Abstract
Ethylene oxide (EtO) is a colorless, flammable gas at room temperature produced by the catalytic oxidation of ethylene. EtO is widely used by medical sterilization facilities to clean medical supplies and equipment. Recent epidemiological studies showed that EtO is a more potent carcinogen than previously documented, leading the Environmental Protection Agency (EPA) to update, in December 2016, the inhalation unit risk estimate for EtO. This resulted in the identification of EtO as a potential health concern in several areas across the US, including the state of Utah. The geography surrounding Salt Lake Valley creates a bowl, which is ideal for collecting air pollution emissions. The region often experiences inversion episodes which inhibit vertical mixing and cause an accumulation of air pollutants, leading to unhealthy pollution levels. Using the EPA’s dispersion modeling software, AERMOD, this study estimated EtO concentrations through facility stack and fugitive emissions modeling results. These values were compared with those of canister-based concentrations from ambient air samples taken near a medical device sterilization facility in Salt Lake Valley. Stainless steel whole-air passivated canisters were used to collect 24 h ambient concentration samples of EtO. Eight locations surrounding a Salt Lake Valley medical device sterilization facility and four background sites were chosen to measure the ambient concentrations. Accounting for potential atmospheric impacts on EtO, measurements were sampled in winter 2022 (January–March) and summer 2022 (July–September). The modeled EtO concentrations were adjusted to account for background values associated with the winter or summer data. Then, the two methodologies were compared using a Wilcoxon signed-ranked paired test. The statistical analysis resulted in six of the eight sample locations surrounding the sterilization facility being significantly different when comparing the canister-based measurements of ambient EtO to modeled estimates. Canister-based measurements taken at sites one, three, and four were statistically greater than the modeled estimates, while sites two, five, and seven were statistically less than the modeled estimates. Also, the summer background value calculated was almost 2.5 times greater than the winter one. The results do not suggest whether one method is more or less conservative than the other. In conclusion, the five of the closest sites and site seven were statistically different when comparing measured and modeled ambient concentrations of EtO. The comparison results do not clearly indicate if a correction factor could be derived for future human exposure to cancer risk assessment modeling. However, it is reasonable that the closer to the sterilization facility, the more total EtO exposure will be realized.
1. Introduction
Ethylene oxide (EtO) is a colorless, flammable gas at room temperature produced by the catalytic oxidation of ethylene [1]. The precursor gas is primarily used as an intermediate in manufacturing other chemicals, most commonly ethylene glycol, which are used to make antifreeze, textile, plastics, detergents, and adhesive products [1,2]. It is also used as a fumigating agent for spices and a sterilizing agent for medical equipment. Medical sterilization facilities, manufacturers, and hospitals use EtO to sterilize medical supplies and equipment instead of steam or radiation [1]. EtO’s alkylating properties prevent microbial contamination and propagation by damaging cellular chromosomal structures [3]. Human exposure to EtO has been observed in healthcare and sterilization workers and is thought to affect community residents living near these facilities using EtO [1].
Inhalation is presumed to be the primary exposure route for affecting human health. The U.S. Environmental Protection Agency (EPA) and the International Agency for Research on Cancer (IARC) both classified EtO as a human carcinogen [2,4]. In 2016, the EPA estimated that concentrations of EtO were associated with a 10−6 to 10−4 increase in inhalation cancer risk, a further increase in toxicity than was previously understood [3,5]. Studies have associated long-term EtO exposure with an increased risk of white blood cells-related cancers in humans, including non-Hodgkin lymphoma, myeloma, and leukemia [5,6]. Studies have also found connections between chronic EtO exposure and women’s risk of breast cancer [6]. Chronic exposure is the primary focus of this study. Acute exposure is not the focus because the measured ambient EtO concentrations are much lower than those concentrations which could cause acute health effects. Animal studies have shown that when exposed to high amounts of EtO for short durations, EtO may cause neurological, reproductive, and developmental effects in addition to lung irritation [1]. Other short-term health effects of EtO can cause headaches, dizziness, nausea, fatigue, respiratory irritation, and gastrointestinal distress [5].
