Protein Phosphorylation Nexus of Cyanobacterial Adaptation and Metabolism
Abstract
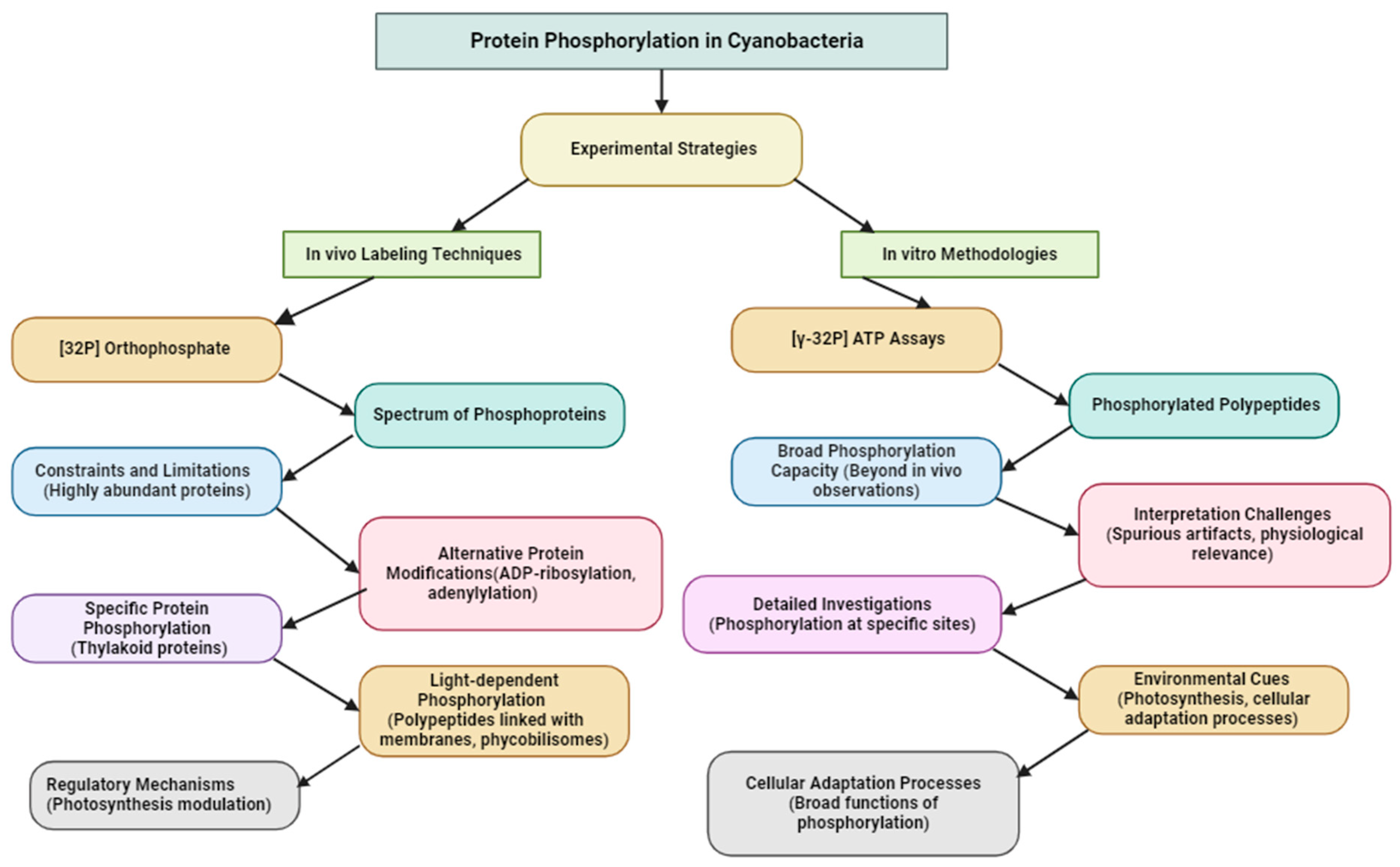
1. Introduction
2. Indications of Protein Phosphorylation: Forms and Functions
3. Historical Perspective: Early Studies on Protein Phosphorylation in Prokaryotes
4. Two-Component Sensory Systems and Protein Phosphorylation
5. Central Nutritional Mode of Cyanobacteria: Photoautotrophy
6. Protein Phosphorylation-Mediated Chromatic Adaptation in Cyanobacteria
7. Protein Phosphorylation in Cyanobacterial Salt Stress Adaptation
8. Risk of Photo-Inhibition and Role of Inorganic Nutrients
9. Integration of Light Harvesting and Nutrient Acquisition
10. Protein Phosphorylation: A Key Player in Metabolic Integration
11. Regulatory Role of Protein Kinases and Phosphatases in Cyanobacteria
12. Future Directions and Implications
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Harrison, M.T.; Zhou, R. Sustainable protein production through genetic engineering of cyanobacteria and use of atmospheric N2 gas. Food Energy Secur. 2024, 13, e536. [Google Scholar] [CrossRef]
- Ditty, J.; Williams, S.; Golden, S. A cyanobacterial circadian timing mechanism. Annu. Rev. Genet. 2003, 37, 513–543. [Google Scholar] [CrossRef]
- Johnson, L.N. The regulation of protein phosphorylation. Biochem. Soc. Trans. 2009, 37, 627–641. [Google Scholar] [CrossRef]
- Babele, P.K.; Kumar, J.; Chaturvedi, V. Proteomic de-regulation in cyanobacteria in response to abiotic stresses. Front. Microbiol. 2019, 10, 429649. [Google Scholar] [CrossRef]
- Steuer, R.; Knoop, H.; Machné, R. Modelling cyanobacteria: From metabolism to integrative models of phototrophic growth. J. Exp. Bot. 2012, 63, 2259–2274. [Google Scholar] [CrossRef]
- Maberly, S.C. The fitness of the environments of air and water for photosynthesis, growth, reproduction and dispersal of photoautotrophs: An evolutionary and biogeochemical perspective. Aquat. Bot. 2014, 118, 4–13. [Google Scholar] [CrossRef]
- Samiotis, G. Wastewater Treatment and Valorization Coupled with Cyanobacterium Synechococcus Elongatus PCC 7942 Cultivation. Ph.D. Thesis, University of Western Macedonia, Kozani, Greece, 2022. [Google Scholar]
- Wu, X.; Xu, M.; Geng, M.; Chen, S.; Little, P.J.; Xu, S.; Weng, J. Targeting protein modifications in metabolic diseases: Molecular mechanisms and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 220. [Google Scholar] [CrossRef]
- Capra, E.J.; Laub, M.T. Evolution of two-component signal transduction systems. Annu. Rev. Microbiol. 2012, 66, 325–347. [Google Scholar] [CrossRef]
- Wolanin, P.M.; Thomason, P.A.; Stock, J.B. Histidine protein kinases: Key signal transducers outside the animal kingdom. Genome Biol. 2002, 3, 1–8. [Google Scholar] [CrossRef]
- Jurdzinski, K.T.; Mehrshad, M.; Delgado, L.F.; Deng, Z.; Bertilsson, S.; Andersson, A.F. Large-scale phylogenomics of aquatic bacteria reveal molecular mechanisms for adaptation to salinity. Sci. Adv. 2023, 9, eadg2059. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy. Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Çelekli, A.; Zariç, Ö.E. Plasma-Enhanced Microalgal Cultivation: A Sustainable Approach for Biofuel and Biomass Production. In Emerging Applications of Plasma Science in Allied Technologies; IGI Global: Hershey, PA, USA, 2024; pp. 243–263. [Google Scholar]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Bleakley, B.; Zhou, R. Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules 2024, 29, 2534. [Google Scholar] [CrossRef]
