Protein Kinases in Copper Homeostasis: A Review on Cu+-ATPase Modulation
Abstract
1. Introduction
1.1. Copper
1.2. Copper in Biology
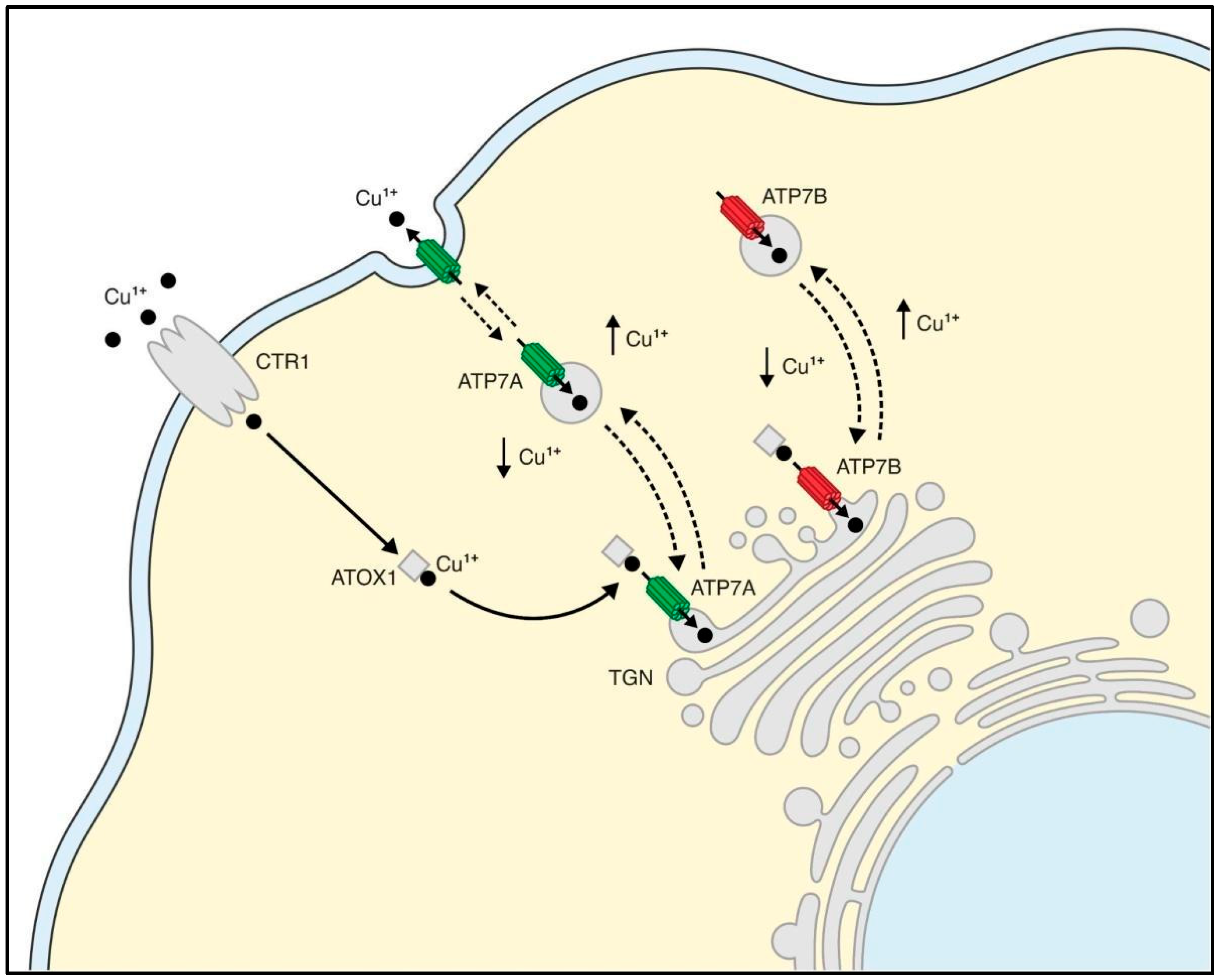
1.3. Cu+-ATPases
2. Indirect Evidence for the Kinase Regulation of Cu+-ATPases
3. The Kinase Regulation of CCC2, the Yeast Cu+-ATPase
4. The Kinase Regulation of ATP7A
5. The Kinase Regulation of ATP7B
5.1. Phosphorylation of the N-Terminal Trigger Intramolecular Interactions
5.2. Multisite Phosphorylation of ATP7B Modulates the Catalytic Cycle
5.3. Phosphorylation of ATP7B by PKD Increases Protein Stability
5.4. The C-Terminal ATP7B Undergoes Multisite Kinase Phosphorylation
5.5. Complex Regulation of ATP7B Trafficking and Phosphorylation by Kinases
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malmström, B.G.; Leckner, J. The chemical biology of copper. Curr. Opin. Chem. Biol. 1998, 2, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Occurrence of copper proteins through the three domains of life: A bioinformatic approach. J. Proteome Res. 2008, 7, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Holmberg, C.G.; Laurell, C.B. Investigations in serum copper. II. Isolation of the copper-containing protein, and a description of some of its properties. Acta Chem. Scand. 1948, 5, 476–478. [Google Scholar] [CrossRef]
- Vander Wende, C.; Wainio, W.W. The state of the copper in cytochrome c oxidase. J. Biol. Chem. 1960, 235, PC11–PC12. [Google Scholar] [CrossRef] [PubMed]
- Udenfriend, S.; Cooper, J.R. The enzymatic conversion of phenylalanine to tyrosine. J. Biol. Chem. 1952, 194, 503–511. [Google Scholar] [CrossRef]
- Harris, E.D.; Gonnerman, W.A.; Savage, J.E.; O’Dell, B.L. Connective tissue amine oxidase. II. Purification and partial characterization of lysyl oxidase from chick aorta. Biochim. Biophys. Acta 1974, 341, 332–344. [Google Scholar] [CrossRef]
- Eipper, B.A.; Milgram, S.L.; Husten, E.J.; Yun, H.Y.; Mains, R.E. Peptidylglycine α-amidating monooxygenase: A multifunctional protein with catalytic, processing, and routing domains. Protein Sci. 1993, 2, 489–497. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Marklund, S.L. Human copper-containing superoxide dismutase of high molecular weight. Proc. Natl. Acad. Sci. USA 1982, 79, 7634–7638. [Google Scholar] [CrossRef]
- Matoba, Y.; Kumagai, T.; Yamamoto, A.; Yoshitsu, H.; Sugiyama, M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 2006, 281, 8981–8990. [Google Scholar] [CrossRef]
- Yoshida, Y.; Furuta, S.; Niki, E. Effects of metal chelating agents on the oxidation of lipids induced by copper and iron. Biochim. Biophys. Acta 1993, 1210, 81–88. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Pase, L.; Voskoboinik, I.; Greenough, M.; Camakaris, J. Copper stimulates trafficking of a distinct pool of the Menkes copper ATPase (ATP7A) to the plasma membrane and diverts it into a rapid recycling pool. Biochem. J. 2004, 378, 1031–1037. [Google Scholar] [CrossRef]
