Nontrivial Impact of Relative Humidity on Organic New Particle Formation from Ozonolysis of cis-3-Hexenyl Acetate
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
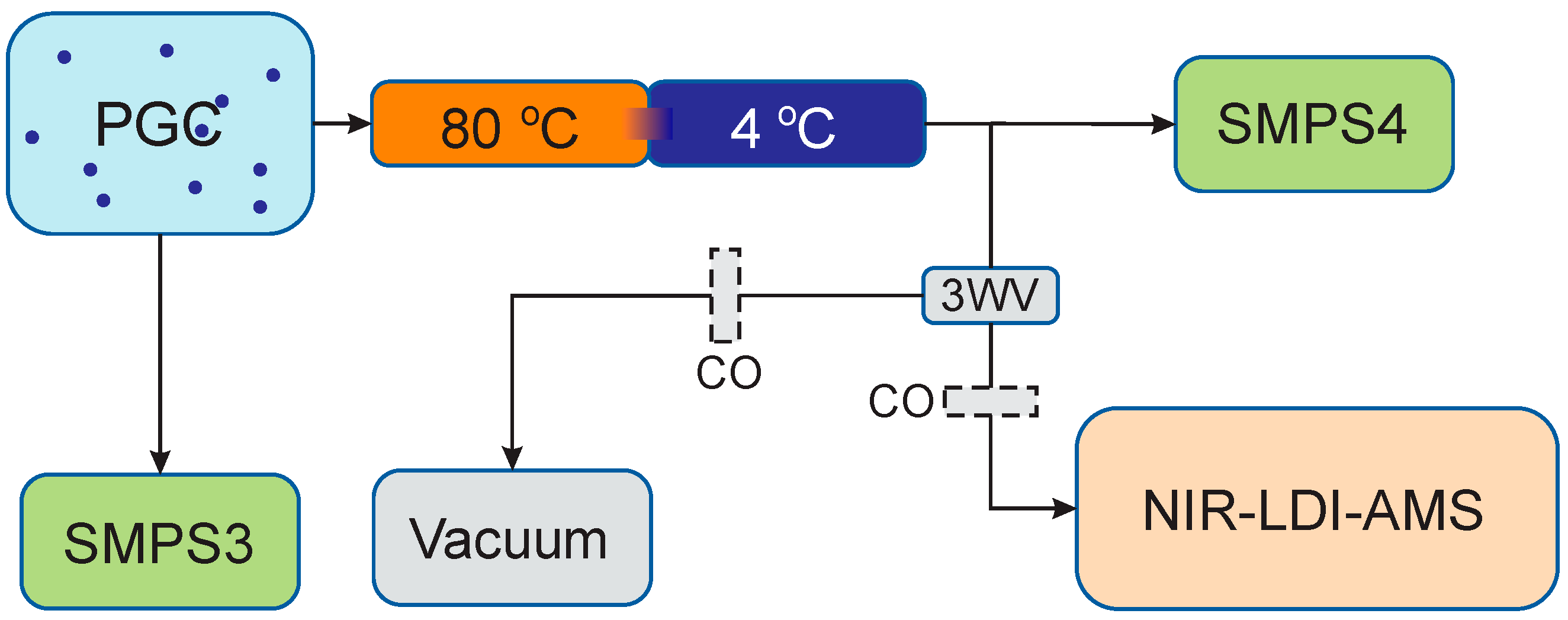
2.2. Experimental Conditions and Instrumentation
2.3. General Methodology
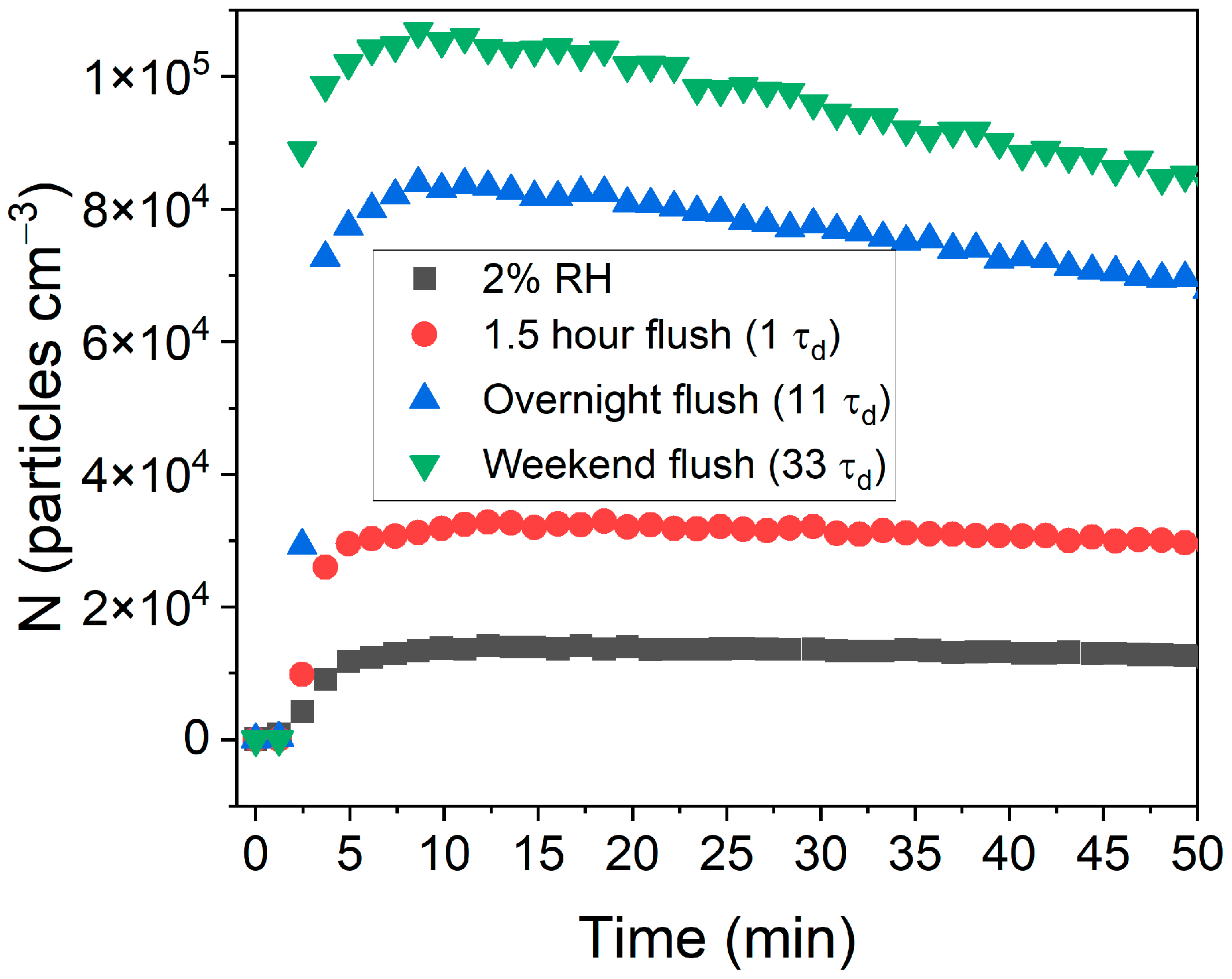
2.4. Humidification of the Chambers
2.5. AMS Methodology
3. Results and Discussion
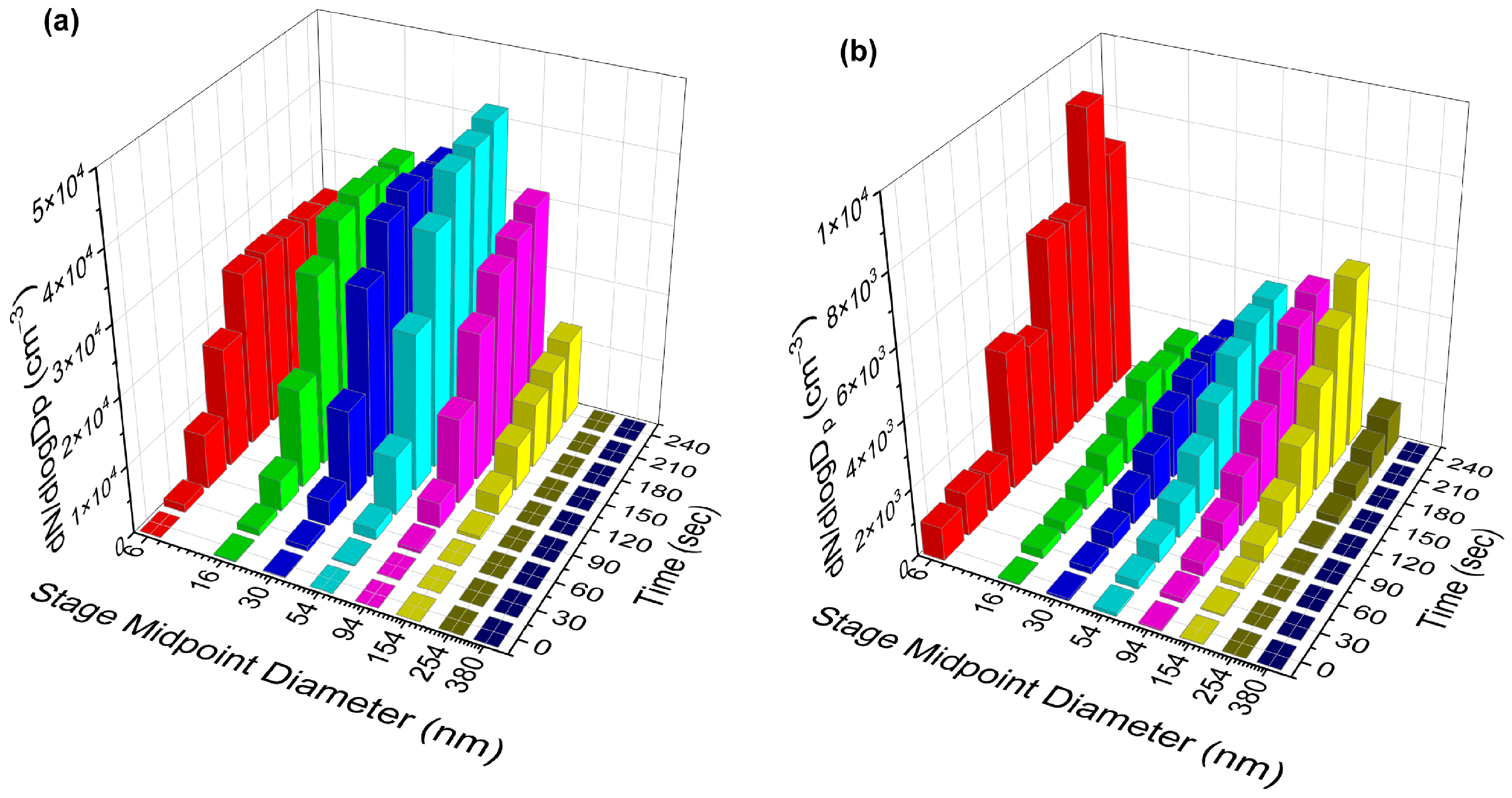
3.1. Effect of Low (<2%) RH on NPF
3.2. Effect of Solubility on Decreased NPF
3.3. Water-Facilitated Chemistry
3.4. Proposed Chemical Mechanisms
3.5. Quaternary System Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model. Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Wiedinmyer, C.; Guenther, A.; Harley, P.; Hewitt, N.; Geron, C.; Artaxo, P.; Steinbrecher, R.; Rasmussen, R. Global Organic Emissions from Vegetation; Springer: Dordrecht, The Netherlands, 2004; pp. 115–170. [Google Scholar]
- Mahilang, M.; Deb, M.K.; Pervez, S. Biogenic secondary organic aerosols: A review on formation mechanism, analytical challenges and environmental impacts. Chemosphere 2021, 262, 127771. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Situ, S.; Hao, Y.F.; Xie, S.D.; Li, L.Y. Enhanced summertime ozone and SOA from biogenic volatile organic compound (BVOC) emissions due to vegetation biomass variability during 1981–2018 in China. Atmos. Chem. Phys. 2022, 22, 2351–2364. [Google Scholar] [CrossRef]
- Sarang, K.; Rudzinski, K.J.; Szmigielski, R. Green Leaf Volatiles in the Atmosphere-Properties, Transformation, and Significance. Atmosphere 2021, 12, 1655. [Google Scholar] [CrossRef]
- Barreira, L.M.F.; Ylisirnio, A.; Pullinen, I.; Buchholz, A.; Li, Z.J.; Lipp, H.; Junninen, H.; Horrak, U.; Noe, S.M.; Krasnova, A.; et al. The importance of sesquiterpene oxidation products for secondary organic aerosol formation in a springtime hemiboreal forest. Atmos. Chem. Phys. 2021, 21, 11781–11800. [Google Scholar] [CrossRef]
- Bianchi, F.; Junninen, H.; Bigi, A.; Sinclair, V.A.; Dada, L.; Hoyle, C.R.; Zha, Q.; Yao, L.; Ahonen, L.R.; Bonasoni, P.; et al. Biogenic particles formed in the Himalaya as an important source of free tropospheric aerosols. Nat. Geosci. 2021, 14, 4–9. [Google Scholar] [CrossRef]
- Donahue, N.M.; Ortega, I.K.; Chuang, W.; Riipinen, I.; Riccobono, F.; Schobesberger, S.; Dommen, J.; Baltensperger, U.; Kulmala, M.; Worsnop, D.R.; et al. How do organic vapors contribute to new-particle formation? Faraday Discuss. 2013, 165, 91–104. [Google Scholar] [CrossRef]
