Abstract
This article is a case study of museum premises at the Museum of King John III’s Palace at Wilanow (Warsaw, Poland), wetted as a result of a failure of the water supply system to the air conditioning unit located in the attic of the building. As a result of flooding, discoloration and cracks appeared on the plaster and stucco decoration of the ceiling, located mainly in the central part of the ceiling of the King’s Library. The paintings (plafonds) mounted on the ceiling of this room also became damp. The article analyzes the microbiological contamination of air and damp paintings in the context of promptly proceeding with the drying of damp building partitions. The obtained results of microbiological air pollution in the flooded rooms were significantly lower than the permissible values recommended by Interdepartmental Commission for Maximum Admissible Concentrations and Intensities for Agents Harmful to Health in the Working Environment. In the King’s Library, i.e., the room with the dampest plaster and stucco as a result of the accident, the concentration of mold spores in the air was only 15 cfu/m3. This means that the immediate commencement of intensive drying of the building partitions (walls, ceilings with wooden floors) brought very good results. The rapid reduction in the moisture of the building partitions contributed to the worsening conditions for the development of microorganisms, which can have an adverse effect on wooden building partitions, plaster, stucco, etc.
1. Introduction
The state of preservation of monuments is affected by many factors, both abiotic and biotic. Abnormal humidity conditions, dampness in the walls, inadequate lighting or high concentrations of microbiological agents can negatively affect the resistance and preservation of monuments. When relative humidity exceeds 70%, the development of mold fungi is observed, the metabolic activity of which can result in the slow biodeterioration of historic objects [1]. Both enzymes and acidic metabolites synthesized by fungi can lead to serious damage of materials and objects made of them, and even to their permanent loss if no action is taken. In order to preserve the permanence of cultural heritage, it is important to comply with conservation standards that define the optimal recommended climatic conditions conducive to the long-term preservation of the monuments.
Among the factors most threatening to monuments are microorganisms, the development of which is closely linked to the environmental factors prevailing in historic buildings. Temperature and humidity, but also human activity affect the physiological activity of microorganisms. Inadequate ventilation, or construction disasters (damage to water supply systems) can lead to an escalation of the growth of microorganisms, and thus intensify the processes of destruction.
The air microbiome is believed to contribute significantly to the deterioration of objects and historic buildings [2]. Currently, there are no standards or directives, concerning the standards of microbiological quality of air in usable rooms, although there are normative documents, specifying the number of colony-forming units (cfu) of bacteria and microscopic fungi in the air, which indicate that the air in a room is highly polluted. Based on the literature, it can be concluded that the standards of microbiological purity of indoor air adopted in different countries vary [3,4]. According to Swedish requirements, the criterion for the acceptability of microbial contamination of indoor air was adopted up to 500 cfu of bacteria and 300 cfu of fungal spores in 1 m3 [5]. The American Industrial Hygiene Association proposes a value of less than 500 cfu/m3 as the limit of fungal spores in a post-dwelling area and less than 250 cfu/m3 in commercial buildings [6]. There is no Polish legislation pertaining to limits on the permissible concentrations of microorganisms for assessing air quality, although outdated and withdrawn standards for determining the number of bacteria and microscopic fungi in ambient air are still used when conducting routine verification tests [7]. When assessing the microbiological quality of air, one can use the recommendations of the Interdepartmental Commission for Maximum Admissible Concentrations and Intensities for Agents Harmful to Health in the Working Environment, which determined the permissible concentration of microbiological agents in the air in the working environment in Poland [8].
According to the literature, it appears that in Polish museums the most frequently detected types of fungi in air microbiological tests are: Alternaria, Aspergillus, Cladosporium, Mucor, Penicillium, Rhizopus and Trichoderma [9]. Bacteria are dominated by species belonging to the genus: Bacillus, Micrococcus and Acinetobacter [10]. Rojas et al. [11] report that the most common types of fungi in cultural facilities were the genera: Aspergillus, Penicillium, Cladosporium, Fusarium and Monilia, and additionally Aspergillus spp. accounted for 29 to 68% of all identified fungi. An air quality analysis conducted at the Historical Museum of Crete confirmed that the presence of fungi of the genera Alternaria and Malassezia in museum premises is associated with human activity [12]. On the other hand, microclimate studies conducted on a 19th century building located in Romania showed that there is a correlation between the identified fungal species and the microclimate conditions of the premises [13].
The most important factor in the microclimate of the rooms determining the growth and physiological activity of the microorganisms is water activity. Water activity in materials rises with increasing relative humidity, but also due to improper maintenance of facilities or negligence, leading to various types of failures, resulting in the rapid growth of microorganisms and progressive bio-degradation of materials. Major water failures, pervious roofing, or overly impermeable window frames are the most common causes of microbial infection of facilities. Therefore, all activities related to the maintenance of historic buildings should be carried out according to holistic planning, taking into account the multiplicity of factors affecting the historic object.