- Nyasae, L.; Bustos, R.; Braiterman, L.; Eipper, B.; Hubbard, A. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: Copper-dependent redistribution between two intracellular sites. Am. J. Phys. Gastr. Liver Physiol. 2007, 292, G1181–G1194. [Google Scholar] [CrossRef]
- Inesi, G. Calcium and copper transport ATPases: Analogies and diversities in transduction and signaling mechanisms. J. Cell Commun. Signal. 2011, 5, 227–237. [Google Scholar] [CrossRef]
- Lutsenko, S.; Kaplan, J.H. Organization of P-type ATPases: Significance of structural diversity. Biochemistry 1995, 34, 15607–15613. [Google Scholar] [CrossRef]
- Kühlbrandt, W. Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 2004, 5, 282–295. [Google Scholar] [CrossRef]
- Silver, S.; Nucifora, G.; Phung, L.T. Human Menkes X-chromosome disease and the staphylococcal cadmium-resistance ATPase: A remarkable similarity in protein sequences. Mol. Microb. 1993, 10, 7–12. [Google Scholar] [CrossRef]
- Odermatt, A.; Suter, H.; Krapf, R.; Solioz, M. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J. Biol. Chem. 1993, 268, 12775–12779. [Google Scholar] [CrossRef]
- Fu, D.; Beeler, T.J.; Dunn, T.M. Sequence, mapping and disruption of CCC2, a gene that cross-complements the Ca2+-sensitive phenotype of csg1 mutants and encodes a P-type ATPase belonging to the Cu2+-ATPase subfamily. Yeast 1995, 11, 283–292. [Google Scholar] [CrossRef]
- Chelly, J.; Tümer, Z.; Tønnesen, T.; Petterson, A.; Ishikawa-Brush, Y.; Tommerup, N.; Horn, N.; Monaco, A.P. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat. Genet. 1993, 3, 14–19. [Google Scholar] [CrossRef]
- Mercer, J.F.; Livingston, J.; Hall, B.; Paynter, J.A.; Begy, C.; Chandrasekharappa, S.; Lockhart, P.; Grimes, A.; Bhave, M.; Siemieniak, D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet. 1993, 3, 20–25. [Google Scholar] [CrossRef]
- Vulpe, C.; Levinson, B.; Whitney, S.; Packman, S.; Gitschier, J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 1993, 3, 7–13. [Google Scholar] [CrossRef]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef]
- Petrukhin, K.; Fischer, S.G.; Pirastu, M.; Tanzi, R.E.; Chernov, I.; Devoto, M.; Brzustowicz, L.M.; Cayans, E.; Vitale, E.; Russo, J.J.; et al. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat. Genet. 1993, 5, 338–343. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Petrukhin, K.; Chernov, I.; Pellequer, J.L.; Wasco, W.; Ross, B.; Romano, D.M.; Parano, E.; Pavone, L.; Brzustowicz, L.M. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993, 5, 344–350. [Google Scholar] [CrossRef]
- Maung, M.T.; Carlson, A.; Olea-Flores, M.; Elkhadragy, L.; Schachtschneider, K.M.; Navarro-Tito, N.; Padilla-Benavides, T. The molecular and cellular basis of copper dysregulation and its relationship with human pathologies. FASEB 2021, 35, e21810. [Google Scholar] [CrossRef]
- Poulsen, H.; Morth, P.; Egebjerg, J.; Nissen, P. Phosphorylation of the Na+,K+-ATPase and the H+,K+-ATPase. FEBS Lett. 2010, 584, 2589–2595. [Google Scholar] [CrossRef]
- Vagin, O.; Turdikulova, S.; Sachs, G. The H,K-ATPase beta subunit as a model to study the role of N-glycosylation in membrane trafficking and apical sorting. J. Biol. Chem. 2004, 279, 39026–39034. [Google Scholar] [CrossRef]