When EtO enters the ambient environment, it will break down over several months [6]. Sterilization facilities the release of EtO into the atmosphere through purge stacks. Also, unintentional fugitive emissions can leak from valves, pumps, storage, tanks, and product-off gassing. An Illinois facility released 1547 lbs of EtO during a quarter of 2019; 52% of these emissions were fugitive [3]. This suggests that fugitive emissions may significantly increase ethylene oxide levels beyond facility-reported stack emissions. Some studies suggest that other environmental sources may contribute to background levels of EtO. Automobile combustion and biological metabolic processes, such as human and plant gaseous by-products, are thought to contribute to background EtO levels, but the EPA has yet to characterize sources and emission levels fully [3]. However, preliminary measurements suggest that background measurements are lower than those observed near sterilization facilities [7].
The geography surrounding Salt Lake Valley creates a mountainous “bowl”, which makes it ideal for collecting and concentrating various air pollutants. The region often experiences inversion episodes under high-pressure weather systems during the winter months. This leads to the colder air mass sinking to the bottom of the valley and becoming sealed and trapped by the warmer air above the surface [6]. Under these conditions, vertical mixing is inhibited, and air pollutants accumulate, resulting in unhealthy pollution levels. Wildfires and high-pressure meteorological systems associated with low-velocity winds can also contribute to poor air quality during the summer months in the western U.S. Because of these unique conditions, EtO may not be adequately diluted and expelled from the area compared to other geographic locations where medical sterilization facilities are currently located. The role of Salt Lake Valley’s seasonal variations in the dispersion of EtO from emission and background sources is unclear; however, preliminary data suggest that background concentrations in the ambient air tend to be higher during the warmer months of the year.
Human exposure model (HEM) is the EPA’s tool for dispersion modeling, estimation of population exposure, estimation of human health risks, and demographic assessment. HEM incorporates the American Meteorological Society—U.S. EPA Regulatory Model (AERMOD) program, which is responsible for the software’s dispersion modeling capabilities. Using point source emissions information supplemented with meteorological data and terrain mapping, AERMOD calculates pollutant dispersion from a given emission source. Windspeed, plume depletion with and without deposition, and building downwash, among other complex plume processes and characteristics, are processed to obtain concentration outputs [8]. The tool estimates ambient concentrations of EtO associated with specific emission sources at defined receptor locations [8]. AERMOD outputs can be used to understand the dispersion of ambient EtO concentrations and inform cancer risk assessments. This research compares dispersion modeling estimates and canister-based measurements of ambient EtO. Differences recorded will aid in the future research of EtO in Salt Lake Valley and quantifying cancer risk. This is not to suggest that one method is better than the other; both canister-based measurements and dispersion modeling have limitations that should be considered. For example, only ambient emissions from a point source are modeled. The AERMOD outputs of EtO concentrations do not include potential background source emissions [8]. Also, data fed into the model do not represent the exact coordinates input into the model. The closest source of meteorological meteorological data is the National Centers for Climate Information, located at the Salt Lake City Regional Airport, about 19 miles north of the point source of EtO in Salt Lake Valley. Canister-based measurements are also subject to collection and handling bias. If a discrepancy is found between the modeled estimates and canister-based measurements, a correction factor could be derived to make more informed conclusions from HEM by providing a correction factor between observed and modeled concentrations.
Specifically, this study compares the USEPA’s AERMOD dispersion modeling estimates of ambient EtO with measured canister-based concentrations around a sterilization facility in Salt Lake Valley. There is scarce information on how EtO distributes spatially and temporally, so the findings of this study will help characterize the emission and dispersion of ambient EtO from medical sterilization facilities. It should be noted that this is the first paper in a series of papers to be written involving ambient air EtO concentration modeling and human exposure modeling in Salt Lake Valley, considering specifically EtO emissions from medical sterilization facilities and background concentrations of this airborne contaminant. Subsequent papers will compare seasonal differences in concentrations, human exposures, and the overall chronic exposure impacts from these emissions.
