Conformational Analysis of Trifluoroacetyl Triflate, CF3C(O)OSO2CF3: Experimental Vibrational and DFT Investigation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis of Trifluoroacetyl Triflate (TFAT)
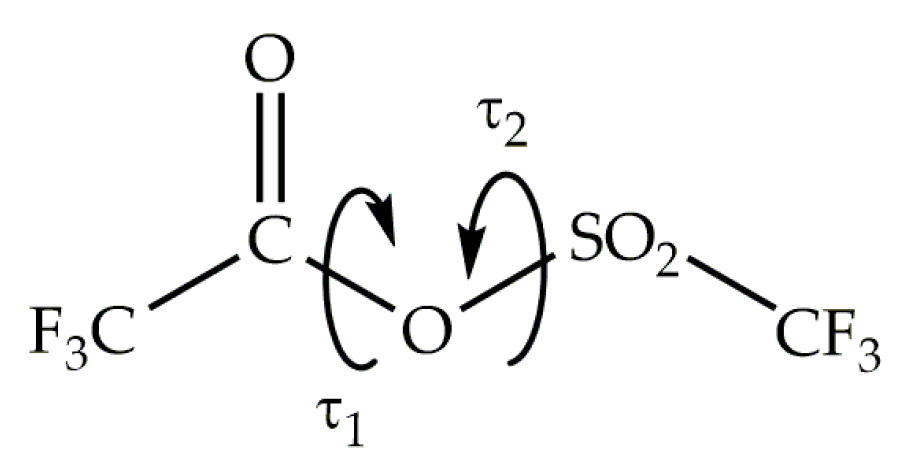
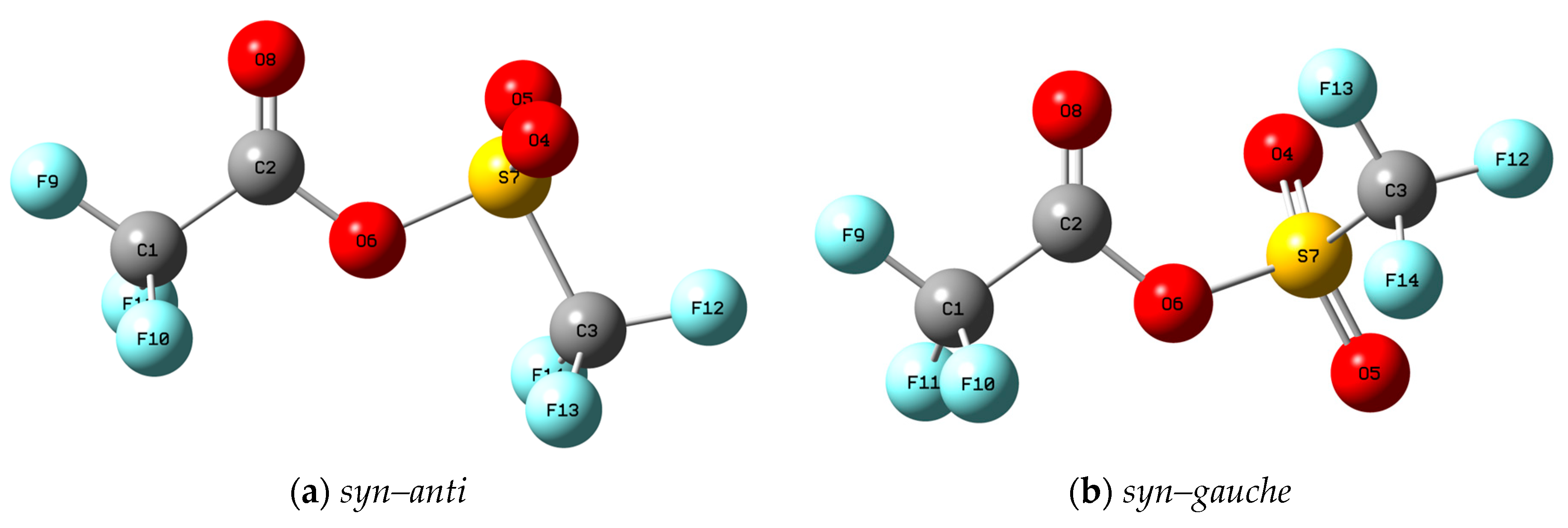
3.2. Quantum Chemical Calculations
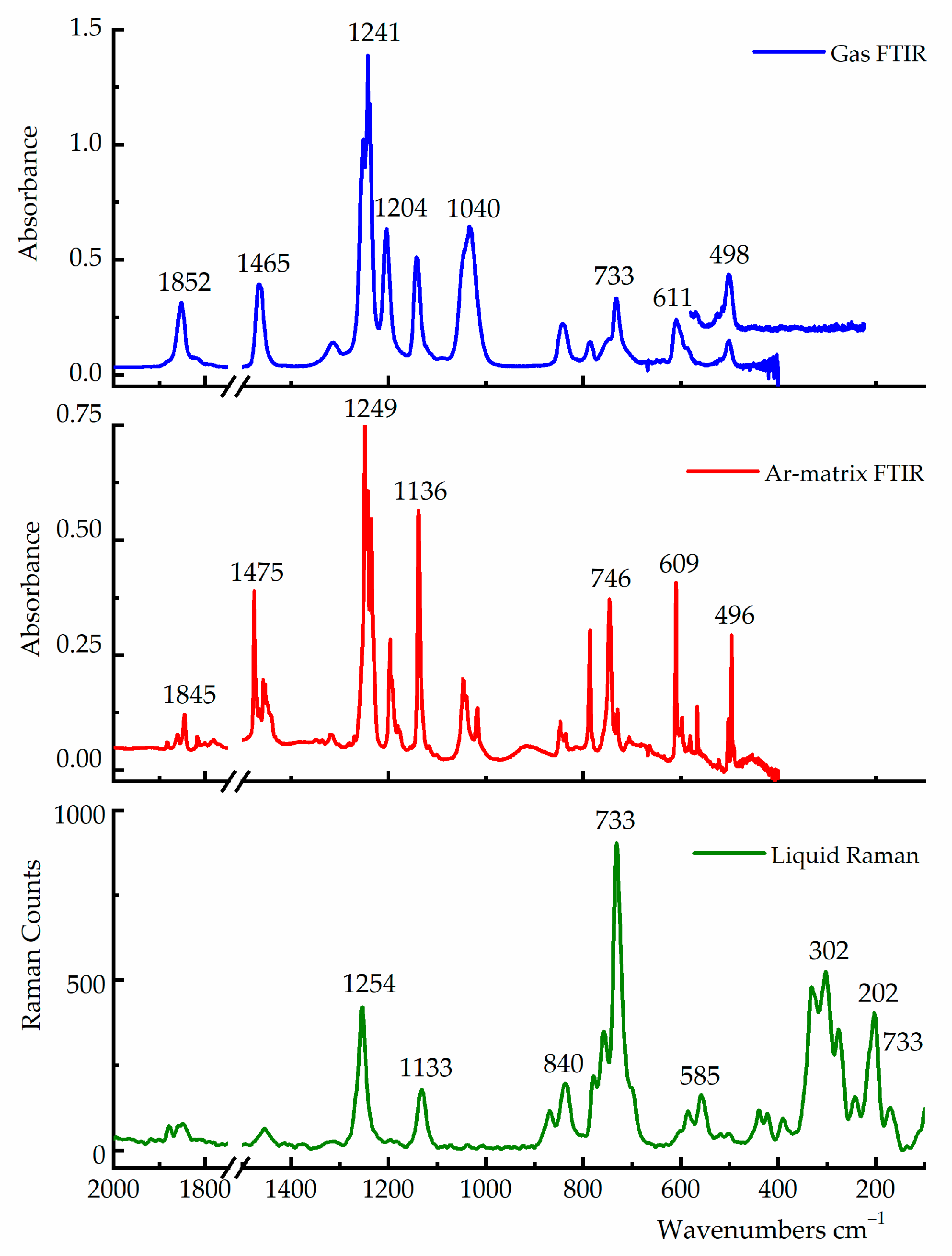
3.3. Vibrational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- López, S.E.; Restrepo, J.; Salazar, J. Trifluoroacetylation in Organic Synthesis: Reagents, Developments and Applications in the Construction of Trifluoromethylated Compounds. Curr. Org. Synth. 2010, 7, 414–432. [Google Scholar] [CrossRef]
- Forbus, T.R.; Taylor, S.L.; Martin, J.C. Reactions of the Readily Accessible Electrophile, Trifluoroacetyl Triflate: A Very Reactive Agent for Trifluoroacetylations at Oxygen, Nitrogen, Carbon, or Halogen Centers. J. Org. Chem. 1987, 52, 4156–4159. [Google Scholar] [CrossRef]
- Kiselyov, A.S.; Harvey, R.G. Acylation of Activated Aromatic Substrates under Mild Conditions with (RCO)2O/Me2S/BF3. Tetrahedron Lett. 1995, 23, 4005–4008. [Google Scholar] [CrossRef]
- Maas, G.; Stang, P.J. Dication disulfides by reaction of thioureas and related compounds with trifluoromethanesulfonic anhydride. The role of triflic anhydride as an oxidizing agent. J. Org. Chem. 1981, 46, 1606–1610. [Google Scholar] [CrossRef]
- Michalak, R.S.; Martin, J.C. Persulfonium salts: The reaction of a difluoropersulfurane with Lewis acids. J. Am. Chem. Soc. 1980, 102, 5921–5923. [Google Scholar] [CrossRef]
- Della Védova, C.O.; Downs, A.J.; Novikov, V.P.; Oberhammer, H.; Parsons, S.; Romano, R.M.; Zawadski, A. Fluorocarbonyl Trifluoromethanesulfonate, FC(O)OSO2CF3: Structure and Conformational Properties in the Gaseous and Condensed Phases. Inorg. Chem. 2004, 43, 4064–4071. [Google Scholar] [CrossRef] [PubMed]