- Veldhuis, N.A.; Gaeth, A.P.; Pearson, R.B.; Gabriel, K.; Camakaris, J. The multi-layered regulation of copper translocating P-type ATPases. Biometals 2009, 22, 177–190. [Google Scholar] [CrossRef]
- Braiterman, L.T.; Gupta, A.; Chaerkady, R.; Cole, R.N.; Hubbard, A.L. Communication between the N and C termini is required for copper-stimulated Ser/Thr phosphorylation of Cu(I)-ATPase (ATP7B). J. Biol. Chem. 2015, 290, 8803–8819. [Google Scholar] [CrossRef]
- Liu, Y.; Pilankatta, R.; Hatori, Y.; Lewis, D.; Inesi, G. Comparative features of copper ATPases ATP7A and ATP7B heterologously expressed in COS-1 cells. Biochemistry 2010, 49, 10006–10012. [Google Scholar] [CrossRef]
- Di Leva, F.; Domi, T.; Fedrizzi, L.; Lim, D.; Carafoli, E. The plasma membrane Ca2+-ATPase of animal cells: Structure, function and regulation. Arch. Biochem. Biophys. 2008, 476, 65–74. [Google Scholar] [CrossRef]
- Cobbold, C.; Ponnambalam, S.; Francis, M.J.; Monaco, A.P. Novel membrane traffic steps regulate the exocytosis of the Menkes disease ATPase. Hum. Mol. Genet. 2002, 11, 2855–2866. [Google Scholar] [CrossRef]
- Pilankatta, R.; Lewis, D.; Inesi, G. Involvement of protein kinase D in expression and trafficking of ATP7B (copper ATPase). J. Biol. Chem. 2011, 286, 7389–7396. [Google Scholar] [CrossRef]
- Wakana, Y.; Campelo, F. The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers. Cells 2021, 10, 1618. [Google Scholar] [CrossRef]
- Hilário-Souza, E.; Cuillel, M.; Mintz, E.; Charbonnier, P.; Vieyra, A.; Cassio, D.; Lowe, J. Modulation of hepatic copper-ATPase activity by insulin and glucagon involves protein kinase A (PKA) signaling pathway. Biochim. Biophys. Acta 2016, 1862, 2086–2097. [Google Scholar] [CrossRef] [PubMed]
- Afton, S.E.; Caruso, J.A.; Britigan, B.E.; Qin, Z. Copper egress is induced by PMA in human THP-1 monocytic cell line. Biometals 2009, 22, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.H.; Britto-Borges, T.; Vieyra, A.; Lowe, J. ATP7B activity is stimulated by PKCe in porcine liver. Int. J. Biochem. Cell Biol. 2014, 54, 60–67. [Google Scholar] [CrossRef][Green Version]
- Chesi, G.; Hegde, R.N.; Iacobacci, S.; Concilli, M.; Parashuraman, S.; Festa, B.P.; Polishchuk, E.V.; Di Tullio, G.; Carissimo, A.; Montefusco, S.; et al. Identification of p38 MAPK and JNK as new targets for correction of Wilson disease-causing ATP7B mutants. Hepatology 2016, 63, 1842–1859. [Google Scholar] [CrossRef]
- Park, G.B.; Choi, Y.; Kim, Y.S.; Lee, H.K.; Kim, D.; Hur, D.Y. ROS-mediated JNK/p38-MAPK activation regulates Bax translocation in Sorafenib-induced apoptosis of EBV-transformed B cells. Int. J. Oncol. 2014, 44, 977–985. [Google Scholar] [CrossRef]
- Hardman, B.; Michalczyk, A.; Greenough, M.; Camakaris, J.; Mercer, J.F.; Ackland, M.L. Hormonal regulation of the Menkes and Wilson copper-transporting ATPases in human placental Jeg-3 cells. Biochem. J. 2007, 402, 241–250. [Google Scholar] [CrossRef]