- Liu, J.; Qian, C.; Cao, X. Post-translational modification control of innate immunity. Immunity 2016, 45, 15–30. [Google Scholar] [CrossRef]
- Cohen, S.E.; Golden, S.S. Circadian rhythms in cyanobacteria. Microbiol. Mol. Biol. Rev. 2015, 79, 373–385. [Google Scholar] [CrossRef]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part I. CRC Crit. Rev. Microbiol. 1982, 10, 317–391. [Google Scholar] [CrossRef] [PubMed]
- Kolodiazhnyi, O.I. Phosphorus compounds of natural origin: Prebiotic, stereochemistry, application. Symmetry 2021, 13, 889. [Google Scholar] [CrossRef]
- Iyer, L.M.; Anantharaman, V.; Krishnan, A.; Burroughs, A.M.; Aravind, L. Jumbo phages: A comparative genomic overview of core functions and adaptions for biological conflicts. Viruses 2021, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Stierum, R.H.; Dianov, G.L.; Bohr, V.A. Single-nucleotide patch base excision repair of uracil in DNA by mitochondrial protein extracts. Nucleic Acids Res. 1999, 27, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Borkovich, K.A.; Alex, L.A.; Yarden, O.; Freitag, M.; Turner, G.E.; Read, N.D.; Seiler, S.; Bell-Pedersen, D.; Paietta, J.; Plesofsky, N.; et al. Lessons from the genome sequence of Neurospora crassa: Tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 2004, 68, 1–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-K.; Qiao, Z.-X.; Zhang, W.-Y.; Xiong, Q.; Zhang, J.; Li, T.; Ge, F.; Zhao, J.-D. Global phosphoproteomic analysis reveals diverse functions of serine/threonine/tyrosine phosphorylation in the model cyanobacterium Synechococcus sp. strain PCC 7002. J. Proteome Res. 2013, 12, 1909–1923. [Google Scholar] [CrossRef]
- Dumas, L.; Zito, F.; Blangy, S.; Auroy, P.; Johnson, X.; Peltier, G.; Alric, J. A stromal region of cytochrome b 6 f subunit IV is involved in the activation of the Stt7 kinase in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2017, 114, 12063–12068. [Google Scholar] [CrossRef]
- Carlberg, I.; Hansson, M.; Kieselbach, T.; Schröder, W.P.; Andersson, B.; Vener, A.V. A novel plant protein undergoing light-induced phosphorylation and release from the photosynthetic thylakoid membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Singh, N.; Kanda, T.; Yadav, S.; Yadav, S.; Atri, N. Cyanobacterial Proteomics: Diversity and Dynamics. J. Proteome Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hao, Y.-H.; Orth, K. A newly discovered post-translational modification–the acetylation of serine and threonine residues. Trends Biochem. Sci. 2007, 32, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.-H. Structural insights into protein regulation by phosphorylation and substrate recognition of protein kinases/phosphatases. Life 2021, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Appleby, J.L. The Activation Mechanism of Response Regulator Chey; The University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 1997. [Google Scholar]