- Della Védova, C.O.; Downs, A.J.; Moschione, E.; Parsons, S.; Romano, R.M. Chlorocarbonyl Trifluoromethanesulfonate, ClC(O)OSO2CF3: Structure and Conformational Properties in the Gaseous and Condensed Phases. Inorg. Chem. 2004, 43, 8143–8149. [Google Scholar] [CrossRef]
- Trautner, F.; Della Védova, C.O.; Romano, R.M.; Oberhammer, H. Gas phase structure and conformational properties of chlorocarbonyl trifluoromethanesulfonate, ClC(O)OSO2CF3. J. Mol. Struct. 2006, 784, 272–275. [Google Scholar] [CrossRef]
- Romano, R.M.; Moreno Betancourt, A.; Della Vedova, C.O.; Zeng, X.; Beckers, H.; Willner, H.; Schwabedissen, J.; Mitzel, N.W. Preparation and properties of chlorosulfuryl chloroformate, ClC(O)OSO2Cl. Inorg. Chem. 2018, 57, 14834–14842. [Google Scholar] [CrossRef]
- Della Védova, C.O.; Mack, H.G. A matrix photochemistry study on (fluorocarbonyl) sulfenyl bromide: The precursor of sulfur bromide fluoride. Inorg. Chem. 1993, 32, 948–950. [Google Scholar] [CrossRef]
- Tamone, L.M.; Picone, A.L.; Romano, R.M. New insights into the Ar-matrix-isolation FTIR spectroscopy and photochemistry of dichloroacetyl chloride, ClC(O)CHCl2: Influence of O2 and comparison with gas-phase photochemistry. J. Photochem. Photobiol. 2021, 6, 100019. [Google Scholar] [CrossRef]
- Bava, Y.B.; Cozzarín, M.V.; Della Védova, C.O.; Willner, H.; Romano, R.M. Preparation of FC(S)SF, FC(S) SeF and FC(Se)SeF through matrix photochemical reactions of F2 with CS2, SCSe, and CSe2. Phys. Chem. Chem. Phys 2021, 23, 20892–20900. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.M.; Della Vedova, C.O.; Downs, A.J.; Greene, T.M. Matrix Photochemistry of syn-(Chlorocarbonyl) sulfenyl Bromide, syn-ClC(O) SBr: Precursor to the Novel Species anti-ClC(O)SBr, syn-BrC(O)SCl, and BrSCl. J. Am. Chem. Soc. 2001, 123, 5794–5801. [Google Scholar] [CrossRef] [PubMed]