- Kammer, J.; Flaud, P.M.; Chazeaubeny, A.; Ciuraru, R.; Le Menach, K.; Geneste, E.; Budzinski, H.; Bonnefond, J.M.; Lamaud, E.; Perraudin, E.; et al. Biogenic volatile organic compounds (BVOCs) reactivity related to new particle formation (NPF) over the Landes forest. Atmos. Res. 2020, 237, 104869. [Google Scholar] [CrossRef]
- Wiedensohler, A.; Ma, N.; Birmili, W.; Heintzenberg, J.; Ditas, F.; Andreae, M.O.; Panov, A. Infrequent new particle formation over the remote boreal forest of Siberia. Atmos. Environ. 2019, 200, 167–169. [Google Scholar] [CrossRef]
- Zhao, B.; Shrivastava, M.; Donahue, N.M.; Gordon, H.; Schervish, M.; Shilling, J.E.; Zaveri, R.A.; Wang, J.; Andreae, M.O.; Zhao, C.; et al. High concentration of ultrafine particles in the Amazon free troposphere produced by organic new particle formation. Proc. Natl. Acad. Sci. USA 2020, 117, 25344–25351. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Zahardis, J.; Petrucci, G.A. Soft Ionization Chemical Analysis of Secondary Organic Aerosol from Green Leaf Volatiles Emitted by Turf Grass. Environ. Sci. Technol. 2014, 48, 4835–4843. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.M.; Bateman, A.P.; Jain, S.; Li, Y.J.; Martin, S.; Petrucci, G.A. Optical Properties of Secondary Organic Aerosol from cis-3-Hexenol and cis-3-Hexenyl Acetate: Effect of Chemical Composition, Humidity, and Phase. Environ. Sci. Technol. 2016, 50, 4997–5006. [Google Scholar] [CrossRef] [PubMed]
- Artaxo, P.; Hansson, H.C.; Andreae, M.O.; Back, J.; Alves, E.G.; Barbosa, H.M.J.; Bender, F.; Bourtsoukidis, E.; Carbone, S.; Chi, J.S.; et al. Tropical and Boreal Forest Atmosphere Interactions: A Review. Tellus B 2022, 74, 24–163. [Google Scholar] [CrossRef]
- Donahue, N.M.; Robinson, A.L.; Pandis, S.N. Atmospheric organic particulate matter: From smoke to secondary organic aerosol. Atmos. Environ. 2009, 43, 94–106. [Google Scholar] [CrossRef]
- Harvey, R.M.; Zahardis, J.; Petrucci, G.A. Establishing the contribution of lawn mowing to atmospheric aerosol levels in American suburbs. Atmos. Chem. Phys. 2014, 14, 797–812. [Google Scholar] [CrossRef]
- Porter, W.C.; Jimenez, J.L.; Barsanti, K.C. Quantifying Atmospheric Parameter Ranges for Ambient Secondary Organic Aerosol Formation. ACS Earth Space Chem. 2021, 5, 2380–2397. [Google Scholar] [CrossRef]
- Lampilahti, J.; Manninen, H.E.; Nieminen, T.; Mirme, S.; Ehn, M.; Pullinen, I.; Leino, K.; Schobesberger, S.; Kangasluoma, J.; Kontkanen, J.; et al. Zeppelin-led study on the onset of new particle formation in the planetary boundary layer. Atmos. Chem. Phys. 2021, 21, 12649–12663. [Google Scholar] [CrossRef]
- Franco, M.A.; Ditas, F.; Kremper, L.A.; Machado, L.A.T.; Andreae, M.O.; Araújo, A.; Barbosa, H.M.J.; de Brito, J.F.; Carbone, S.; Holanda, B.A.; et al. Occurrence and growth of sub-50 nm aerosol particles in the Amazonian boundary layer. Atmos. Chem. Phys. 2022, 22, 3469–3492. [Google Scholar] [CrossRef]