The purpose of this study is to investigate the emission and dispersion of ambient EtO from the sterilization facility’s stack and fugitive emissions in Salt Lake Valley during the winter and summer months. In addition, the study will compare EPA’s HEM dispersion modeling estimates of EtO to measured canister-based measurements to establish a correction factor for future HEM cancer risk assessment research.
2. Methods
A sterilization facility with ten stacks was identified as a point source of ambient EtO emissions in Salt Lake Valley. The facility provided information regarding the controlled and uncontrolled EtO emissions for dispersion modeling estimates. Based on preliminary data, daily canisters which measured ambient EtO concentrations were a hundred times below the range of acute health effects. This suggests that the sterilization facility’s emission rate does not warrant concern for acute health concerns; however, it may still chronically affect community members who live in close proximity to the sterilization facility.
Whole-air passivated canisters were used to collect 24 h grab samples. The stainless-steel canisters were lined with silonite for a clean collection surface for EtO. Each canister was fitted with a flow controller and timer to allow unattended, 24 h sample collection. The canisters were blank-checked before collection. Based on EPA’s national sampling calendar, the canister-based measurements were conducted on a 1-in-3-day sampling schedule at eight (8) discrete locations around the sterilization facility. These locations are described in Table 1 and geographically pinpointed in Figure 1. After the canisters collected a 24 h sample, they were sent to the Eastern Research Group (ERG) lab. The total EtO volume was analyzed using the EPA TO-15 method, which employs a gas chromatography–mass spectrometry (GC/MS) procedure. This method is used for volatile organic compounds identified as hazardous air pollutants by the Clean Air Act, which applies to EtO. The method provides higher analytical sensitivity and accuracy to decrease uncertainties with spectrum information collected for selected ion monitoring (SIM).

Table 1.
Descriptions of the eight sample locations near the sterilization facility and the five background sites.
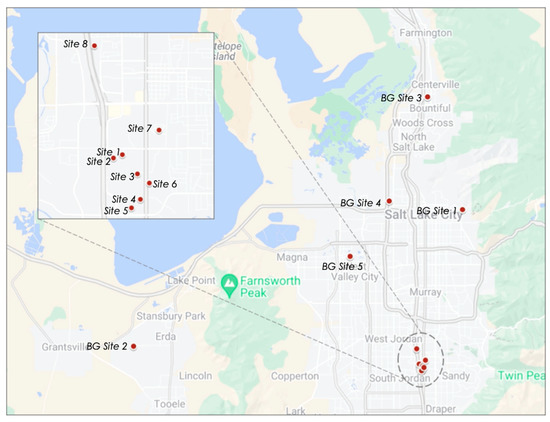
Figure 1.
Map showing where the canister-based measurements were taken across Utah’s Salt Lake Valley. There are 8 sampling sites near the medical sterilization facility (see blown up image) and 5 background site locations (Google Maps, 2022 [9]).
Seasonal wind trajectory data from the years 2015 to 2019 helped inform sampling location sites, both upwind and downwind from the sterilization facility. A temporalization analysis partially ensured that the selected locations were valid representations of the meteorological conditions during most hours of the day (a 24 h period) in addition to both summer and winter seasons. In addition, AERMOD accounted for wind trajectory and velocity conditions based upon its EtO dispersion modeling capabilities to track the pollutants’ ambient characteristics. All were considered to determine the eight sites, varying in distance from the sterilization facility for canister-based measurements and dispersion modeling. Additional sites were chosen to represent potential sources of EtO in the background, separate from the sterilization facility’s emissions. Since background sources of EtO are largely unknown, the sites accounted for potential emitters near biogenic-impacted, industrial, urban, and near-freeway rural sources (i.e., away from the influence of any significant anthropogenic sources). To account for potential atmospheric impacts on EtO, canister-based measurements were collected during winter 2022 (January–March) and summer 2022 (July–September). Table 1 describes where the five (5) winter background canister-based measurement sites and four (4) summer background sites were sampled for EtO ambient concentrations. These locations are visually represented in Figure 1. During the summer sampling period, background site 5 (BG Site 5) was replaced by a background site close to another facility for a second study involving EtO in the ambient air. Due to resource constraints, it was not possible to continue with the background sampling during the summer months at BG Site 5. The background sites were used to help account for EtO concentrations not associated with the medical sterilization point source.