- Marijuán, P.C.; Navarro, J. From molecular recognition to the “vehicles” of evolutionary complexity: An informational approach. Int. J. Mol. Sci. 2021, 22, 11965. [Google Scholar] [CrossRef] [PubMed]
- Getz, L.J.; Runte, C.S.; Rainey, J.K.; Thomas, N.A. Tyrosine phosphorylation as a widespread regulatory mechanism in prokaryotes. J. Bacteriol. 2019, 201, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Boehi, F.; Manetsch, P.; Hottiger, M.O. Interplay between ADP-ribosyltransferases and essential cell signaling pathways controls cellular responses. Cell Discov. 2021, 7, 104. [Google Scholar] [CrossRef]
- Falke, J.J.; Bass, R.B.; Butler, S.L.; Chervitz, S.A.; Danielson, M.A. The two-component signaling pathway of bacterial chemotaxis: A molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu. Rev. Cell Dev. Biol. 1997, 13, 457–512. [Google Scholar] [CrossRef]
- Buschiazzo, A.; Trajtenberg, F. Two-component sensing and regulation: How do histidine kinases talk with response regulators at the molecular level? Annu. Rev. Microbiol. 2019, 73, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Bissett, A.; Brown, M.V.; Siciliano, S.D.; Thrall, P.H. Microbial community responses to anthropogenically induced environmental change: Towards a systems approach. Ecol. Lett. 2013, 16, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Mascher, T.; Helmann, J.D.; Unden, G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 910–938. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Beyer, H.M.; Zurbriggen, M.D.; Gärtner, W. The red edge: Bilin-binding photoreceptors as optogenetic tools and fluorescence reporters. Chem. Rev. 2021, 121, 14906–14956. [Google Scholar] [CrossRef] [PubMed]
- Schluchter, W.M. The Characterization of Photosystem I and Ferredoxin-NADP (+) Oxidoreductase in the Cyanobacterium Synechococcus sp. PCC 7002; The Pennsylvania State University: University Park, PA, USA, 1994. [Google Scholar]
- Koskinen, S.; Kurkela, J.; Linhartová, M.; Tyystjärvi, T. The genome sequence of Synechocystis sp. PCC 6803 substrain GT-T and its implications for the evolution of PCC 6803 substrains. FEBS Open Bio 2023, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Vakonakis, I.; Klewer, D.A.; Williams, S.B.; Golden, S.S.; LiWang, A.C. Structure of the N-terminal domain of the circadian clock-associated histidine kinase SasA. J. Mol. Biol. 2004, 342, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, N.; Tran, C.N.; Brumlow, C.; DebRoy, S.; Yao, H.; Gonzalez, G.N.; Makthal, N.; Kumaraswami, M.; Shelburne, S.A. Phosphatase activity of the control of virulence sensor kinase CovS is critical for the pathogenesis of group A streptococcus. PLoS Pathog. 2018, 14, e1007354. [Google Scholar] [CrossRef] [PubMed]
- Deussing, J.M. Targeted mutagenesis tools for modelling psychiatric disorders. Cell Tissue Res. 2013, 354, 9–25. [Google Scholar] [CrossRef]
- May, J.P. Characterisation of the slr1212 Genomic Region of the Freshwater Cyanobacterium Synechocystis sp. PCC 6803; University of Warwick: Coventry, UK, 2001. [Google Scholar]
- Lezhneva, L. Identification of Novel Nuclear Factors Required for Chloroplast Gene Expression and Photosystem I Assembly. Ph.D. Thesis, LMU, Munich, Germany, 2005. [Google Scholar]
- Samuels, D.S.; Lybecker, M.C.; Yang, X.F.; Ouyang, Z.; Bourret, T.J.; Boyle, W.K.; Stevenson, B.; Drecktrah, D.; Caimano, M.J. Gene regulation and transcriptomics. Curr. Issues Mol. Biol. 2021, 42, 223–266. [Google Scholar]