- Lee, S.H.; Gordon, H.; Yu, H.; Lehtipalo, K.; Haley, R.; Li, Y.X.; Zhang, R.Y. New Particle Formation in the Atmosphere: From Molecular Clusters to Global Climate. J. Geophys. Res.-Atmos. 2019, 124, 7098–7146. [Google Scholar] [CrossRef]
- Baalbaki, R.; Pikridas, M.; Jokinen, T.; Laurila, T.; Dada, L.; Bezantakos, S.; Ahonen, L.; Neitola, K.; Maisser, A.; Bimenyimana, E.; et al. Towards understanding the characteristics of new particle formation in the Eastern Mediterranean. Atmos. Chem. Phys. 2021, 21, 9223–9251. [Google Scholar] [CrossRef]
- Zimmerman, A.; Petters, M.D.; Meskhidze, N. Observations of new particle formation, modal growth rates, and direct emissions of sub-10 nm particles in an urban environment. Atmos. Environ. 2020, 242, 117835. [Google Scholar] [CrossRef] [PubMed]
- Sulo, J.; Sarnela, N.; Kontkanen, J.; Ahonen, L.; Paasonen, P.; Laurila, T.; Jokinen, T.; Kangasluoma, J.; Junninen, H.; Sipila, M.; et al. Long-term measurement of sub-3 nm particles and their precursor gases in the boreal forest. Atmos. Chem. Phys. 2021, 21, 695–715. [Google Scholar] [CrossRef]
- Snyder, C.N.; Flueckiger, A.C.; Petrucci, G.A. Relative Humidity Impact on Organic New Particle Formation from Ozonolysis of α- and β-Pinene at Atmospherically Relevant Mixing Ratios. Atmosphere 2023, 14, 173. [Google Scholar] [CrossRef]
- Jonsson, A.M.; Hallquist, M.; Ljungstrom, E. Impact of humidity on the ozone initiated oxidation of limonene, Delta(3)-carene, and alpha-pinene. Environ. Sci. Technol. 2006, 40, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Chee, S.; Hao, J.M.; Abbatt, J.P.D.; Jiang, J.K.; Smith, J.N. Relative humidity effect on the formation of highly oxidized molecules and new particles during monoterpene oxidation. Atmos. Chem. Phys. 2019, 19, 1555–1570. [Google Scholar] [CrossRef]
- Pommer, L.; Fick, J.; Andersson, B.; Nilsson, C. The influence of O-3, relative humidity, NO and NO2 on the oxidation of alpha-pinene and Delta(3)-carene. J. Atmos. Chem. 2004, 48, 173–189. [Google Scholar] [CrossRef]
- Ma, Y.; Russell, A.T.; Marston, G. Mechanisms for the formation of secondary organic aerosol components from the gas-phase ozonolysis of alpha-pinene. Phys. Chem. Chem. Phys. 2008, 10, 4294–4312. [Google Scholar] [CrossRef]
- Emanuelsson, E.U.; Watne, A.K.; Lutz, A.; Ljungstrom, E.; Hallquist, M. Influence of Humidity, Temperature, and Radicals on the Formation and Thermal Properties of Secondary Organic Aerosol (SOA) from Ozonolysis of beta-Pinene. J. Phys. Chem. A 2013, 117, 10346–10358. [Google Scholar] [CrossRef]