- Schlief, M.L.; West, T.; Craig, A.M.; Holtzman, D.M.; Gitlin, J.D. Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc. Natl. Acad. Sci. USA 2006, 103, 14919–14924. [Google Scholar] [CrossRef]
- Valverde, R.H.; Morin, I.; Lowe, J.; Mintz, E.; Cuillel, M.; Vieyra, A. Cyclic AMP-dependent protein kinase controls energy interconversion during the catalytic cycle of the yeast copper-ATPase. FEBS Lett. 2008, 582, 891–895. [Google Scholar] [CrossRef]
- Valverde, R.H.; Britto-Borges, T.; Lowe, J.; Einicker-Lamas, M.; Mintz, E.; Cuillel, M.; Vieyra, A. Two serine residues control sequential steps during catalysis of the yeast copper ATPase through different mechanisms that involve kinase-mediated phosphorylations. J. Biol. Chem. 2011, 286, 6879–6889. [Google Scholar] [CrossRef]
- Pilankatta, R.; Lewis, D.; Adams, C.M.; Inesi, G. High yield heterologous expression of wild-type and mutant Cu+-ATPase (ATP7B, Wilson disease protein) for functional characterization of catalytic activity and serine residues undergoing copper-dependent phosphorylation. J. Biol. Chem. 2009, 284, 21307–21316. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Fernando, R.; Veldhuis, N.; Hannan, K.M.; Marmy-Conus, N.; Pearson, R.B.; Camakaris, J. Protein kinase-dependent phosphorylation of the Menkes copper P-type ATPase. Biochem. Biophys. Res. Commun. 2003, 303, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, N.A.; Kuiper, M.J.; Dobson, R.C.; Pearson, R.B.; Camakaris, J. In silico modeling of the Menkes copper-translocating P-type ATPase 3rd metal binding domain predicts that phosphorylation regulates copper-binding. Biomet. Int. J. Role Met. Ions Biol. Biochem. Med. 2011, 24, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Holloway, Z.G.; Velayos-Baeza, A.; Howell, G.J.; Levecque, C.; Ponnambalam, S.; Sztul, E.; Monaco, A.P. Trafficking of the Menkes copper transporter ATP7A is regulated by clathrin-, AP-2-, AP-1-, and Rab22-dependent steps. Mol. Biol. Cell 2013, 24, 1735–1748. [Google Scholar] [CrossRef]
- Zhu, S.; Shanbhag, V.; Hodgkinson, V.L.; Petris, M.J. Multiple di-leucines in the ATP7A copper transporter are required for retrograde trafficking to the trans-Golgi network. Metallomics 2016, 8, 993–1001. [Google Scholar] [CrossRef]
- Sudhahar, V.; Okur, M.N.; Bagi, Z.; O’Bryan, J.P.; Hay, N.; Makino, A.; Patel, V.S.; Phillips, S.A.; Stepp, D.; Ushio-Fukai, M.; et al. Akt2 (Protein Kinase B Beta) Stabilizes ATP7A, a Copper Transporter for Extracellular Superoxide Dismutase, in Vascular Smooth Muscle: Novel Mechanism to Limit Endothelial Dysfunction in Type 2 Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 529–541. [Google Scholar] [CrossRef]
- Vanderwerf, S.M.; Cooper, M.J.; Stetsenko, I.V.; Lutsenko, S. Copper specifically regulates intracellular phosphorylation of the Wilson’s disease protein, a human copper-transporting ATPase. J. Biol. Chem. 2001, 276, 36289–36294. [Google Scholar] [CrossRef]
- Tsivkovskii, R.; Eisses, J.F.; Kaplan, J.H.; Lutsenko, S. Functional properties of the copper-transporting ATPase ATP7B (the Wilson’s disease protein) expressed in insect cells. J. Biol. Chem. 2002, 277, 976–983. [Google Scholar] [CrossRef]