- Laub, M.T. The Role of Two-Component Signal Transduction Systems in Bacterial Stress Responses. In Bacterial Stress Responses; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 45–58. [Google Scholar]
- Rachedi, R.; Foglino, M.; Latifi, A. Stress signaling in cyanobacteria: A mechanistic overview. Life 2020, 10, 312. [Google Scholar] [CrossRef]
- Nawaz, T.; Saud, S.; Gu, L.; Khan, I.; Fahad, S.; Zhou, R. Cyanobacteria: Harnessing the Power of Microorganisms for Plant Growth Promotion, Stress Alleviation, and Phytoremediation in the Era of Sustainable Agriculture. Plant Stress 2024, 11, 100399. [Google Scholar] [CrossRef]
- Ringsmuth, A.K.; Landsberg, M.J.; Hankamer, B. Can photosynthesis enable a global transition from fossil fuels to solar fuels, to mitigate climate change and fuel-supply limitations? Renew. Sustain. Energy Rev. 2016, 62, 134–163. [Google Scholar] [CrossRef]
- Stitt, M.; Sulpice, R.; Keurentjes, J. Metabolic networks: How to identify key components in the regulation of metabolism and growth. Plant Physiol. 2010, 152, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Gurrieri, L.; Fermani, S.; Velie, L.; Sparla, F.; Crozet, P.; Henri, J.; Zaffagnini, M. Ribulose-1, 5-bisphosphate regeneration in the Calvin-Benson-Bassham cycle: Focus on the last three enzymatic steps that allow the formation of Rubisco substrate. Front. Plant Sci. 2023, 14, 1130430. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Burnap, R.L. Systems and photosystems: Cellular limits of autotrophic productivity in cyanobacteria. Front. Bioeng. Biotechnol. 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Cray, J.A.; Bell, A.N.; Bhaganna, P.; Mswaka, A.Y.; Timson, D.J.; Hallsworth, J.E. The biology of habitat dominance; can microbes behave as weeds? Microb. Biotechnol. 2013, 6, 453–492. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, R.; PriyaDharshini, L.C.; Sakthivel, K.M.; Rasmi, R.R. Role and regulation of autophagy in cancer. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166400. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Y.; Teng, W.-K.; Zhao, L.; Hu, C.-X.; Zhou, Y.-K.; Han, B.-P.; Song, L.-R.; Shu, W.-S. Comparative genomics reveals insights into cyanobacterial evolution and habitat adaptation. ISME J. 2021, 15, 211–227. [Google Scholar] [CrossRef]
- Hawkins, R.D.; Hon, G.C.; Ren, B. Next-generation genomics: An integrative approach. Nat. Rev. Genet. 2010, 11, 476–486. [Google Scholar] [CrossRef]
- Wiltbank, L.B.; Kehoe, D.M. Diverse light responses of cyanobacteria mediated by phytochrome superfamily photoreceptors. Nat. Rev. Microbiol. 2019, 17, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Larkum, A.W. Light-harvesting in cyanobacteria and eukaryotic algae: An overview. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms; Springer: Cham, Switzerland, 2020; pp. 207–260. [Google Scholar]
- Kehoe, D.M.; Grossman, A.R. Complementary chromatic adaptation: Photoperception to gene regulation. In Seminars in Cell Biology; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- de Marsac, N.T. Differentiation of hormogonia and relationships with other biological processes. In The Molecular Biology of Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 1994; pp. 825–842. [Google Scholar]