- Caudillo, L.; Rorup, B.; Heinritzi, M.; Marie, G.; Simon, M.; Wagner, A.C.; Muller, T.; Granzin, M.; Amorim, A.; Ataei, F.; et al. Chemical composition of nanoparticles from alpha-pinene nucleation and the influence of isoprene and relative humidity at low temperature. Atmos. Chem. Phys. 2021, 21, 17099–17114. [Google Scholar] [CrossRef]
- Yu, K.P.; Lin, C.C.; Yang, S.C.; Zhao, P. Enhancement effect of relative humidity on the formation and regional respiratory deposition of secondary organic aerosol. J. Hazard. Mater. 2011, 191, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Korhonen, H.; Sihto, S.L.; Joutsensaari, J.; Jarvinen, H.; Petaja, T.; Arnold, F.; Nieminen, T.; Kulmala, M.; Smith, J.N.; et al. The role of relative humidity in continental new particle formation. J. Geophys. Res.-Atmos. 2011, 116, D03202. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, G.H.; Cao, J.J.; Wang, X.M.; Zhang, R.J. Observation of biogenic secondary organic aerosols in the atmosphere of a mountain site in central China: Temperature and relative humidity effects. Atmos. Chem. Phys. 2013, 13, 11535–11549. [Google Scholar] [CrossRef]
- Liang, L.L.; Engling, G.; Cheng, Y.; Zhang, X.Y.; Sun, J.Y.; Xu, W.Y.; Liu, C.; Zhang, G.; Xu, H.; Liu, X.Y.; et al. Influence of High Relative Humidity on Secondary Organic Carbon: Observations at a Background Site in East China. J. Meteorol. Res. 2019, 33, 905–913. [Google Scholar] [CrossRef]
- von Hessberg, C.; von Hessberg, P.; Poschl, U.; Bilde, M.; Nielsen, O.J.; Moortgat, G.K. Temperature and humidity dependence of secondary organic aerosol yield from the ozonolysis of beta-pinene. Atmos. Chem. Phys. 2009, 9, 3583–3599. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Le, C.; Xu, Q.; Peng, W.H.; Jiang, H.H.; Lin, Y.H.; Cocker, D.R.; Zhang, H.F. Compositional Evolution of Secondary Organic Aerosol as Temperature and Relative Humidity Cycle in Atmospherically Relevant Ranges. ACS Earth Space Chem. 2019, 3, 2549–2558. [Google Scholar] [CrossRef]
- Geddes, S.; Nichols, B.; Todd, K.; Zahardis, J.; Petrucci, G.A. Near-infrared laser desorption/ionization aerosol mass spectrometry for measuring organic aerosol at atmospherically relevant aerosol mass loadings. Atmos. Meas. Tech. 2010, 3, 1175–1183. [Google Scholar] [CrossRef]
- Nakao, S.; Tang, P.; Tang, X.C.; Clark, C.H.; Qi, L.; Seo, E.; Asa-Awuku, A.; Cocker, D. Density and elemental ratios of secondary organic aerosol: Application of a density prediction method. Atmos. Environ. 2013, 68, 273–277. [Google Scholar] [CrossRef]
- Geddes, S.; Nichols, B.; Flemer, S.; Eisenhauer, J.; Zahardis, J.; Petrucci, G.A. Near-Infrared Laser Desorption/Ionization Aerosol Mass Spectrometry for Investigating Primary and Secondary Organic Aerosols under Low Loading Conditions. Anal. Chem. 2010, 82, 7915–7923. [Google Scholar] [CrossRef]