AERMOD’s dispersion modeling uses three input files of emission source information to estimate the ambient concentrations of EtO at a designated location. The “HAP Emissions” file describes the tons of EtO emitted per year into the ambient air. The “Emission Location” file characterizes the source type and location using facility coordinates, building dimensions, stack dimensions, emission velocity, etc. The “Facility List Options” file gives the model parameters. For example, a 24 h timeline pulled the corresponding meteorological data for an informed dispersion model estimate of the same 24 h the canister-based measurements were deployed. Additionally, the exact geographical coordinates of the canisters were input into the modeling software. The canister-based measurements of EtO were expressed in parts per billion volume (ppbv) and had to be converted into micrograms per meter cubed (μg/m3) to match the modeled estimates. Volume (V2) was derived from the daily averages of pressure (P2) and temperature (T2) obtained from the 2022 MET data. Also, a value of 0.81 was inputted for P1V1/T1 given the following standard atmospheric conditions: 1 atm, 24.45 L, and 298 K. Then, multipliers were extrapolated using the pressure output from Equation (1) divided by EtO’s molecular weight of 44.05 g/mol. Multipliers were applied to the measured EtO concentrations to directly compare to the modeled estimates. Please see Equation (1) below for details.
Equation (1) was used to determine Pressure2 accounting for varying changes in temperature and pressure over the sampled 24 h timeline.
AERMOD dispersion modeling does not include contributions from background sources of ambient EtO in its estimates; it only models the emissions from the point source. The background sites were used to compute a summer and winter background concentration using a mean-of-the-medians method. The median EtO concentration was determined for each background site (sites B1–B5) for both seasons (summer and winter). Then, the five winter and four summer background site medians were averaged together to establish a single EtO background concentration value for winter and summer, respectively. The corresponding seasonal background value in μg/m3 was added to AERMOD’s estimated concentrations. Rather than using a mean-of-the-means method, this procedure removed the significant bias that a few measurement outliers would have on the data/analysis and, in essence, established values closer to the central tendency of the true background value. A Wilcoxon signed-ranked test for paired, non-parametric data was used to compare the modeled EtO estimates to the canister-based EtO measurements for each of the eight sites. RStudio was used for all statistical analyses [10].
3. Results and Discussion
The Wilcoxon signed-Ranked test results comparing the measured and modeling concentrations are outlined in Table 2. The results indicated that six of the eight sample locations surrounding the sterilization facility significantly differed when comparing dispersion-modeled estimates of ambient EtO to canister-based measurements. Modeled estimates at sites 1, 3, and 4 were statistically higher than the canister-based measurements, whereas estimated ambient EtO at sites 2, 5, and 7 were statistically lower than the canister-based measurements. Sites 6 and 8 showed no statistical difference. Figure 2 mirrors these findings and visually shows that neither method, modeled or measured ambient EtO, was more conservative than the other. A conservative method would have a lower median concentration of ambient EtO between the modeled and measured boxplots for each of the eight sites. The median concentration is lower at sites 1, 3, and 4 when plotted next to the median of the measured method, whereas sites 2, 5, and 7 are higher than the modeled median concentration. The dispersion modeling estimation method appears to have been the conservative collection method for three sites, especially at site 1. However, canister-based measurements were more conservative at the other three significantly different sites. Therefore, the results do not clearly indicate whether one method is consistently more or less conservative than the other. This suggests that the calculation of a correction factor to relate the modeled and measured concentrations consistently is not possible. Figure 2 does not include an extreme outlier measured from the canister-based collection method at site 6, reported at 7.0 μg/m3. Although the value was removed from Figure 2, it was included in the analysis. The difference seen between the modeled and measured median at site 6 may have been prevented from producing a statistically significant result because of outliers in the data. The last interpretation from Figure 2 was that ambient EtO concentrations are expected to be higher closer to the sterilization facility, as seen at site 1, and gradually decrease as distance increases away from the source.