- Cohen, P. The origins of protein phosphorylation. Nat. Cell Biol. 2002, 4, E127–E130. [Google Scholar] [CrossRef] [PubMed]
- Buljan, M.; Ciuffa, R.; van Drogen, A.; Vichalkovski, A.; Mehnert, M.; Rosenberger, G.; Lee, S.; Varjosalo, M.; Pernas, L.E.; Spegg, V.; et al. Kinase interaction network expands functional and disease roles of human kinases. Mol. Cell 2020, 79, 504–520.e9. [Google Scholar] [CrossRef] [PubMed]
- Valente-Paterno, M. Spatial and Temporal Patterns of Localized Thylakoid Biogenesis in the Chloroplast of Chlamydomonas reinhardtii; Concordia University: Montreal, QC, Canada, 2018. [Google Scholar]
- Mann, N.H. Protein phosphorylation in cyanobacteria. Microbiology 1994, 140, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Salomon, A.R.; Ficarro, S.B.; Brill, L.M.; Brinker, A.; Phung, Q.T.; Ericson, C.; Sauer, K.; Brock, A.; Horn, D.M.; Schultz, P.G.; et al. Profiling of tyrosine phosphorylation pathways in human cells using mass spectrometry. Proc. Natl. Acad. Sci. USA 2003, 100, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhan, J.; Chen, Y.; Yang, M.; He, C.; Ge, F.; Wang, Q. Effects of phosphorylation of β subunits of phycocyanins on state transition in the model cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2015, 56, 1997–2013. [Google Scholar] [CrossRef]
- Teoh, F.K.Y. Membrane Proteins and Protein-Protein Interactions in Marine Cyanobacteria; Macquarie University: Sydney, Australia, 2022. [Google Scholar]
- Li, S.; Dean, S.; Li, Z.; Horecka, J.; Deschenes, R.J.; Fassler, J.S. The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol. Biol. Cell 2002, 13, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Gibbons, N. The glycerol dehydrogenases of Pseudomonas salinaria, Vibrio costicolus, and Escherichia coli in relation to bacterial halophilism. Can. J. Biochem. Physiol. 1954, 32, 206–217. [Google Scholar] [CrossRef]
- Batelli, G.; Verslues, P.E.; Agius, F.; Qiu, Q.; Fujii, H.; Pan, S.; Schumaker, K.S.; Grillo, S.; Zhu, J.-K. SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol. Cell. Biol. 2007, 27, 7781–7790. [Google Scholar] [CrossRef]
- Eberhard, S.; Finazzi, G.; Wollman, F.-A. The dynamics of photosynthesis. Annu. Rev. Genet. 2008, 42, 463–515. [Google Scholar] [CrossRef]
- Muhseen, Z.T.; Xiong, Q.; Chen, Z.; Ge, F. Proteomics studies on stress responses in diatoms. Proteomics 2015, 15, 3943–3953. [Google Scholar] [CrossRef] [PubMed]
- Atakkatan, A.; Innesent, S.; Prajapat, S.P.; Pandit, S.; Khanna, N. Potential of Extremophilic Algae for the Synthesis of Value-Added Products, in Extremophiles; CRC Press: Boca Raton, FL, USA, 2023; pp. 80–114. [Google Scholar]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D.J. The potential of manipulating light for the commercial production of carotenoids from algae. Algal Res. 2023, 71, 103047. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Price, G.D.; Badger, M.R.; Woodger, F.J.; Long, B.M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 2008, 59, 1441–1461. [Google Scholar] [CrossRef] [PubMed]