- Presto, A.A.; Huff Hartz, K.E.; Donahue, N.M. Secondary Organic Aerosol Production from Terpene Ozonolysis. 1. Effect of UV Radiation. Environ. Sci. Technol. 2005, 39, 7036–7045. [Google Scholar] [CrossRef]
- Flueckiger, A.; Petrucci, G.A. Methodological advances to improve repeatability of SOA generation in enivronmental chambers. Aerosol. Sci. Technol. 2023, 57, 925–933. [Google Scholar] [CrossRef]
- Masi, J.M. Artificial Growth of Aerosol Particles for Mass Spectrometry Measurement. Bachelor’s Thesis, University of Vermont, Burlington, VT, USA, 2020. [Google Scholar]
- Kerecman, D.E.; Apsokardu, M.J.; Talledo, S.L.; Taylor, M.S.; Haugh, D.N.; Zhang, Y.; Johnston, M.V. Online Characterization of Organic Aerosol by Condensational Growth into Aqueous Droplets Coupled with Droplet-Assisted Ionization. Anal. Chem. 2021, 93, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Pankow, J.F.; Marks, M.C.; Barsanti, K.C.; Mahmud, A.; Asher, W.E.; Li, J.Y.; Ying, Q.; Jathar, S.H.; Kleeman, M.J. Molecular view modeling of atmospheric organic particulate matter: Incorporating molecular structure and co-condensation of water. Atmos. Environ. 2015, 122, 400–408. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Erdakos, G.B.; Asher, W.E.; Pankow, J.F. Modeling the formation of secondary organic aerosol (SOA). 2. The predicted effects of relative humidity on aerosol formation in the alpha-pinene-, beta-pinene-, sabinene-, Delta(3)-Carene-, and cyclohexene-ozone systems. Environ. Sci. Technol. 2001, 35, 1806–1817. [Google Scholar] [CrossRef]
- Surdu, M.; Lamkaddam, H.; Wang, D.S.; Bell, D.M.; Xiao, M.; Lee, C.P.; Li, D.; Caudillo, L.; Marie, G.; Scholz, W.; et al. Molecular Understanding of the Enhancement in Organic Aerosol Mass at High Relative Humidity. Environ. Sci. Technol. 2023, 57, 2297–2309. [Google Scholar] [CrossRef]
- Dixon, M.; Grace, J. Water-Uptake by Some Chamber Materials. Plant Cell Environ. 1982, 5, 323–327. [Google Scholar] [CrossRef]
- Sacher, E.; Susko, J.R. Water Permeation of Polymer Films. IV. Teflon FEP. J. Appl. Polym. Sci. 1982, 27, 3893–3902. [Google Scholar] [CrossRef]
- Shepherd, W. Moisture Absorption by Some Instrumental Materials. Rev. Sci. Instrum. 1973, 44, 234. [Google Scholar] [CrossRef]
- Liebe, H.J.; Wolfe, V.L.; Howe, D.A. Test of wall coatings for controlled moist air experiments. Rev. Sci. Instrum. 1984, 55, 1702–1705. [Google Scholar] [CrossRef][Green Version]
- Showing Metabocard for cis-3-Hexenyl Acetate. Human Metabolome Database. Available online: https://hmdb.ca/metabolites/HMDB0040215 (accessed on 24 January 2023).