Biological harmful factors are a very important problem in the museum environment. On the one hand, they may lead to the risk of biodeterioration of important and valuable cultural assets, and on the other hand, they may emit substances hazardous to human health into the air, thus threatening the health of museum employees and visitors. Protection of the microclimate and museum objects against the development of harmful microorganisms is one of the most important procedures in buildings where historical collections are exhibited or stored.
This study analyzes the microbiological contamination of the air and paintings mounted on the ceilings that were dampened as a result of a water supply system failure in part of the building at the Museum of King John III’s Palace at Wilanów (Warsaw, Poland).
2. Materials and Methods
Description of the object. The case in question concerns rooms in the main body of a Baroque palace, which was built in Wilanów near Warsaw between 1677 and 1696 as the summer residence of the Polish King John III, designed by Augustine Locci, in the style of a Palladian villa. The main body was built based on the plan of an already existing building at the site in the style of a Polish manor house, a type of building characterized by a quadrangular one-story central body with four adjoining alcoves. Several stages of expansion of the original establishment resulted in the addition of 2 more stories to the initial structure, without changing its layout. The case in question happened within the southwest alcove. The decorative ceiling and floor of the ground-floor room of this alcove, historically and currently known as the King’s Library, are among the oldest surviving areas in the palace [14]. The second floor of the alcove is occupied by an exhibition room, known as the Historical Cabinet, in which no historical decorations have survived.
Description of the failure and the remedial actions taken. The failure consisted of a leak in the water supply to the humidifier of the air handling unit located in the attic of the alcove (Figure 1), and it occurred on the morning of 15 December 2020. At the time of the palace’s commission opening, the water had already reached the first floor through a room on the second floor. Its rapid arrival on the first floor was possible due to the presence of light and smoke detection installations in the ceiling of the second floor. The ducts of these installations allowed significant amounts of water to quickly pass through the ceiling of the first-floor room and flood the wooden floor of that room. After passing through the floor, the water found its way into the inter-ceiling space between the second floor and the first floor, and had reached the stucco and painting decoration of the King’s Library ceiling directly. There, due to the technical characteristics of the ceiling’s painting decoration (the historical strong duplication of the canvases on wax, which caused them to become an air- and watertight membrane), part of the water was “channeled” and flowed out at the edges of the oil plafond paintings onto the surface of the ceiling, falling onto the marble floor of the King’s Library, and began to seep into the stucco decoration.
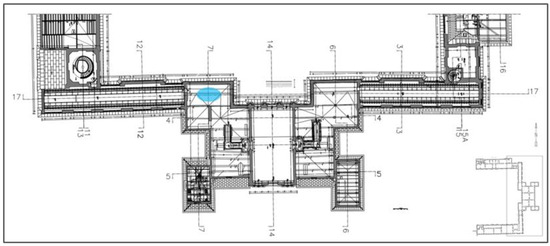
Figure 1.
Floor plan of the second floor of the building water system failure location (blue ellipse).
A certain amount of water penetrated the walls of the alcove in parallel, and there, its penetration was already slow, typical of the gravitational movement of water reserves through porous structures. This resulted in moisture on both sides of the eastern wall of the upstairs room and the mural paintings on the other side of it that decorated the former staircase, now called the “Room above the Chapel”.
Immediately after the damage occurred and for the next 4 months, a series of rescue and security measures were carried out to alleviate the negative changes and to secure the movable objects and rooms, and make them safely accessible to visitors. The measures taken by the museum were as follows:
- Immediate shut-off of the water supply to the system;
- Immediate shut-off of the electricity supply to this part of the palace;
- Immediate dismantling of the display and mobile decorations of the alcove (paintings on canvas incorporated into stucco framing of the ceiling-forming plafonds);
- Drying the wall paintings in the Room above the Chapel using conservation methods;
- Involvement of a professional service experienced in repairing damage after floods and inundations;
- Involvement of an expert mycologist, due to the development of microorganisms being considered the biggest threat, followed by the danger of indirect physical and chemical effects of water on historic decorative materials.
As a result of a multifaceted analysis of the situation, after ascertaining the proper condition of the electrical system and implementing a procedure for 24 h direct monitoring of the affected rooms, the process of air dehumidification and intensive forced ventilation of the flooded rooms was launched using high-efficiency dehumidifiers and ventilators supplied by a third party. As a supportive measure, the removal of successive, non-heritage wall finish layers gradually began, which primarily affected the upstairs room. Thus, a multidisciplinary task force was formed, consisting of art conservators employed by the museum, microbiologists and technicians, in order to conduct independent, but previously agreed-upon, countermeasures, including moisture measurements of walls and architectural surfaces. The results of the measurements were mutually exchanged and discussed among the members of the task force.