- Balparda, M.; Bouzid, M.; Martinez, M.d.P.; Zheng, K.; Schwarzländer, M.; Maurino, V.G. Regulation of plant carbon assimilation metabolism by post-translational modifications. Plant J. 2023, 114, 1059–1079. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.J.; Easson, C.G.; Fiore, C.L.; Thacker, R.W. Sponge–microbe interactions on coral reefs: Multiple evolutionary solutions to a complex environment. Front. Mar. Sci. 2021, 8, 705053. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Colloff, M.J.; Gibb, N.L.; Wakelin, S.A. The abundance of microbial functional genes in grassy woodlands is influenced more by soil nutrient enrichment than by recent weed invasion or livestock exclusion. Appl. Environ. Microbiol. 2010, 76, 5547–5555. [Google Scholar] [CrossRef] [PubMed]
- Carini, P.J. Genome-Enabled Investigation of the Minimal Growth Requirements Andphosphate Metabolism for Pelagibacter Marine bacteria. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2013. [Google Scholar]
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant Biol. 2014, 56, 192–220. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cotera, L.B.; Chávez-Cabrera, C.; Martínez-Cárdenas, A.; Sánchez, S.; García-Flores, O.U. Deciphering the mechanism by which the yeast Phaffia rhodozyma responds adaptively to environmental, nutritional, and genetic cues. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab048. [Google Scholar] [CrossRef]
- Trevisan, R.; Mello, D.F. Redox control of antioxidants, metabolism, immunity, and development at the core of stress adaptation of the oyster Crassostrea gigas to the dynamic intertidal environment. In Free Radical Biology and Medicine; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Battchikova, N.; Angeleri, M.; Aro, E.-M. Proteomic approaches in research of cyanobacterial photosynthesis. Photosynth. Res. 2015, 126, 47–70. [Google Scholar] [CrossRef]
- Kirilovsky, D.; Kaňa, R.; Prášil, O. Mechanisms modulating energy arriving at reaction centers in cyanobacteria. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2014; pp. 471–501. [Google Scholar]
- Calderon, R.H. More than just a pair of blue genes: How cyanobacteria adapt to changes in their light environment. Physiol. Plant. 2020, 170, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hernandez-Prieto, M.A.; Loughlin, P.C.; Li, Y.; Willows, R.D. Genome and proteome of the chlorophyll f-producing cyanobacterium Halomicronema hongdechloris: Adaptative proteomic shifts under different light conditions. BMC Genom. 2019, 20, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Zhou, Y.; Zhang, Y.; Su, Y.H.; Xu, T. Protein phosphorylation: A molecular switch in plant signaling. Cell Rep. 2023, 42, 112729. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.-D.; Ogé, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.J.; James, D.E.; Mann, M. Protein phosphorylation: A major switch mechanism for metabolic regulation. Trends Endocrinol. Metab. 2015, 26, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Berla, B.M.; Saha, R.; Immethun, C.M.; Maranas, C.D.; Moon, T.S.; Pakrasi, H.B. Synthetic biology of cyanobacteria: Unique challenges and opportunities. Front. Microbiol. 2013, 4, 59403. [Google Scholar] [CrossRef] [PubMed]