- Shi, X.L.; Huang, G.X.Z.; Yang, D.H.; Zhang, Q.Z.; Zong, W.S.; Cheng, J.M.; Sui, X.; Yuan, F.H.; Wang, W.X. Theoretical study of the formation and nucleation mechanism of highly oxygenated multi-functional organic compounds produced by alpha-pinene. Sci. Total Environ. 2021, 780, 146422. [Google Scholar] [CrossRef]
- Simon, M.; Dada, L.; Heinritzi, M.; Scholz, W.; Stolzenburg, D.; Fischer, L.; Wagner, A.C.; Kurten, A.; Rorup, B.; He, X.C.; et al. Molecular understanding of new-particle formation from alpha-pinene between-50 and+25 degrees C. Atmos. Chem. Phys. 2020, 20, 9183–9207. [Google Scholar] [CrossRef]
- Schervish, M.; Donahue, N.M. Peroxy radical chemistry and the volatility basis set. Atmos. Chem. Phys. 2020, 20, 1183–1199. [Google Scholar] [CrossRef]
- Perakyla, O.; Riva, M.; Heikkinen, L.; Quelever, L.; Roldin, P.; Ehn, M. Experimental investigation into the volatilities of highly oxygenated organic molecules (HOMs). Atmos. Chem. Phys. 2020, 20, 649–669. [Google Scholar] [CrossRef]
- Quelever, L.L.J.; Kristensen, K.; Jensen, L.N.; Rosati, B.; Teiwes, R.; Daellenbach, K.R.; Perakyla, O.; Roldin, P.; Bossi, R.; Pedersen, H.B.; et al. Effect of temperature on the formation of highly oxygenated organic molecules (HOMs) from alpha-pinene ozonolysis. Atmos. Chem. Phys. 2019, 19, 7609–7625. [Google Scholar] [CrossRef]
- Qi, X.M.; Ding, A.J.; Roldin, P.; Xu, Z.N.; Zhou, P.T.; Sarnela, N.; Nie, W.; Huang, X.; Rusanen, A.; Ehn, M.; et al. Modelling studies of HOMs and their contributions to new particle formation and growth: Comparison of boreal forest in Finland and a polluted environment in China. Atmos. Chem. Phys. 2018, 18, 11779–11791. [Google Scholar] [CrossRef]
- Bianchi, F.; Trostl, J.; Junninen, H.; Frege, C.; Henne, S.; Hoyle, C.R.; Molteni, U.; Herrmann, E.; Adamov, A.; Bukowiecki, N.; et al. New particle formation in the free troposphere: A question of chemistry and timing. Science 2016, 352, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.L.; Wang, W.N.; Liu, F.Y.; Lu, J.; Wang, W.L. Oligomerization reactions for precursors to secondary organic aerosol: Comparison between two formation mechanisms for the oligomeric hydroxyalkyl hydroperoxides. Atmos. Environ. 2017, 166, 1–8. [Google Scholar] [CrossRef]
- Kenseth, C.M.; Huang, Y.L.; Zhao, R.; Dalleska, N.F.; Hethcox, C.; Stoltz, B.M.; Seinfeld, J.H. Synergistic O-3 + OH oxidation pathway to extremely low-volatility dimers revealed in beta-pinene secondary organic aerosol. Proc. Natl. Acad. Sci. USA 2018, 115, 8301–8306. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Xue, Y.G.; Shen, Z.X.; Cao, J.J.; Wang, W.L. Mechanistic and kinetics investigations of oligomer formation from Criegee intermediate reactions with hydroxyalkyl hydroperoxides. Atmos. Chem. Phys. 2019, 19, 4075–4091. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Zhang, W.; Alexander, T.; Zhang, X.; Martin, D.B.C.; Zhang, H.F. Isolating a-Pinene Ozonolysis Pathways Reveals New Insights into Peroxy Radical Chemistry and Secondary Organic Aerosol Formation. Environ. Sci. Technol. 2021, 55, 6700–6709. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, S.; Wang, D.; Zabalegui, N.; Li, D.; Lamkaddam, H.; Bachmeier, F.; Vogel, A.; Monge, M.E.; Perrier, S.; Baltensperger, U.; et al. Structures and reactivity of peroxy radicals and dimeric products revealed by online tandem mass spectrometry. Nat. Commun. 2021, 12, 300. [Google Scholar] [CrossRef]
- Inomata, S. New Particle Formation Promoted by OH Reactions during alpha-Pinene Ozonolysis. ACS Earth Space Chem. 2021, 5, 1929–1933. [Google Scholar] [CrossRef]