The biggest problem was the control of the processes taking place in the aforementioned inter-ceiling space on the decorative ceiling of the King’s Library. It was, as in many other places in the palace, designed and constructed in the 1960s during the large-scale restoration of the palace, and modernization of its technical infrastructure occurred. The goal, in line with the trend of the time, was to duplicate the original wooden ceiling elements by introducing and concealing a steel structure. As a result, at the time of flooding, there was a hidden, flattened volume of about 12 m3 to deal with, containing a conglomerate of inorganic and organic materials beyond air that had not been fully recognized ever before. Since the removal of the wooden floor on the second floor was out of the question due to the cost, maintenance of the exposition and the need to preserve communication tracts, a process was set in motion to stimulate the flow of air through the inaccessible space based on the assumption that it would “follow the path of water”, picking up moisture from the affected excess areas along the way. To this end, holes with a diameter of 5 cm were made in the floor of the upstairs room, at locations corresponding to the outlets of the electrical system in the ceiling, and the air was pumped into the inter-ceiling space with the help of the unit. After a slight overpressure was created there, it escaped through all possible gaps into the King’s Library. It should be noted that all the countermeasures described above were carried out for 10 weeks.
Description of the realization of microbiological measurements. The general principles of microbiological air testing were taken from the national guidelines for museum facilities [1,15]. In the selected rooms (Figure 2 and Figure 3), 4 bioaerosol samples each were collected using an AQUARIA Microflow α 90c device: sampling head with 380 conical holes ø 1 mm (Aquaria Srl, Lacchiarella, Italy). The operating parameters of the device during bioaerosol sampling were as follows: airflow 100 L/min, exposure time 60 s, air sampling was performed at a height of 100 cm from the floor. Samples were taken in Petri dishes (90 mm) on a maltose–agar medium (Biomxima, Lublin, Poland). The samples were then incubated for 7 days at 22–35 °C and 80% air humidity. The limiting incubation temperatures of the samples were adopted following the methodology of molds growing on building materials [16] and pathogenic molds [17]. Microscopy slides were made from the proliferated mold colonies. Morphological features of individual mycelial cultures were observed using the Delta Optical Evolution 100 light microscope (Delta Optical Sp. z o. o., Warsaw, Poland), using a 60x magnification, equipped with a Levenhuk M1000Plus camera (Levenhuk Poland Sp. z o. o., Warsaw, Poland).
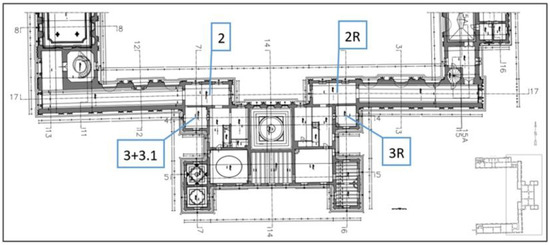
Figure 2.
Floor plan of the first floor of the building air sampling location (2, 3, 3.1—air samples symbol; 2R, 3R—symbol of the reference air samples).
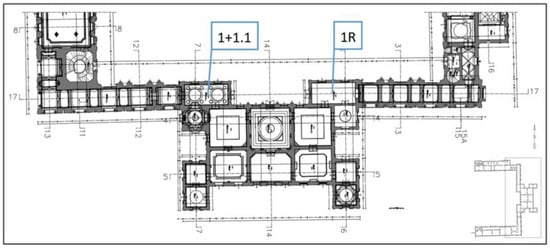
Figure 3.
Plan of the ground floor of the building air sampling location (1, 1.1—air samples symbol; 1R—symbol of the reference air sample).
Samples were taken from the surface of the paintings installed on the ceiling of the King’s Library and from the stucco surface by swabbing. Sterile swabs with transport containers without a substrate (Deltalab, Rubí, Spain) were used for this purpose. Samples were taken from areas of 25 cm2. Due to the sampling of museum objects, a so-called dry swab was used, i.e., without wetting the swab with sterile saline. Samples were collected, stored and transported according to the recommendations of test procedure I-01/PO-03 [18]. The samples were then packed into a bulk transport container and sent to the accredited testing laboratory of the Provincial Sanitary and Epidemiological Station in Olsztyn, where the determinations were performed according to instruction PB-OBP-019 [19].
Description of the implementation of moisture measurements (walls and ceilings). Moisture measurements were taken with a Greisinger moisture meter GMH 3831 (GHM Messtechnik GmbH, Remscheid, Germany) equipped with probes, both invasive and non-invasive.
3. Results
3.1. Evaluation of the Actual Technical Condition and Description of the Observed Defects
The King’s Library (room no. 08). At the time of inspection, the humidity in the room was 43.8% and its temperature was 21.1 °C. Two fans and one dehumidification device were operating in the room. As a result of the failure, the ceiling and the walls of the room were flooded. Cracks and separations in the plaster and stucco were located mainly in the central part of the ceiling. In this zone, in addition to the yellowish discoloration, cracks and spalling of the plaster and stucco were visible (Figure 3, Figure 4, Figure 5 and Figure 6). Approximately a quarter of the ceiling area was in the affected zone. On the west facade wall, damp patches were visible from water running down the wall surface (Figure 7). In addition to the discoloration of the stucco, there were also innumerable cracks and spalling behind the surface. Mold colonies were found in the cavities of the stucco (Figure 8). The remaining surfaces of the building partitions were free of mold. The moisture content of the ceiling and walls was in the range of 0.4–3.0%. Locally, the west facade wall showed a moisture content of 0.9–3%. In Figure 6, the extent of this zone is marked with a black dashed line. Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13 show the results of the moisture content measurements of individual building partitions of the King’s Library. The extent and depth of the cracks caused by the failure overlaid their previously benign structure.