Table 2.
Wilcoxon signed-ranked test results from comparing median concentrations of EtO estimated with AERMOD modeled to canister-based measurements. Significant p-values are noted (*).
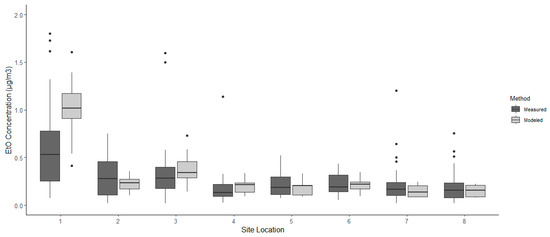
Figure 2.
Ethylene oxide (EtO) concentrations measured with passive, whole-air canisters and estimated from air dispersion modeling at eight locations near the emission source.
The background site concentrations are shown in Table 3. Interestingly, the summer mean-of-the-median value calculated was almost two and a half times greater than the winter background value. Figure 3 shows the total ambient EtO concentration after adding the corresponding winter and summer background values, 0.0779 and 0.1962 μg/m3, to modeled EtO concentration estimates for the five background sites. The background modeled and measured EtO concentrations shown in Figure 3 were uniform in concentration across all sites in comparison to Figure 2. The results indicate that there may be a potential EtO background source; however, they do not suggest residential, industrial, or rural locations as being sole contributors to the ambient EtO concentration. Although background sources are unknown, there may be evidence of seasonal effects on environmental EtO. The rural background site, BG 2, was deployed to capture potential biogenic EtO sources. BG 2 also showed a two and a half times greater median value over summer when compared to winter. This finding may support the idea that greater hours of sunlight could result in higher biological metabolic processes contributing to environmental EtO levels. Future research will continue to explore the seasonal differences in ambient EtO concentrations.

Table 3.
Background site medians and mean-of-median values for winter and summer.
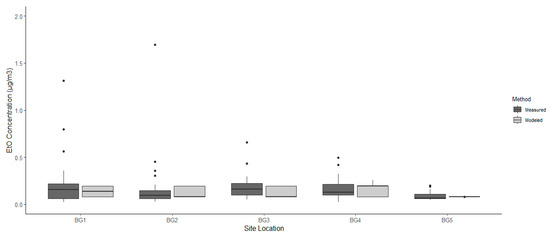
Figure 3.
Ethylene oxide (EtO) concentrations measured with passive, whole-air canisters and estimated with air dispersion modeling at five background locations.
4. Strengths and Limitations
The EPA’s TO-15 method is the best practice for collecting ambient EtO; however, there are limitations. Winter collection months were more challenging than summer due to the value timers freezing at night and extended transient times due to weather conditions. Many samples were flagged for attenuation concerns, which future research will attempt to investigate. The EPA’s HEM-4 program also did not allow for a single day of modeling, so the 24 h were accounted for over two days. This may have biased the modeled estimate concentrations; however, the next edition of HEM will remove the error and be used for future modeling. Despite the limitations, the results suggest that neither method is more conservative than the other. This finding may benefit future ambient pollutant research in Salt Lake City conducted by researchers or point sources due to the generalizability of pollutant dispersion modeling characterized by the research of EtO dispersion from a point source’s stack and fugitive emissions.