- Jia, L.; Xu, Y.F. The role of functional groups in the understanding of secondary organic aerosol formation mechanism from alpha-pinene. Sci. Total Environ. 2020, 738, 139831. [Google Scholar] [CrossRef]
- Roldin, P.; Ehn, M.; Kurten, T.; Olenius, T.; Rissanen, M.P.; Sarnela, N.; Elm, J.; Rantala, P.; Hao, L.Q.; Hyttinen, N.; et al. The role of highly oxygenated organic molecules in the Boreal aerosol-cloud-climate system. Nat. Commun. 2019, 10, 4370. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Kurten, T.; Riva, M.; Mohr, C.; Rissanen, M.P.; Roldin, P.; Berndt, T.; Crounse, J.D.; Wennberg, P.O.; Mentel, T.F.; et al. Highly Oxygenated Organic Molecules (HOM) from Gas-Phase Autoxidation Involving Peroxy Radicals: A Key Contributor to Atmospheric Aerosol. Chem. Rev. 2019, 119, 3472–3509. [Google Scholar] [CrossRef]
- Frege, C.; Ortega, I.K.; Rissanen, M.P.; Praplan, A.P.; Steiner, G.; Heinritzi, M.; Ahonen, L.; Amorim, A.; Bernhammer, A.K.; Bianchi, F.; et al. Influence of temperature on the molecular composition of ions and charged clusters during pure biogenic nucleation. Atmos. Chem. Phys. 2018, 18, 65–79. [Google Scholar] [CrossRef]
- Crounse, J.D.; Nielsen, L.B.; Jorgensen, S.; Kjaergaard, H.G.; Wennberg, P.O. Autoxidation of Organic Compounds in the Atmosphere. J. Phys. Chem. Lett. 2013, 4, 3513–3520. [Google Scholar] [CrossRef]
- Caravan, R.L.; Vansco, M.F.; Lester, M.I. Open questions on the reactivity of Criegee intermediates. Commun. Chem. 2021, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Chhantyal-Pun, R.; Rotavera, B.; McGillen, M.R.; Khan, M.A.H.; Eskola, A.J.; Caravan, R.L.; Blacker, L.; Tew, D.P.; Osborn, D.L.; Percival, C.J.; et al. Criegee Intermediate Reactions with Carboxylic Acids: A Potential Source of Secondary Organic Aerosol in the Atmosphere. ACS Earth Space Chem. 2018, 2, 833–842. [Google Scholar] [CrossRef]
- Zhong, J.; Kumar, M.; Zhu, C.Q.; Francisco, J.S.; Zeng, X.C. Surprising Stability of Larger Criegee Intermediates on Aqueous Interfaces. Angew. Chem. Int. Edit 2017, 56, 7740–7744. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yajima, R.; Inomata, S.; Hirokawa, J. Water vapour effects on secondary organic aerosol formation in isoprene ozonolysis. Phys. Chem. Chem. Phys. 2017, 19, 3165–3175. [Google Scholar] [CrossRef]
- Lin, J.J.M.; Chao, W. Structure-dependent reactivity of Criegee intermediates studied with spectroscopic methods. Chem. Soc. Rev. 2017, 46, 7483–7497. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Tyndall, G.S.; Crounse, J.D.; Teng, A.P.; Bates, K.H.; Schwantes, R.H.; Coggon, M.M.; Zhang, L.; Feiner, P.; Milller, D.O.; et al. Atmospheric fates of Criegee intermediates in the ozonolysis of isoprene. Phys. Chem. Chem. Phys. 2016, 18, 10241–10254. [Google Scholar] [CrossRef]
- Gong, Y.W.; Chen, Z.M. Quantification of the role of stabilized Criegee intermediates in the formation of aerosols in limonene ozonolysis. Atmos. Chem. Phys. 2021, 21, 813–829. [Google Scholar] [CrossRef]
- Sheps, L.; Rotavera, B.; Eskola, A.J.; Osborn, D.L.; Taatjes, C.A.; Au, K.; Shallcross, D.E.; Khan, M.A.H.; Percival, C.J. The reaction of Criegee intermediate CH2OO with water dimer: Primary products and atmospheric impact. Phys. Chem. Chem. Phys. 2017, 19, 21970–21979. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Bao, J.L.; Truhlar, D.G. Unimolecular reaction of acetone oxide and its reaction with water in the atmosphere. Proc. Natl. Acad. Sci. USA 2018, 115, 6135–6140. [Google Scholar] [CrossRef] [PubMed]
- Taatjes, C.A. Criegee Intermediates: What Direct Production and Detection Can Teach Us About Reactions of Carbonyl Oxides. Annu. Rev. Phys. Chem. 2017, 68, 183–207. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.M.; Kroll, J.H.; Pandis, S.N.; Robinson, A.L. A two-dimensional volatility basis set—Part 2: Diagnostics of organic-aerosol evolution. Atmos. Chem. Phys. 2012, 12, 615–634. [Google Scholar] [CrossRef]