- Bartee, M.Y.; Ralle, M.; Lutsenko, S. The loop connecting metal-binding domains 3 and 4 of ATP7B is a target of a kinase-mediated phosphorylation. Biochemistry 2009, 48, 5573–5581. [Google Scholar] [CrossRef]
- Ruturaj Mishra, M.; Saha, S.; Maji, S.; Rodriguez-Boulan, E.; Schreiner, R.; Gupta, A. Regulation of the apico-basolateral trafficking polarity of the homologous copper-ATPases ATP7A and ATP7B. J. Cell Sci. 2024, 137, jcs261258. [Google Scholar] [CrossRef]
- Hasan, N.M.; Gupta, A.; Polishchuk, E.; Yu, C.H.; Polishchuk, R.; Dmitriev, O.Y.; Lutsenko, S. Molecular events initiating exit of a copper-transporting ATPase ATP7B from the trans-Golgi network. J. Biol. Chem. 2012, 287, 36041–36050. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yin, J.Y.; Liu, Z.Q.; Li, X.P. Copper efflux transporters ATP7A and ATP7B: Novel biomarkers for platinum drug resistance and targets for therapy. IUBMB Life 2018, 70, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Janardhanan, P.; Somasundaran, A.K.; Balakrishnan, A.J.; Pilankatta, R. Sensitization of cancer cells towards Cisplatin and Carboplatin by protein kinase D inhibitors through modulation of ATP7A/B (copper transport ATPases). Cancer Treat. Res. Commun. 2022, 32, 100613. [Google Scholar] [CrossRef] [PubMed]
- Vanderwerf, S.M.; Lutsenko, S. The Wilson’s disease protein expressed in Sf9 cells is phosphorylated. Biochem. Soc. Trans. 2002, 30, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.; Bartee, M.Y.; Braiterman, L.; Gupta, A.; Ustiyan, V.; Zuzel, V.; Kaplan, J.H.; Hubbard, A.L.; Lutsenko, S. Cell-specific trafficking suggests a new role for renal ATP7B in the intracellular copper storage. Traffic 2009, 10, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Labbozzetta, V.; Giuffrida, A.E.; Peritore, L.; Calabrese, V.; Spinella, C.; Stancanelli, M.R.; Spallino, E.; Visconti, L.; Santoro, D. Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites 2023, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Plattner, F.; Bibb, J. Serine and Threonine Phosphorylation. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D.L., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 467–492. ISBN 9780123749475. [Google Scholar] [CrossRef]
- Samet, J.M.; Graves, L.M.; Quay, J.; Dailey, L.A.; Devlin, R.B.; Ghio, A.J.; Wu, W.; Bromberg, P.A.; Reed, W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. 1998, 275, L551–L558. [Google Scholar] [CrossRef] [PubMed]
- Barthel, A.; Ostrakhovitch, E.A.; Walter, P.L.; Kampkötter, A.; Klotz, L.O. Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: Mechanisms and consequences. Arch. Biochem. Biophys. 2007, 463, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Turski, M.L.; Brady, D.C.; Kim, H.J.; Kim, B.E.; Nose, Y.; Counter, C.M.; Winge, D.R.; Thiele, D.J. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell Biol. 2012, 32, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, J.; Zheng, N.; Zhang, X.; Dai, X.; Zhang, L.; Hu, C.; Wu, X.; Jiang, Q.; Wu, D.; et al. Copper Promotes Tumorigenesis by Activating the PDK1-AKT Oncogenic Pathway in a Copper Transporter 1 Dependent Manner. Adv. Sci. 2021, 8, e2004303. [Google Scholar] [CrossRef] [PubMed]