- Forbus, T.R.; Martin, J.C. Trifluoroacetyl Triflate: An Easily Accessible, Highly Electrophilic Trifluoroacetylating Agent. J. Org. Chem. 1979, 44, 313–314. [Google Scholar] [CrossRef]
- Taylor, S.L.; Forbus, T.R.; Martin, J.C. Trifloroacetyl Triflate: Acetic Acid, Trifluoro-, Anhydride with Trifluoromethanesulfonic Acid. Organic Syntheses; Kende, A.S., Freeman, J.P., Eds.; Wiley: Hoboken, NJ, USA, 2003; p. 217. [Google Scholar]
- Taylor, S.L.; Forbus, T.R., Jr.; Martin, J.C. Trifluoroacetyl Triflate. Org. Synth. 1986, 64, 217. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Glendening, E.D.; Devin, M.H.; Weinhold, F. Natural Bond Orbital Analysis of chemical structure, spectroscopy, and reactivity: How it works. In Comprehensive Computational Chemistry, 1st ed.; Yáñez, M., Boyd, R.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 406–421. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar]
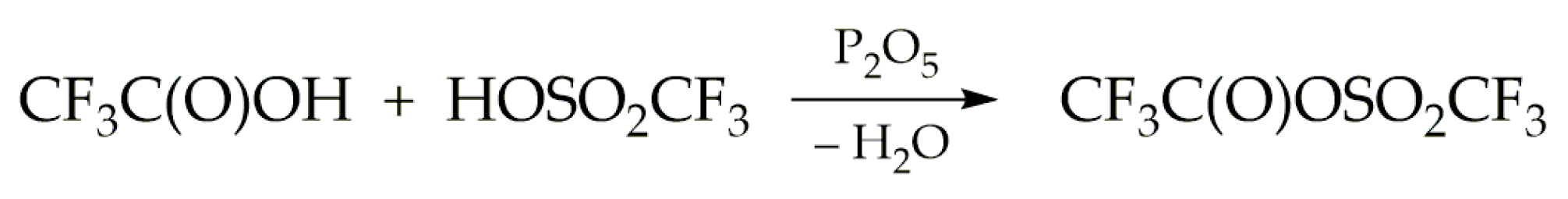





| Theoretical Approximation | ΔE (Kcal·mol−1) 1 | ΔG° (Kcal·mol−1) 2 | % syn–anti 3 |
|---|---|---|---|
| B3LYP/6-31++G(d) | 1.26 | 2.22 | 96 |
| B3LYP/6-311++G(d) | 1.23 | 2.02 | 94 |
| B3LYP/tzvp | 1.11 | 1.34 | 83 |
| B3LYP/cc-pvtz | 0.87 | 0.88 | 69 |
| MP2/6-31++G(d) | 0.70 | 0.98 | 72 |
| Orbital Interaction 1 | syn–anti | syn–gauche |
|---|---|---|
| lpπO6 → π*C2=O8 | 39.20 | 35.65 |
| lpσO6 → σ*C2=O8 | 7.20 | 7.48 |
| lpσO6 → σ*S=O4 | 5.42 | − |
| lpσO6 → σ*S=O5 | 5.42 | − |
| lpσO6 → σ*S−C3 | − | 5.93 |
| Total anomeric effect | 18.04 | 13.41 |
| Total hyperconjugative effect | 39.20 | 35.65 |
| Total | 57.24 | 49.06 |
| syn–anti | syn–gauche | |
|---|---|---|
| Wavelength (nm) | 226 | 229 |