5. Conclusions
This study aimed to identify the difference between the two ways of measuring ambient EtO from point source emissions, estimating the concentration through EPA’s dispersion modeling and canister-based measurement collection. However, the results do not clearly indicate if a correction factor could be derived for future human exposure to cancer risk assessment modeling using HEM-4. There was an indication that the results predicted similar spatial variation in ambient EtO from the point source. The eight sites sampled around the sterilization facility, five sites closest to the point source, and site seven were statistically different when comparing modeled ambient concentrations of EtO to the measured concentrations. The data did show a difference between seasonal background concentrations. Therefore, investigating the phenomenon behind seasonal changes in EtO is of future interest. The intention is to compare the seasonal variations in the ambient EtO concentration from modeled estimates to canister-based measurements. This paper is the first of a three-part study investigating ambient EtO dispersion modeling, looking at seasonal differences and human exposure modeling for cancer risk.
Author Contributions
Conceptualization, N.D.; methodology, S.S.; software; R.H.; validation, T.H.; formal analysis, T.H. and S.S.; investigation, N.D. and R.E.; resources, N.D. and R.E.; data curation, N.D., R.E. and T.H.; writing—original draft preparation, S.S.; writing—review and editing, S.S., R.H., D.S., N.D. and R.E.; visualization, N.D.; supervision, R.H.; project administration, N.D.; funding acquisition, N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Utah Department of Air Quality subcontract #220517 with the University of Utah, Salt Lake City, UT, USA.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This project was funded by the U.S. EPA and completed through a joint partnership with the Utah Division of Air Quality and the University of Utah Department of Family and Preventive Medicine.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ATSDR. Toxicological Profile for Ethylene Oxide. Agency for Toxic Substances and Disease Registry. 2022. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=734&tid=133#bookmark04 (accessed on 7 February 2023).
- EPA. Our Current Understanding of the Human Health and Environmental Risks of Ethylene Oxide. U.S. Environmental Protection Agency. 2022. Available online: https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/our-current-understanding-human-health-and-environmental (accessed on 7 February 2023).
- Sheehan, P.J.; Lewis, R.C.; Kirman, C.R.; Watson, H.N.; Winegar, E.D.; Bus, J.S. Ethylene Oxide Exposure in U.S. Populations Residing Near Sterilization and Other Industrial Facilities: Context Based on Endogenous and Total Equivalent Concentration Exposures. Int. J. Environ. Res. Public Health 2021, 18, 607. [Google Scholar] [CrossRef] [PubMed]
- IARC. Ethylene oxide. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Chemical Agents and Related Occupations; International Agency for Research on Cancer: Lyon, France, 2012; 100F; pp. 73–159, 379–400. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Chemical-Agents-And-Related-Occupations-2012 (accessed on 9 February 2023).
- EPA. Evaluation of the Inhalation Carcinogenicity of Ethylene Oxide; U.S. Environmental Protection Agency: Washington, DC, USA, 2016. Available online: https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=329730 (accessed on 7 February 2023).
- Steenland, K.; Stayner, L.; Deddens, J. Mortality analyses in a cohort of 18 235 ethylene oxide expose workers: Follow up extended from 1987 to 1998. Occup. Environ. Med. 2004, 61, 2–7. [Google Scholar] [PubMed]
- EPA. Pre Proposal: Outreach to Communities on Ethylene Oxide Commercial Sterilizers Fact Sheet. U.S. Environmental Protection Agency. 2022. Available online: https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/pre-proposal-outreach-communities-ethylene-oxide-commercial (accessed on 7 February 2023).
- EPA. The HEM4 User Guide: Instructions for Using the Human Exposure Model for Singel and Multiple Facility Exposure and Risk Modeling; U.S. Environmental Protection Agency: Chapel Hill, NC, USA, 2021. Available online: https://www.epa.gov/fera/human-exposure-model-users-guides (accessed on 7 February 2023).
- Google Maps. 2022. Available online: https://www.maps.google.com (accessed on 13 March 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 13 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).