Figure 4.
Cracks in the plaster and stucco in the central ceiling of the King’s Library.

Figure 5.
Cracking and chipping of plaster in the southern part of the ceiling of the King’s Library.

Figure 6.
Discoloration of the stucco and plaster on the ceiling of the King’s Library.

Figure 7.
Stains on the west wall of the King’s Library.

Figure 8.
Mold colonies on the stucco of the west wall of the King’s Library.
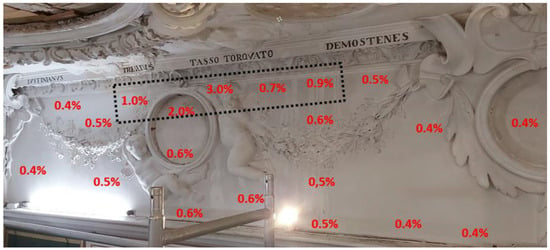
Figure 9.
King’s Library: the results of moisture measurements of the west facade wall.

Figure 10.
King’s library: the results of moisture measurements of the south wall.

Figure 11.
King’s library: the results of moisture measurements of the east wall.
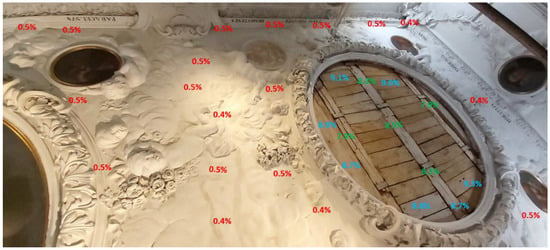
Figure 12.
King’s Library: the results of measurements of the ceiling moisture, western part.

Figure 13.
King’s library: the results of the ceiling moisture measurements, eastern part.
Historical Cabinet (room no. 44). Two fans, a compressor to ventilate the floor and one dehumidification device were operating in the room. As a result of the failure, the ceiling above the room, the walls of the room and the wooden floor were flooded. Plaster was removed from about a quarter of the interior wall (on the side of the Room above the Chapel). In the area of the removed plaster, there were cracks in the wall. The plaster was partially removed from the ceiling of the U-shaped room. There were also cracks in the zone of the removed plaster. There was no other visible damage on the walls and ceiling that could be attributed to moisture in the partitions as a result of the failure. The moisture content of the ceiling and walls was in the range of 0.5–0.6% (Figure 14, Figure 15, Figure 16, Figure 17 and Figure 18). As a result of the failure, the wooden floor was dampened. The oak floor elements were locally deformed and discolored due to fungi (Figure 19 and Figure 20). The moisture content of the wooden floor on the day of the visual inspection of the room was between 7.9 and 10.0% (correct moisture content). Figure 21 and Figure 22 shows the results of measurements of the moisture content of the wooden floor.

Figure 14.
Historical Cabinet: the wall on the side of the Room above the Chapel, part 1.

Figure 15.
Historical Cabinet: the wall on the side of the Room above the Chapel, part 2.

Figure 16.
Historical Cabinet: the facade wall, part 1.

Figure 17.
Historical Cabinet: the facade wall, part 2.

Figure 18.
Historical Cabinet: the ceiling on the side of the Room above the Chapel.

Figure 19.
Deformation and discoloration of the oak flooring in the Historical Cabinet, part 1.

Figure 20.
Deformation and discoloration of the oak flooring in the Historical Cabinet, part 2.

Figure 21.
Historical Cabinet: the oak flooring, part 1.

Figure 22.
Historical Cabinet: the oak flooring, part 2.
Room above the Chapel (room no. 45). Two fans and one dehumidification device were operating in the room. As a result of the failure, the ceiling above the room and the walls of the room (mainly the walls on the interior side of no. 44 and the corner on the interior side of 46A) were flooded. Numerous cracks in the plaster were found on the walls of the post-room. Polychromes covering the walls of the room were also damaged. The room showed no macroscopic signs of mold growth on the surfaces of the building partitions. The moisture content of the ceiling and walls was in the range of 0.4 to 0.7%. The moisture content of the floor was 0.5–0.6%. The moisture content of the wooden floor elements ranged from 7.3 to 7.6%. Figure 23, Figure 24 and Figure 25 show the results of the moisture measurements of the building partitions.

