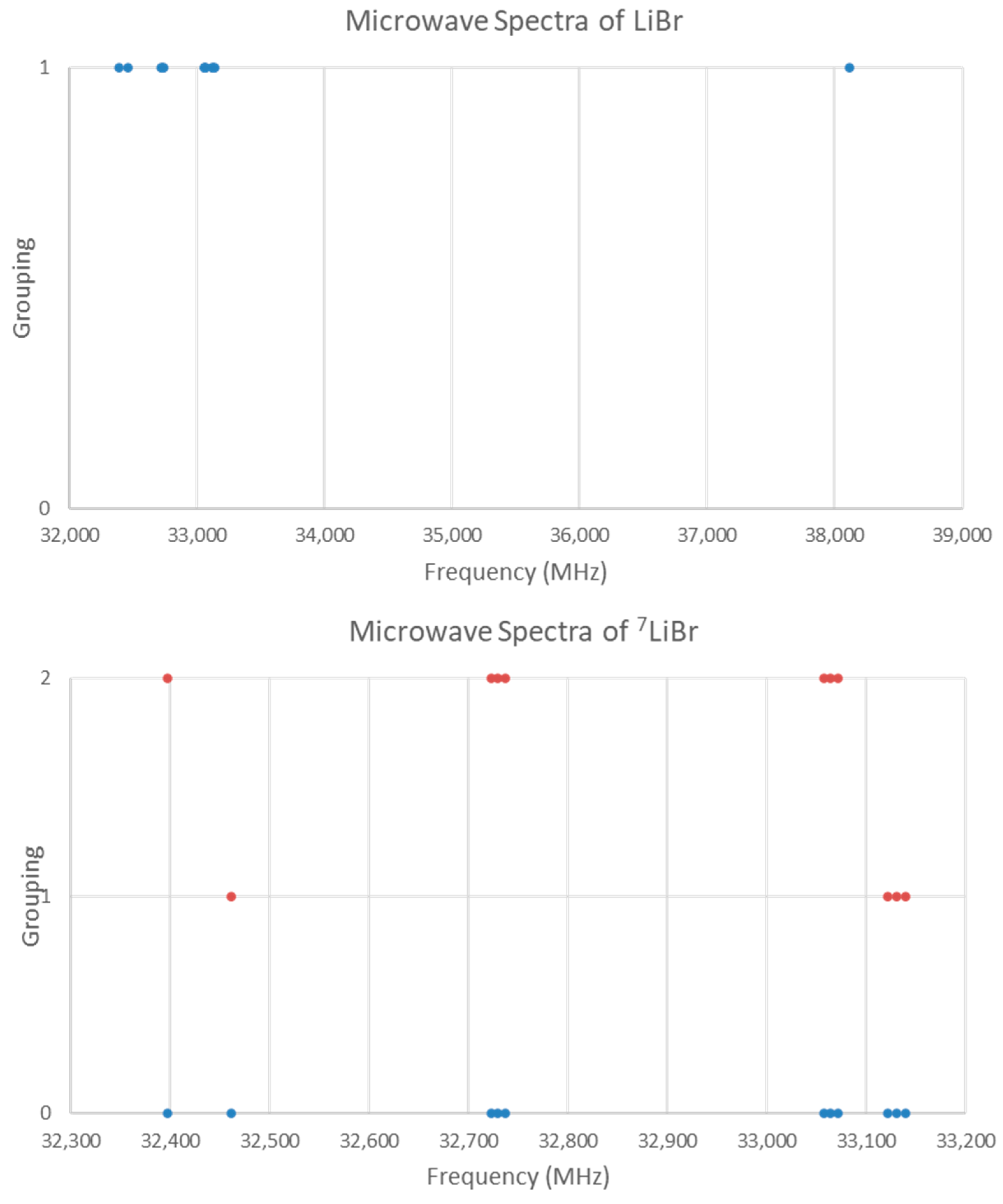
| Model | Be | αe | γe | eQq | SSE |
|---|---|---|---|---|---|
| 1 | 3530.486 | 19.218 | n/a | n/a | 8741.1 |
| 1-1 | 3531.567 | 19.226 | n/a | −268.133 | 8.099 |
| 2 | 3532.010 | 21.298 | −0.452 | n/a | 8260.4 |
| 2-1 | 3531.736 | 19.465 | +0.052 | −266.624 | 2.033 |
| 1-2 | 3531.553 | 19.219 | n/a | −268.5 −271.9 −270.0 −267.2 −258.9 | 5.597 |
| 2-2 | 3531.715 | 10.450 | 0.050 | −265.7 −271.1 −267.3 −264.7 −259.9 | 0.187 |
| Model | Be | αe | γe | eQq | SSE |
|---|---|---|---|---|---|
| 6Li127I | |||||
| 1 | 15,377.363 | 142.544 | n/a | n/a | 2058 |
| 1-1 | 15,380.614 | 151.044 | n/a | −199.993 | 0.203 |
| 1-2 | 15,380.779 | 151.369 | n/a | −206.8 −199.4 | 0.0002 |
| 2-1 | 15,380.977 | 152.012 | 0.484 | −199.993 | 0.203 |
| 7Li127I | |||||
| 1 | 13,286.504 | 121.179 | n/a | n/a | 6050 |
| 1-1 | 13,285.474 | 121.179 | n/a | −206.141 | 7.820 |
| 1-2 | 13,285.532 | 121.218 | n/a | −213.9 −206.1 −198.4 | 2.101 |
| 2-1 | 13,286.240 | 122.632 | 0.484 | −206.140 | 5.945 |
| 2-2 | 13,286.303 | 122.678 | 0.486 | −213.7 −206.5 −198.2 | 0.218 |
| MX | R (Å) 1 | T (K) | Ref. |
|---|---|---|---|
| ClF | 1.628(1) | 85 | [14] |
| BrF | 1.822(2) 2 | 123 | [15] |
| IF | 2.0 (est.) | ||
| BrCl | 2.178(2) 3 | 133 | [15] |
| 2.205(1) 4 | |||
| ICl | 2.37(4), 2.44(4) 5 | 195 | [16] |
| 2.351, 2.440 6 | 253 | [17] | |
| IBr | 2.521 | 293 | [18] |
| Model | Be | αe | eQq | SSE |
|---|---|---|---|---|
| 35Cl19F | ||||
| 1 | 15,486.061 | 130.666 | n/a | 4332 |
| 1-1 | 15,483.628 | 130.666 | −145.968 | 0.018 |
| 37Cl19F | ||||
| 1 | 15,191.085 | 126.958 | n/a | 2687 |
| 1-1 | 15,189.169 | 126.959 | −114.964 | 0.002 |
| Model | Be | αe | eQq | SSE |
|---|---|---|---|---|
| 79Br19F | ||||
| 1 | 10,649.717 | 78.267 | n/a | 241,356 |
| 1-1 | 10,667.875 | 78.267 | +1089.5 | 1.49 |
| 1-2 | 10,667.851 | 78.243 | +1090.2 +1088.8 | 1.39 |
| 81Br 19F | ||||
| 1 | 10,601.692 | 77.950 | n/a | 168,159 |
| 1-1 | 10,616.848 | 77.950 | +909.4 | 0.12 |
| 1-2 | 10,616.838 | 77.939 | +909.7 +909.1 | 0.10 |
| Model | Be | αe | γe | De 1 | eQq | SSE |
|---|---|---|---|---|---|---|
| 0 | 8317.031 | n/a | n/a | n/a | n/a | 4,605,220 |
| 0-1 | 8326.929 | n/a | n/a | n/a | −3406.72 | 352,033 |
| 1 | 8371.848 | 51.862 | n/a | n/a | n/a | 4,309,414 |