- Chubukov, V.; Gerosa, L.; Kochanowski, K.; Sauer, U. Coordination of microbial metabolism. Nat. Rev. Microbiol. 2014, 12, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.L.; Matiolli, C.C.; Soares, R.C.; Almadanim, M.C.; Oliveira, M.M.; Abreu, I.A. Carbon/nitrogen metabolism and stress response networks–calcium-dependent protein kinases as the missing link? J. Exp. Bot. 2021, 72, 4190–4201. [Google Scholar] [CrossRef]
- Lasonder, E.; Green, J.L.; Camarda, G.; Talabani, H.; Holder, A.A.; Langsley, G.; Alano, P. The Plasmodium falciparum schizont phosphoproteome reveals extensive phosphatidylinositol and cAMP-protein kinase A signaling. J. Proteome Res. 2012, 11, 5323–5337. [Google Scholar] [CrossRef]
- Vaga, S.; Bernardo-Faura, M.; Cokelaer, T.; Maiolica, A.; Barnes, C.A.; Gillet, L.C.; Hegemann, B.; van Drogen, F.; Sharifian, H.; Klipp, E.; et al. Phosphoproteomic analyses reveal novel cross-modulation mechanisms between two signaling pathways in yeast. Mol. Syst. Biol. 2014, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Mast, F.D.; Ratushny, A.V.; Aitchison, J.D. Systems cell biology. J. Cell Biol. 2014, 206, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Schmelling, N.M.; Scheurer, N.; Köbler, C.; Wilde, A.; Axmann, I.M. Diversity of timing systems in cyanobacteria and beyond. In Circadian Rhythms in Bacteria and Microbiomes; Springer: Berlin/Heidelberg, Germany, 2021; pp. 179–202. [Google Scholar]
- Grangeasse, C.; Nessler, S.; Mijakovic, I. Bacterial tyrosine kinases: Evolution, biological function and structural insights. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2640–2655. [Google Scholar] [CrossRef] [PubMed]
- Av-Gay, Y.; Jamil, S.; Drews, S.J. Expression and characterization of the Mycobacterium tuberculosis serine/threonine protein kinase PknB. Infect. Immun. 1999, 67, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.H.; Shenoy, A.R.; Dutta, A.; Visweswariah, S.S. The evolution of guanylyl cyclases as multidomain proteins: Conserved features of kinase-cyclase domain fusions. J. Mol. Evol. 2009, 68, 587–602. [Google Scholar] [CrossRef]
- Xie, W.Q.; Whitton, B.A.; Simon, J.W.; Jäger, K.; Reed, D.; Potts, M. Nostoc commune UTEX 584 gene expressing indole phosphate hydrolase activity in Escherichia coli. J. Bacteriol. 1989, 171, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Lundby, A.; Secher, A.; Lage, K.; Nordsborg, N.B.; Dmytriyev, A.; Lundby, C.; Olsen, J.V. Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat. Commun. 2012, 3, 876. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Kaur, P.; Nandicoori, V.K. Targeting the messengers: Serine/threonine protein kinases as potential targets for antimycobacterial drug development. IUBMB Life 2018, 70, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Veaudor, T.; Blanc-Garin, V.; Chenebault, C.; Diaz-Santos, E.; Sassi, J.-F.; Cassier-Chauvat, C.; Chauvat, F. Recent advances in the photoautotrophic metabolism of cyanobacteria: Biotechnological implications. Life 2020, 10, 71. [Google Scholar] [CrossRef]
- Qin, S.; Kitty, I.; Hao, Y.; Zhao, F.; Kim, W. Maintaining genome integrity: Protein kinases and phosphatases orchestrate the balancing act of DNA double-strand breaks repair in cancer. Int. J. Mol. Sci. 2023, 24, 10212. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Aryal, U.K.; Stöckel, J.; Krovvidi, R.K.; Gritsenko, M.A.; Monroe, M.E.; Moore, R.J.; Koppenaal, D.W.; Smith, R.D.; Pakrasi, H.B.; Jacobs, J.M. Dynamic proteomic profiling of a unicellular cyanobacterium Cyanothece ATCC51142 across light-dark diurnal cycles. BMC Syst. Biol. 2011, 5, 194. [Google Scholar] [CrossRef] [PubMed]