- Chojnowski, J.E.; Li, R.; Tsang, T.; Alfaran, F.H.; Dick, A.; Cocklin, S.; Brady, D.C.; Strochlic, T.I. Copper Modulates the Catalytic Activity of Protein Kinase CK2. Front. Mol. Biosci. 2022, 9, 878652. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Posimo, J.M.; Gudiel, A.A.; Cicchini, M.; Feldser, D.M.; Brady, D.C. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol. 2020, 22, 412–424. [Google Scholar] [CrossRef]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef]
- Brady, D.C.; Crowe, M.S.; Greenberg, D.N.; Counter, C.M. Copper Chelation Inhibits BRAFV600E-Driven Melanomagenesis and Counters Resistance to BRAFV600E and MEK1/2 Inhibitors. Cancer Res. 2017, 77, 6240–6252. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Casio, M.; Range, D.E.; Sosa, J.A.; Counter, C.M. Copper Chelation as Targeted Therapy in a Mouse Model of Oncogenic BRAF-Driven Papillary Thyroid Cancer. Clin. Cancer Res. 2018, 24, 4271–4281. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Taveira-da-Silva, R.; Hilário-Souza, E. Dissecting copper homeostasis in diabetes mellitus. IUBMB Life 2017, 69, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Chen, L.; Kong, Y.; Zhuo, J.F.; Sun, Q.; Chang, J. The association between serum copper concentration and prevalence of diabetes among US adults with hypertension (NHANES 2011–2016). J. Cell Mol. Med. 2024, 28, e18270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Amarsingh, G.V.; Cheung, C.C.H.; Hogl, S.; Narayanan, U.; Zhang, L.; McHarg, S.; Xu, J.; Gong, D.; et al. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc. Diabetol. 2014, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Lu, J.; Chen, X.; Reddy, S.; Crossman, D.J.; Glyn-Jones, S.; Choong, Y.S.; Kennedy, J.; Barry, B.; Zhang, S.; et al. A copper(II)-selective chelator ameliorates diabetes-evoked renal fibrosis and albuminuria, and suppresses pathogenic TGF-beta activation in the kidneys of rats used as a model of diabetes. Diabetologia 2008, 51, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Farrant, J.; Dodd, S.; Vaughan, C.; Reid, A.; Schmitt, M.; Garratt, C.; Akhtar, M.; Mahmod, M.; Neubauer, S.; Cooper, R.M.; et al. TEMPEST investigators. Rationale and design of a randomised trial of trientine in patients with hypertrophic cardiomyopathy. Heart 2023, 109, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.; Butler, J.; Cleland, J.; Felker, M.; Mentz, R.; Wang, Y.; Zhang, Y.; Mou, H.; Yu, J.; Guo, L.; et al. Effect Of Trientine-hydrochloride In Heart Failure With Lower Left Ventricular Ejection Fraction: The TRACER-HF Trial. J. Card. Fail. 2024, 30, 314–315. [Google Scholar] [CrossRef]
- Scholefield, M.; Church, S.J.; Xu, J.; Patassini, S.; Roncaroli, F.; Hooper, N.M.; Unwin, R.D.; Cooper, G.J.S. Widespread Decreases in Cerebral Copper Are Common to Parkinson’s Disease Dementia and Alzheimer’s Disease Dementia. Front. Aging Neurosci. 2021, 13, 641222. [Google Scholar] [CrossRef]