| 1-1 | 8386.775 | 56.567 | n/a | n/a | −3430.00 | 335.828 |
| 2-1 | 8386.464 | 55.877 | 0.285 | n/a | −3430.15 | 332.485 |
| 3-1 | 8389.135 | 56.449 | n/a | 0.640 | −3429.45 | 294.911 |
| 4-1 | 8388.806 | 55.580 | 0.358 | 0.656 | −3429.64 | 289.642 |
| Model | Be | αe | γe | eQq | SSE |
|---|---|---|---|---|---|
| 2H79Br | |||||
| 0 | 127,343.95 | n/a | n/a | n/a | 29,098 |
| 1 | 127,434.21 | 60.175 | n/a | n/a | 129.7 |
| 2 | 127,445.26 | 81.100 | 6.975 | n/a | exact |
| 0-1 | 127,352.87 | n/a | n/a | 534.87 | 0.222 |
| 2H81Br | |||||
| 0 | 127,267.35 | n/a | n/a | n/a | 19,817 |
| 1 | 127,341.82 | 49.65 | 96.0 | ||
| 2 | 127,351.33 | 67.650 | 6.000 | n/a | exact |
| 0-1 | 127,274.71 | n/a | n/a | 441.49 | 0.620 |
| Model | Be | αe | γe | De 1 | eQq | SSE |
|---|---|---|---|---|---|---|
| 23Na79Br | ||||||
| 1 | 4534.486 | 27.868 | n/a | n/a | n/a | 514.10 |
| 1-1 | 4533.933 | 27.718 | n/a | n/a | 55.351 | 12.89 |
| 2-1 | 4534.451 | 28.278 | −0.114 | n/a | 55.865 | 3.18 |
| 3-1 | 4534.148 | 27.672 | n/a | 0.042 | 54.983 | 10.95 |
| 4-1 | 4534.465 | 28.261 | −0.111 | 0.0048 | 55.812 | 3.15 |
| 23Na81Br | ||||||
| 1 | 4507.996 | 27.170 | n/a | n/a | n/a | 74.44 |
| 1-1 | 4508.673 | 27.454 | n/a | n/a | 43.525 | 5.06 |
| 2-1 | 4509.110 | 27.932 | −0.109 | n/a | 48.461 | 3.67 |
| 3-1 | 4508.992 | 27.402 | n/a | 0.052 | 46.984 | 4.01 |
| 4-1 | 4509.213 | 27.790 | −0.085 | 0.033 | 49.545 | 3.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pye, C.C. Interpreting the Microwave Spectra of Diatomic Molecules—Part II: Nuclear Quadrupole Coupling of One Nucleus. Spectrosc. J. 2024, 2, 82-104. https://doi.org/10.3390/spectroscj2030006
Pye CC. Interpreting the Microwave Spectra of Diatomic Molecules—Part II: Nuclear Quadrupole Coupling of One Nucleus. Spectroscopy Journal. 2024; 2(3):82-104. https://doi.org/10.3390/spectroscj2030006
Chicago/Turabian StylePye, Cory C. 2024. "Interpreting the Microwave Spectra of Diatomic Molecules—Part II: Nuclear Quadrupole Coupling of One Nucleus" Spectroscopy Journal 2, no. 3: 82-104. https://doi.org/10.3390/spectroscj2030006
APA StylePye, C. C. (2024). Interpreting the Microwave Spectra of Diatomic Molecules—Part II: Nuclear Quadrupole Coupling of One Nucleus. Spectroscopy Journal, 2(3), 82-104. https://doi.org/10.3390/spectroscj2030006