- Moxon, R.; Bayliss, C.; Hood, D. Bacterial contingency loci: The role of simple sequence DNA repeats in bacterial adaptation. Annu. Rev. Genet. 2006, 40, 307–333. [Google Scholar] [CrossRef] [PubMed]
- Puthiyaveetil, S.; Tsabari, O.; Lowry, T.; Lenhert, S.; Lewis, R.R.; Reich, Z.; Kirchhoff, H. Compartmentalization of the protein repair machinery in photosynthetic membranes. Proc. Natl. Acad. Sci. USA 2014, 111, 15839–15844. [Google Scholar] [CrossRef]
- Longoni, F.P.; Goldschmidt-Clermont, M. Thylakoid protein phosphorylation in chloroplasts. Plant Cell Physiol. 2021, 62, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.-M.; Ohad, I. Redox regulation of thylakoid protein phosphorylation. Antioxid. Redox Signal. 2003, 5, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Gibon, Y.; Usadel, B.; Blaesing, O.E.; Kamlage, B.; Hoehne, M.; Trethewey, R.; Stitt, M. Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol. 2006, 7, R76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Jang, J.; Sakr, S.; Wang, L. Protein phosphorylation on Ser, Thr and Tyr residues in cyanobacteria. J. Mol. Microbiol. Biotechnol. 2005, 9, 154–166. [Google Scholar] [CrossRef]
- Whitford, D.S. Function of the Synechocystis RNA Helicase, CrhR, and Its Cyanobacterial Homologs. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2020. [Google Scholar]
- Wittkopp, T.M.; Saroussi, S.; Yang, W.; Grossman, A.R.; Kirchhoff, H. The GreenCut: Functions and relationships of proteins conserved in green lineage organisms. In Chloroplasts: Current Research and Future Trends; Institute of Biological Chemistry, Washington State University: Pullman, WA, USA, 2016; pp. 241–278. [Google Scholar]
- Ferrari, R.C.; Freschi, L. C4/CAM facultative photosynthesis as a means to improve plant sustainable productivity under abiotic-stressed conditions: Regulatory mechanisms and biotechnological implications. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–532. [Google Scholar]
- Satta, A.; Esquirol, L.; Ebert, B.E. Current metabolic engineering strategies for photosynthetic bioproduction in cyanobacteria. Microorganisms 2023, 11, 455. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, T.; Fahad, S.; Zhou, R. Protein Phosphorylation Nexus of Cyanobacterial Adaptation and Metabolism. Kinases Phosphatases 2024, 2, 209-223. https://doi.org/10.3390/kinasesphosphatases2020013
Nawaz T, Fahad S, Zhou R. Protein Phosphorylation Nexus of Cyanobacterial Adaptation and Metabolism. Kinases and Phosphatases. 2024; 2(2):209-223. https://doi.org/10.3390/kinasesphosphatases2020013
Chicago/Turabian StyleNawaz, Taufiq, Shah Fahad, and Ruanbao Zhou. 2024. "Protein Phosphorylation Nexus of Cyanobacterial Adaptation and Metabolism" Kinases and Phosphatases 2, no. 2: 209-223. https://doi.org/10.3390/kinasesphosphatases2020013
APA StyleNawaz, T., Fahad, S., & Zhou, R. (2024). Protein Phosphorylation Nexus of Cyanobacterial Adaptation and Metabolism. Kinases and Phosphatases, 2(2), 209-223. https://doi.org/10.3390/kinasesphosphatases2020013