- Heinritzi, M.; Dada, L.; Simon, M.; Stolzenburg, D.; Wagner, A.C.; Fischer, L.; Ahonen, L.R.; Amanatidis, S.; Baalbaki, R.; Baccarini, A.; et al. Molecular understanding of the suppression of new-particle formation by isoprene. Atmos. Chem. Phys. 2020, 20, 11809–11821. [Google Scholar] [CrossRef]
- Bonn, B.; Schuster, G.; Moortgat, G.K. Influence of water vapor on the process of new particle formation during monoterpene ozonolysis. J. Phys. Chem. A 2002, 106, 2869–2881. [Google Scholar] [CrossRef]
- Jonsson, A.M.; Hallquist, M.; Ljungstrom, E. Influence of OH scavenger on the water effect on secondary organic aerosol formation from ozonolysis of limonene, Delta(3)-carene, and alpha-pinene. Environ. Sci. Technol. 2008, 42, 5938–5944. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.; Jensen, L.N.; Quelever, L.L.J.; Christiansen, S.; Rosati, B.; Elm, J.; Teiwes, R.; Pedersen, H.B.; Glasius, M.; Ehn, M.; et al. The Aarhus Chamber Campaign on Highly Oxygenated Organic Molecules and Aerosols (ACCHA): Particle formation, organic acids, and dimer esters from alpha-pinene ozonolysis at different temperatures. Atmos. Chem. Phys. 2020, 20, 12549–12567. [Google Scholar] [CrossRef]

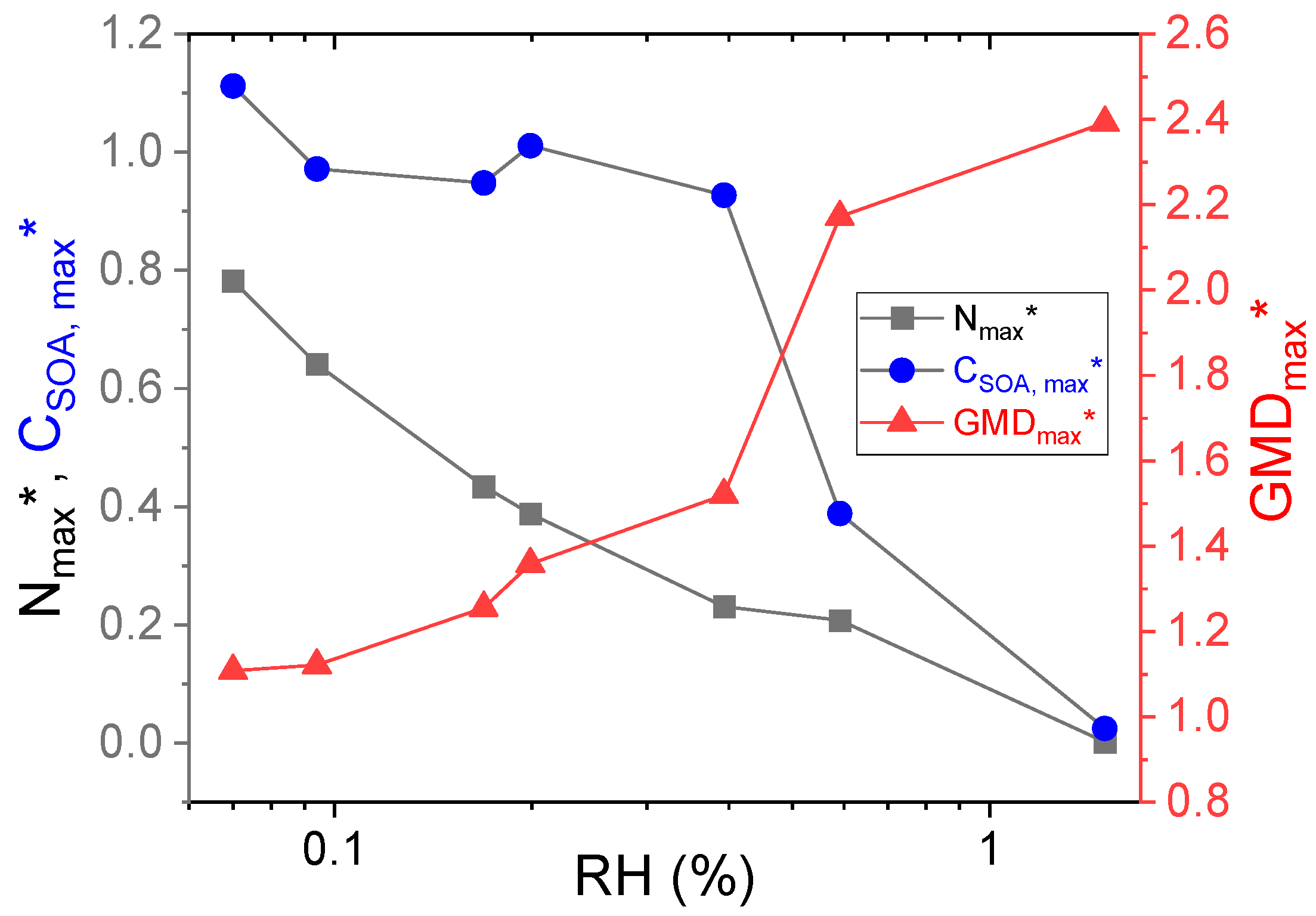



| RH (%) | ξCHA (ppbv) | ξO3 (ppbv) | Nmax (103 cm−3) | Nmax * | CSOA,max (µg m−3) | CSOA,max * | GMDmax (nm) | GMDmax * |
|---|---|---|---|---|---|---|---|---|
| 0.0 | 99.5 | 297.7 | 89.2 | 1 | 31.5 | 1 | 83.5 | 1 |
| 0.070 | 99.2 | 302.4 | 69.8 | 0.78 | 35.0 | 1.11 | 92.5 | 1.11 |
| 0.094 | 99.9 | 293.2 | 57.2 | 0.64 | 30.6 | 0.97 | 93.6 | 1.12 |
| 0.169 | 98.6 | 295.6 | 38.7 | 0.43 | 29.8 | 0.95 | 104.8 | 1.26 |
| 0.199 | 99.6 | 284.0 | 34.6 | 0.39 | 31.8 | 1.01 | 113.4 | 1.36 |
| 0.393 | 100.3 | 294.0 | 20.6 | 0.23 | 29.2 | 0.93 | 126.9 | 1.52 |
| 0.591 | 100.1 | 297.1 | 18.5 | 0.21 | 12.2 | 0.39 | 181.3 | 2.17 |
| 1.5 | 99.2 | 292.3 | 0.1 | 0.002 | 0.78 | 0.025 | 199.8 | 2.39 |
| Product MW (g mol−1) | Estimated VP (Pa) | Volatility Class |
|---|---|---|
| 148 | 10−2 | IVOC |
| 184 (pinonic acid) | 10−3 | SVOC |
| 204 | 10−4 | SVOC |
| 262 | 10−8 | LVOC |
| 318 | 10−10 | ELVOC |
| 320 | 10−13 | ULVOC |
| 348 * | 10−15 | ULVOC |
| ξα-Pinene:CHA:RH | α-Pinene (ppbv) | CHA (ppbv) | %RH | Ozone (ppbv) | Nmax (×103) (#/cm3) | ΔNmax * | CSOA (µg/m3) | GMD (nm) |
|---|---|---|---|---|---|---|---|---|
| ξ6:0:0 | 6 | 0 | 0 | 500 | 32.7 | −82% | 4.32 | 49.9 |
| ξ6:12:0 | 6 | 12 | 0 | 500 | 5.8 | 2.33 | 64.5 | |
| ξ6:0:60 | 6 | 0 | 60 | 500 | 144 | −20% | 11.5 | 49.1 |
| ξ6:12:60 | 6 | 12 | 60 | 500 | 115 | 6.89 | 43.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flueckiger, A.C.; Snyder, C.N.; Petrucci, G.A. Nontrivial Impact of Relative Humidity on Organic New Particle Formation from Ozonolysis of cis-3-Hexenyl Acetate. Air 2023, 1, 222-236. https://doi.org/10.3390/air1040017
Flueckiger AC, Snyder CN, Petrucci GA. Nontrivial Impact of Relative Humidity on Organic New Particle Formation from Ozonolysis of cis-3-Hexenyl Acetate. Air. 2023; 1(4):222-236. https://doi.org/10.3390/air1040017
Chicago/Turabian StyleFlueckiger, Austin C., Christopher N. Snyder, and Giuseppe A. Petrucci. 2023. "Nontrivial Impact of Relative Humidity on Organic New Particle Formation from Ozonolysis of cis-3-Hexenyl Acetate" Air 1, no. 4: 222-236. https://doi.org/10.3390/air1040017
APA StyleFlueckiger, A. C., Snyder, C. N., & Petrucci, G. A. (2023). Nontrivial Impact of Relative Humidity on Organic New Particle Formation from Ozonolysis of cis-3-Hexenyl Acetate. Air, 1(4), 222-236. https://doi.org/10.3390/air1040017