Figure 23.
Room above the Chapel: the wall from the side of the Historical Cabinet.

Figure 24.
Room above the Chapel: the wall from the side of the Cabinet with portraits of the Czartoryskis.

Figure 25.
Moisture content of the ceiling boards in the Room above the Chapel.
3.2. Assessment of Microbiological Air Pollution
Table 1 shows the results of quantitative studies, and Table 2 shows the results of qualitative studies of measurements of microbial contamination of the air.

Table 1.
Assessment of mold and bacterial contamination of air quantitative analysis.

Table 2.
Assessment of the mold contamination of air qualitative analysis.
Only a few molds and bacteria were found in the air samples collected. The amount of microorganisms found in the air was much lower than the permissible air pollution proposed by the Interdepartmental Commission for Maximum Admissible Concentrations and Intensities for Agents Harmful to Health in the Working Environment [20].
Among the molds found were both allergy-causing species and species that can produce mycotoxins [21,22,23]. Molds, proposed as indicators of water damage in the building, such as P. chrysogenum and A. versicolor, were found in the air of almost all water-damaged rooms [24].
The mycobiota of the control rooms was not analyzed in terms of the species composition, as the study mainly focused on analyzing the microbiological risk of the rooms flooded as a result of the failure of the water supply system to the air conditioning unit.
3.3. Evaluation of Microbial Contamination of Stucco Surfaces and Painting Surfaces
On the day of the visual inspection, a swab was taken from the stucco cavities (Figure 5) of the west wall in the King’s Library. The results are shown in Table 3. The result of the swab sample complements the results of the air sample analysis.

Table 3.
Results of mold analysis taken from the stuccos of the King’s Library.
On the day of the visual inspection, swabs were taken from the paintings removed from the ceiling of the King’s Library. The results are shown in Table 4.