| Oscillator strength | 0.0013 | 0.0014 |
| Assignment | HOMO → LUMO | HOMO → LUMO |
| lppO → π*C=O | lppO → π*C=O |
| Experimental a | B3LYP/cc-pvtz c,d | Tentative Assignment | |||
|---|---|---|---|---|---|
| Gas-FTIR ν (cm−1) | Ar matrix FTIR ν (cm−1) b | Liquid Raman ν (cm−1) | syn–anti | syn–gauche | |
| 1852 m | 1860 w | 1880 | 1892 (37) | ν C=O syn–gauche | |
| 1845 w | 1851 | 1882 (38) | ν C=O syn–anti | ||
| 1465 m | 1475 m | 1455 | 1434 (33) | νas SO2 syn–anti | |
| 1453 m | 1426 (35) | νas SO2 syn–gauche | |||
| 1314 w | 1319 vw | 1317 | 1301 (7) | ν C−CF3 syn–anti | |
| 1315 vw | 1293 (9) | ν C−CF3 syn–gauche | |||
| 1252 s | 1249 s | 1242 (35) | νas CF3 (−SO2) syn–anti | ||
| 1248 s | 1241 (64) | νas CF3 (−SO2)syn–gauche | |||
| 1241 s | 1243 s | 1266 | 1236 (41) | νas CF3 (−C=O) syn–anti | |
| 1241 s | 1234 (36) | νas CF3 (−C=O) syn–gauche | |||
| 1234 sh | 1236 m | 1231 (31) | 1231 (29) | νas CF3 (−SO2) | |
| 1228 m | 1254 | 1225 (27) | 1225 (6) | νs SO2 syn–anti | |
| 1204 m | 1200 w | 1182 (44) | νas CF3 (−C=O)syn–gauche | ||
| 1196 m | 1179 (35) | νas CF3 (−C=O)syn–anti | |||
| 1142 m | 1136 s | 1133 | 1106 (36) | 1106 (42) | νs CF3 (−SO2) |
| 1040 s | 1047 m | 1061 (100) | ν C−Osyn–anti | ||
| 1018 m | 1043 (100) | ν C−Osyn–gauche | |||
| 842 w | 847 w | 840 | 852 (15) | δ OCO syn–anti | |
| 836 w | 843 (6) | δ OCO syn–gauche | |||
| 788 w | 788 w | 780 | 774 (7) | δo.o.p. (C=O) syn–gauche | |
| 786 m | 765 (2) | δo.o.p. (C=O) syn–anti | |||
| 779 sh | 780 vw | 770 (<1) | 770 (1) | νs CF3 (−SO2) | |
| 751 w | 746 m | 759 | 741 (9) | δs CF3 (−C=O) syn–anti | |
| 733 m | 730 w | 734 (8) | δs CF3 (−C=O) syn–gauche | ||
| 704 sh | 705 vw | 733 | 686 (46) | 686 (46) | ν S−O |
| 611 m | 611 m | 594 (20) | ω SO2 syn–gauche | ||