- Patwa, J.; Flora, S.J.S. Copper: From enigma to therapeutic target for neurological disorder. Basic Clin. Pharmacol. Toxicol. 2024, 134, 778–791. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Huang, Z.; Duan, J.; Nice, E.C.; Lin, J.; Huang, C. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol. Oncol. 2021, 15, 3527–3544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, M.; Zhang, J.; Abdalla, M.; Sun, P.; Yang, Z.; Zhang, C.; Liu, Y.; Chen, C.; Jiang, X. Syphilis mimetic nanoparticles for cuproptosis-based synergistic cancer therapy via reprogramming copper metabolism. Int. J. Pharm. 2023, 640, 123025. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, J.; Yang, Y.; Fleishman, J.S.; Wang, Y.; Wang, J.; Chen, J.; Li, Y.; Wang, H. Cuproptosis: A novel therapeutic target for overcoming cancer drug resistance. Drug Resist. Updates 2024, 72, 101018. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Huang, L.; Gan, Y.; Xia, Y.; Yu, W. The Molecular Mechanisms of Cuproptosis and Small-Molecule Drug Design in Diabetes Mellitus. Molecules 2024, 29, 2852. [Google Scholar] [CrossRef] [PubMed]

| Enzyme | Function |
|---|---|
| Ceruloplasmin [5] | Serum ferroxidase activity, responsible for 95% copper transport in the blood. |
| Cytochrome c oxidase [6] | Conversion of molecular oxygen to water. Complex IV of the respiratory chain, involved in energy metabolism. |
| Dopamine-β-hydroxylase [7] | Catalyzes the conversion of dopamine to norepinephrine. Biosynthesis of neurotransmitters. |
| Lysyl oxidase [8] | Crosslinking of collagen and elastin. Biogenesis of connective tissue matrices. |
| Peptidylglycine α-amidating monooxygenase [9] | Catalyzes the C-terminal amidation of glycine-extended peptide precursors. Bioactivity of hormones and neuropeptides. |
| Superoxide dismutase 1 [10] and 3 [11] | Conversion of superoxide anion to hydrogen peroxide. Regulation of redox balance. |
| Tyrosinase [12] | Conversion of tyrosine to L-DOPA. Regulation of melanin synthesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valverde, R.H.F.; Lowe, J. Protein Kinases in Copper Homeostasis: A Review on Cu+-ATPase Modulation. Kinases Phosphatases 2024, 2, 240-254. https://doi.org/10.3390/kinasesphosphatases2030015
Valverde RHF, Lowe J. Protein Kinases in Copper Homeostasis: A Review on Cu+-ATPase Modulation. Kinases and Phosphatases. 2024; 2(3):240-254. https://doi.org/10.3390/kinasesphosphatases2030015
Chicago/Turabian StyleValverde, Rafael Hospodar Felippe, and Jennifer Lowe. 2024. "Protein Kinases in Copper Homeostasis: A Review on Cu+-ATPase Modulation" Kinases and Phosphatases 2, no. 3: 240-254. https://doi.org/10.3390/kinasesphosphatases2030015
APA StyleValverde, R. H. F., & Lowe, J. (2024). Protein Kinases in Copper Homeostasis: A Review on Cu+-ATPase Modulation. Kinases and Phosphatases, 2(3), 240-254. https://doi.org/10.3390/kinasesphosphatases2030015