Table 4.
Results of mold tests taken from the paintings from the King’s Library.
Fewer species and a different species composition of molds were found on the stucco and plafonds than in the air. This was particularly noticeable for the surface of the plafonds and was likely related to the dust accumulated on the back surfaces of the plafonds. As is well known, dust is a reservoir of mold spores [25].
4. Discussion
The generally accepted key to work in the field of conservatorial prevention in museums is a catalogue of conservatorial risks, compiled in the 1990s by Stefan Michalski of the Canadian Conservation Institute. The list consists of nine heterogenous factors [26], including water and microbiological threats as well. In the envisaged case of water, beyond the usual physico-chemical impact to the materials which the collection objects are made of, the humidity level of the air was raised (which is included in the list of risks independently) and triggered a microbiological invasion, which also posed a threat to humans. Immediate and methodical reaction of the museum preventive team has minimized these risks, and work to improve air quality has proven to be a key effort in tackling the broader crisis.
Microbiological air pollution in the analyzed rooms, flooded by a failure involving a leak in the water supply to the humidifier of the air handling unit located in the alcove attic, was very low. After 10 weeks of drying the walls and ceilings of the water-damaged rooms, the air contamination with mold was 15–85 cfu/m3, with bacteria at 15–40 cfu/m3.
In the world literature, the normative values for fungal contamination of the air of residential rooms and non-industrial work environments ranges from 1.0 × 101 to 1.0 × 104 cfu/m3, so there is a very large discrepancy on this issue among the applicable standards. Unanimity, however, prevails over one very important aspect of microbiological purity of indoor air. The amount of pathogenic fungi and those producing toxic metabolites (mycotoxins) in the bioaerosol should be 0 cfu/m3.
None of the legal and normative acts currently enforce concerning biological agents in the air specified threshold values for air pollution of residential and non-industrial premises in Poland. In view of this situation, publications on the problem of microbiological air pollution cite the proposal of the Interdepartmental Commission for Maximum Admissible Concentrations and Intensities for Agents Harmful to Health in the Working Environment. It concerns the adoption of recommended values for the permissible total concentration of fungi and bacteria found in the air of residential, working and public spaces at a certain level. According to the proposal of the above-mentioned Expert Panel, the permissible concentration of mesophilic bacteria and mold in the air of residential and public spaces is 5000 cfu/m3, while in archives, museums, museum warehouses and historic preservation studios, the Expert Panel on Biological Agents recommends half the allowable concentration for fungi [20]. However, in the national literature, you can also find more restrictive guidelines stating that the level of indoor air pollution should not exceed 200 cfu/m3 [27]. However, the indicated norms can only be treated as facultative standards or auxiliary reference values.
Ubiquitous molds thrive on a variety of substrates, covering them with delicate or dense, white or colored mycelium. The oligotrophic way of life of ubiquitous molds means that they find enough food to grow and reproduce under almost any conditions. Fungi usually grow best in damp, shaded areas and places with limited air circulation. Mold fungi are among the heterotrophs most dangerous to wall polychromes. The decisive factor determining their growth is moisture. Molds cause a number of adverse changes in the materials they overgrow. They secrete large amounts of hydrolytic enzymes, decomposing carbohydrates over large areas around the colonies they form [28]. They can also cause discoloration of paint layers, with not only the type of pigment, but also the binder used being important in this case [29,30]. Mycelium growing deep into the substrate damages its structure and causes the deformation of the paint layer or its detachment. The growing mycelium forms colorful, difficult-to-remove stains on the surface of the painting [31]. Biological corrosion mainly affects the plaster, mortar and stucco decorations. The cause of biodeterioration of these elements is mainly bacteria and fungi [1,15].
Molds that develop on damp building materials can also have harmful effects on human health [17]. Molds can cause many ailments, such as shortness of breath, headache, lethargy, weakness, chronic upper respiratory diseases and mycoses [32]. However, it should be kept in mind that the impact of mold on the human body is an individual matter, and the resulting consequences vary and depend on the health condition and capacity of the human immune system.
The dehumidification treatments carried out in the flooded rooms had a good effect. Measurements taken on the day of the inspection of the flooded rooms showed a low level of moisture on the surface of the building partitions. After 10 weeks of operation of dehumidification equipment, the moisture of the building partitions prevented the development of mold on their surfaces. It should be noted here that not only industrial dehumidifiers, but also fans were working in the flooded rooms. Forced air circulation significantly contributed not only to a faster reduction in the moisture of the building partitions, but also reduced the intensity of fungal development, since air flow is an outstandingly unfavorable factor for the development of fungal morphological structures (both mold and basal).