| 609 m | 587 (10) | ω SO2 syn–anti | |||
| 600 w | 601 w | 585 | 575 (7) | ν C−S syn–gauche | |
| 598 w | 566 (8) | ν C−S syn–anti | |||
| 585 sh | 580 vw | 556 (<1) | 554 (<1) | δ CF3 (−SO2) | |
| 566 vw | 566 w | 559 | 544 (1) | 547 (<1) | δ CF3 (−SO2) |
| 522 vw | 521 vw | 519 (1) | 517 (<1) | δ CF3 (−C=O) | |
| 498 w | 496 m | 489 (7) | δ SO2 syn–anti | ||
| 492 w | 487 (10) | δ SO2 syn–gauche | |||
| 438 | 432 | 428 | δ O=S=O | ||
| 421 | 415 | 381 | δ C−C=O | ||
| 330 | 325 | 335 | δ F−C−C | ||
| 302 | 300 | 284 | δ F−C−C | ||
| 276 | 257 | 277 | δ F−C−S | ||
| 242 | 236 | 243 | ω CF3 (−C=O) | ||
| 202 | 186 | 200 | δ C−O−S | ||
| 170 | 159 | 149 | δ O−S−C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spaltro, A.; Peluas, M.G.; Della Védova, C.O.; Romano, R.M. Conformational Analysis of Trifluoroacetyl Triflate, CF3C(O)OSO2CF3: Experimental Vibrational and DFT Investigation. Spectrosc. J. 2024, 2, 68-81. https://doi.org/10.3390/spectroscj2020005
Spaltro A, Peluas MG, Della Védova CO, Romano RM. Conformational Analysis of Trifluoroacetyl Triflate, CF3C(O)OSO2CF3: Experimental Vibrational and DFT Investigation. Spectroscopy Journal. 2024; 2(2):68-81. https://doi.org/10.3390/spectroscj2020005
Chicago/Turabian StyleSpaltro, Agustín, Melina G. Peluas, Carlos O. Della Védova, and Rosana M. Romano. 2024. "Conformational Analysis of Trifluoroacetyl Triflate, CF3C(O)OSO2CF3: Experimental Vibrational and DFT Investigation" Spectroscopy Journal 2, no. 2: 68-81. https://doi.org/10.3390/spectroscj2020005
APA StyleSpaltro, A., Peluas, M. G., Della Védova, C. O., & Romano, R. M. (2024). Conformational Analysis of Trifluoroacetyl Triflate, CF3C(O)OSO2CF3: Experimental Vibrational and DFT Investigation. Spectroscopy Journal, 2(2), 68-81. https://doi.org/10.3390/spectroscj2020005