The results obtained for microbiological purity of indoor air are significantly lower than the permissible value proposed by the Expert Panel on Biological Agents of the Interdepartmental Commission on Occupational Safety and Health for both mold and bacteria [19]. This indicates that there are currently no sources of microbial contamination in the rooms flooded as a result of the accident. However, allergenic mold spores, as well as potentially mycotoxigenic mold spores, were found among the fungi present in the flooded rooms of the museum. In the inspected reference rooms, which were not flooded as a result of the accident, no spores of potentially mycotoxigenic fungi were found, as there are no conditions conducive to their growth there.
Unlike most chemical and physical agents, there are no globally accepted criteria for assessing the exposure to biological agents, as well as no generally recognized normative values [33]. This is primarily due to the fact that there is still no satisfactory epidemiological data defining the relationship between exposure to an agent and the health effect caused by its action. In addition, the sensitivity of each organism exposed to a given biologically harmful agent is an individual feature of that organism, which translates into difficulty in unambiguously determining the effects of such an action. Due to the limited access to data describing the relationship between the concentration of a biologically harmful agent and the health effect caused by its action, there are still no developed guidelines on the MRL and safe time of exposure to mold fungi.
The reason for the appearance of elevated (compared to the reference rooms) concentrations of mold spores, and especially pathogenic species, in the rooms of the Palace (the King’s Library, the Historical Cabinet, the Room above the Chapel, and the inter-ceiling space above the King’s Library and the space above the ceiling of the Room above the Chapel) is the dampness of the building envelope as a result of the failure of the water supply system. This is evidenced, among other things, by the fact that the fungal species found belong mainly to weak and moderate xerophiles. This means that these species can develop only with higher water activity (higher than the acceptable moisture content of the building materials) [10,34]. Once the moisture content of the materials is lowered to the correct (acceptable) level, the conditions conducive to mold growth will disappear.
5. Conclusions
This paper presents the results of a study of air pollution by mold and bacteria, using the example of a museum building flooded by water as a result of the failure of the water supply system to the air conditioning unit located in the attic of the building. After immediate removal of the causes of the failure and drying of the walls and ceilings, it was found that microbiological contamination of the air in the building was lower than the permissible value proposed by Interdepartmental Commission for Maximum Admissible Concentrations and Intensities for Agents Harmful to Health in the Working Environment. This means that the rapid start of drying the damp building partitions has reduced their moisture content, thereby reducing the threat of fungal and bacterial growth in the flooded rooms. The rapid reduction of the microbiological threat to people (museum employees and tourists), building materials and museum exhibits contributed to the quicker reopening of the water-damaged rooms to visitors.
Author Contributions
Conceptualization, B.A.; methodology, B.A.; software, B.A. and I.B.; validation, B.A.; formal analysis, B.A.; investigation, B.A.; resources, B.A.; data curation, B.A.; writing—original draft preparation, B.A. and I.B.; writing—review and editing, B.A., I.B. and W.B.; visualization, B.A. and I.B.; supervision, W.B.; project administration, I.B.; funding acquisition, I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Aleksandra Wójcik-Mészároš for technical and substantive support regarding the analysis of the microbiological quality of the air, and to the staff of the Museum of King John III’s Palace at Wilanow, in particular to Elżbieta Modzelewska, the head of the Museum’s Prevention and Conservation Department, and Agnieszka Pawlak, the deputy and now head of this department, for organizational and substantive support in the field of conservation issues provided during the event described above. We thank the museum director, Paweł Jaskanis, for permission to use this event as a case study for the safety of other historic buildings.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dyda, M. Zagrożenia Mikrobiologiczne Zbiorów Muzealnych; Narodowy Instytut Muzealnictwa i Zbiorów Muzealnych: Warsaw, Poland, 2020; pp. 1–92. [Google Scholar]
- Skóra, J.; Zduniak, K.; Gutarowska, B.; Rembisz, D. Harmful biological agents at museum workposts. Med. Pract. 2012, 63, 153–165. [Google Scholar]
- Stryjakowska-Sekulska, M.; Piotraszewska-Pająk, A.; Szyszka, A.; Nowicki, M.; Filipiak, M. Microbiological quality of indoor air in university rooms. Polish J. Environ. Stud. 2007, 16, 623–632. [Google Scholar]
- Kumar, P.; Singh, A.B.; Singh, R. Comprehensive health risk assessment of microbial indoor air quality in microenvironments. PLoS ONE 2022, 17, e0264226. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.; Andersson, J.V.; Dawidowicz, N.; Christophersen, E.; Hanssen, S.O.; Lindèn, A.-L.; Lindvall, T.; Pasanen, A.-L. The Swedish key action “the healthy building”—Research results achieved during the frst three years period 1998–2000. In Indoor Air 2002, Proceedings of the 9th International Conference on Indoor Air Quality and Climate, Monterey/Santa Cruz, CA, USA, 30 June–5 July 2002; Levin, H., Ed.; The International Academy of Indoor Air Sciences: Santa Cruz, CA, USA, 2002; pp. 996–1001. [Google Scholar]
- Information Series Post-Remediation Guidelines; Wonder Makers Environmental, Inc.: Kolamazoo, MI, USA, 2001; p. 147. Available online: www.wondermakers.com (accessed on 1 January 2023).
- Gutarowska, B. Grzyby strzępkowe zasiedlające materiały budowlane. Wzrost oraz produkcja mikotoksyn i alergenów. Zesz. Nauk. Rozpr. Nauk. 2010, 398, 3–161. [Google Scholar]
- Międzyresortowa Komisja do Spraw Najwyższych Dopuszczalnych Stężeń i Natężeń Czynników Szkodliwych dla Zdrowia w Środowisku Pracy. Available online: www.ciop.pl (accessed on 20 February 2023).
- Wiszniewska, M.; Walusiak, J.; Gutarowska, B.; Żakowska, Z.; Pałczyński, C. Grzyby pleśniowe w środowisku komunalnym i miejscu pracy—Istotne zagrożenie zdrowotne. Med. Pract. 2004, 55, 257–266. [Google Scholar]
- Dziurzyński, M.; Ciuchcinski, K.; Dyda, M.; Szych, A.; Drabik, P.; Laudy, A.; Dziewit, Ł. Assessment of bacterial contamination of air at the museum of King John III’s Palace at Wilanow (Warsaw, Poland): Selection of an optimal growth medium for analyzing airborne bacteria diversity. Appl. Sci. 2020, 10, 7128. [Google Scholar] [CrossRef]
- Rojas, T.I.; Martínez, E.; Gómez, Y.; Alvarado, Y. Airborne spores of Aspergillus species in cultural institutions at Havana University. Grana 2002, 41, 190–193. [Google Scholar] [CrossRef]
- Saridaki, A.; Glytsos, T.; Raisi, L.; Katsivela, E.; Tsiamis, G.; Kalogerakis, N.; Lazaridis, M. Airborne particles, bacterial and fungal communities insights of two museum exhibition halls with diverse air quality characteristics. Areobiologia 2022, 39, 69–86. [Google Scholar] [CrossRef]
- Ilieş, D.C.; Marcu, F.; Caciora, T.; Indrie, L.; Ilieş, A.; Albu, A.; Costea, M.; Burtă, L.; Baias, Ş.; Ilieş, M.; et al. Investigations of museum indoor microclimate and air quality. Case study from Romania. Atmosphere 2021, 12, 286. [Google Scholar] [CrossRef]
- Morawski, K. W Stronę Uniwersalnej Nauki. Dekoracja Rzeźbiarsko-Malarska Biblioteki Jana III w Pałacu w Wilanowie; Muzeum Pałacu Króla Jana III w Wilanowie: Warsaw, Poland, 2022; p. 12. [Google Scholar]
- Górny, R.L.; Cyprowski, M.; Stobnicka, A.; Gołofit-Szymczak, M.; Ławniczek-Wałczyk, A. Bezpieczeństwo Biologiczne w Muzeach i Pracowniach Konserwacji Zabytków; CIOP-PIB: Warsaw, Poland, 2013; pp. 1–40. [Google Scholar]
- Piontek, M. Grzyby Pleśniowe; Uniwersytet Zielonogórski: Zielona Góra, Poland, 1999; p. 32. [Google Scholar]
- Krzyściak, P.; Skóra, M.; Macura, A.B. Atlas Grzybów Chorobotwórczych Człowieka; MedPharm Polska: Wrocław, Poland, 2011; pp. 1–342. [Google Scholar]
- Instrukcja I-01/PO-03: Pobieranie, Transport i Przechowywanie Próbek do Badań. Available online: https://www.gov.pl/attachment/48e0e97d-da96-42c5-bbde-497d46f4603d (accessed on 18 February 2023).
- Procedura badawcza PB-OBP-019: Pobór, Wykrywanie i Identyfikacja Oraz Oznaczanie Liczby Bakterii i Grzybów w Próbkach Środowiskowych. Available online: https://www.gov.pl/attachment/16f0cfd5-d07f-41b6-b8ac-a31d6fedc7f8 (accessed on 18 February 2023).
- Prośniak, M.; Skowroń, J. (Eds.) Czynniki Szkodliwe w Środowisku Pracy. Wartości Dopuszczalne 2022; Międzyresortowa Komisja ds. Najwyższych Dopuszczalnych Stężeń i Natężeń Czynników Szkodliwych dla Zdrowia w Środowisku Pracy; CIOP-PIB: Warsaw, Poland, 2022; pp. 1–396. [Google Scholar]
- Twaroch, T.E.; Curin, M.; Valenta, W.; Swoboda, J. Mold Allergens in Respiratory Allergy: From Structure to Therapy. Allergy Asthma Immunol. Res. 2015, 7, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.F. Mycotoxin production by indoor molds. Fungal Genet. Biol. 2003, 39, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, J.W.; Mycotoxins, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Frisvad, J.C.; Søndergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between Fungal Species and Water-Damaged Building Materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Hirooka, Y.; Tanney, J.B.; Whitfield, E.; Mwange, K.; Meijer, M.; Amend, A.S.; Seifert, K.A.; Samson, R.A. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 2014, 78, 63–139. [Google Scholar] [CrossRef] [PubMed]
- Waller, R. Conservation Risk Assessment: A Strategy for Managing Resources for Preventive Conservation, 39, Suplement 2: Preventive Conservation: Practice, Theory and Research; Taylor & Francis: Abingdon, UK, 1999; pp. 12–16. [Google Scholar]
- Wójcik, A.; Laudy, A.; Andres, B.; Oleksiewicz, A. Fungi in museum obiects: A case study of Museum of King Jan III’s Palace at Wilanów. J. Herit. Conserv. 2015, 41, 92–98. [Google Scholar]
- Gutarowska, B.; Piotrowska, M.; Koziróg, A. Grzyby w Budynkach-Zagrożenie, Ochrona, Zwalczanie; PWN: Warsaw, Poland, 2019; pp. 1–166. [Google Scholar]
- Andres, B.; Gierasimiuk, E. Wyniki wstępnych badań nad wpływem grzybów pleśniowych na pigmenty stosowane w XV w. w małopolskim malarstwie tablicowym. Ochr. Zabyt. 2009, 62, 91–95. [Google Scholar]
- Andres, B.; Wojciechowska, J. The impact of mold fungi on the pigments used for wall and ceiling decoration based on the example of the wooden church of St Stanislaus the Bishop in Boguszyce. Ochr. Zabyt. 2017, 1, 223–238. [Google Scholar]
- Strzelczyk, A.B.; Karbowska-Berent, J. Drobnoustroje i Owady Niszczące Zabytki i ich Zwalczanie; Uniwersytet Mikołaja Kopernika: Toruń, Poland, 2004; pp. 1–250. [Google Scholar]
- Brągoszewska, E.; Pastuszka, J.S. Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia 2018, 34, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Górny, R.L. Biologiczne czynniki szkodliwe: Normy, zalecenia i propozycje wartości dopuszczalnych. Podst. Metod. Oceny Środ. Pract. 2004, 3, 17–39. [Google Scholar]
- Pinzari, F.; Gutarowska, B. Extreme colonizers and rapid profiteers: The challenging world of microorganisms that attack paper and parchment. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Springer: Cham, Switzerland, 2021; pp. 79–113. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).