1. Introduction
Alkaline phosphatase is an important enzyme that plays a crucial role in various biological processes. It is commonly used as a model enzyme in separation studies and has several advantages for enzyme detection. Alkaline phosphatase is found in many living organisms, including bacteria, plants, and animals, making its molecular weight (MW) differ. However, human ALP has an MW of 86,000 Da. Its widespread occurrence makes it a convenient enzyme for research and detection purposes. In the medical field, alkaline phosphatase is used as a biomarker for various health conditions, especially those related to the liver and bones. Detecting its presence and activity can help to diagnose and monitor diseases like liver disorders, bone diseases, and certain cancers. Alkaline phosphatase is relatively stable under certain conditions, allowing researchers to study its behavior without significant degradation or loss of activity. This enzyme has been extensively studied and characterized, making it easier for researchers to compare their experimental results with existing knowledge and data. Detection methods for alkaline phosphatase can be highly sensitive, allowing for the detection of small amounts of the enzyme even in complex biological samples. Alkaline phosphatase detection methods can be designed to be highly specific, ensuring that the measured activity or presence of the enzyme is not influenced by other interfering substances. Alkaline phosphatase exhibits specific enzymatic properties, such as substrate specificity and reaction kinetics, which can be studied during separation experiments to gain insights into enzyme behavior [
1,
2,
3]. Overall, using alkaline phosphatase as a model enzyme in separation studies helps researchers to develop and optimize detection methods that can be applicable to other enzymes as well. The insights gained from these studies can have broader implications in various scientific fields, including biotechnology, medicine, and biochemistry.
In addition, alkaline phosphatase is responsible for the dephosphorylating process in cells. ALP comprises a group of enzymes that facilitate the breakdown of numerous organic phosphate esters under alkaline pH conditions, with zinc serving as a crucial cofactor. It therefore plays a significant role in various human pathological and normal conditions. The serum alkaline phosphatase activity primarily emanates from three primary sources: the liver (accounting for more than 80 percent), bone tissue, and, in some cases, the intestinal tract. This turns it into four main isoenzymes: germ cell alkaline phosphatase (GCAP); intestinal alkaline phosphatase (IAP); placental alkaline phosphatase (PLAP); and tissue-nonspecific alkaline phosphatase (TNAP), whereby all of which have their own physiological role. For example, TNAP is expressed mostly in the liver, the kidney, and bones. The functional role of bone isoenzyme is seen in bone mineralization, while the function of liver and kidney isoenzymes remains unknown [
4,
5]. The functional roles of the other isoenzymes—GCAP, IAP, and PLAP—are still not known [
6].
When elevated levels of alkaline phosphatase are detected, it can be fractionated to ascertain whether it originates from the liver or bones [
3]. However, in clinical practice, a liver source is typically confirmed by simultaneously observing elevated levels of other cholestasis markers, such as gamma-glutamyl transpeptidase. Additional factors can also contribute to serum alkaline phosphatase levels. For instance, women in their third trimester of pregnancy often exhibit heightened serum alkaline phosphatase levels due to the introduction of placental alkaline phosphatase into the bloodstream. Individuals with blood types O and B may experience increased serum alkaline phosphatase levels after consuming a fatty meal, owing to the influx of intestinal alkaline phosphatase. Moreover, infants and toddlers may occasionally display temporary, substantial increases in alkaline phosphatase levels without any detectable bone or liver disorders. Elevated alkaline phosphatase levels have also been observed in patients diagnosed with diabetes mellitus. Additionally, there have been reports of benign familial occurrences of elevated serum alkaline phosphatase attributed to intestinal alkaline phosphatase. So far, the primary clinical utility of serum alkaline phosphatase lies in its role in diagnosing liver disorders, particularly in identifying cholestatic diseases characterized by impaired bile flow. It is believed that a deep understanding of isoenzyme analysis of ALP will aid the understanding of the physiological cellular process and cancer. Therefore, it is important to facilitate isoenzyme analysis of ALP. It is well known that separation techniques are widely used for enzyme and protein detection, especially in pharmaceutical research and the study of post-translational modifications (PTMs). These methods enable the identification, quantification, and characterization of enzymes and proteins, providing valuable insights into their structure and function. This is often achieved through techniques like electrophoresis, chromatography, or gel filtration, which allow researchers to separate different molecules based on their size, charge, or other properties.
With a focus on capillary electrophoresis, the review paper aims to explore the recent advancements in this analytical technique, examining how it can improve the sensitivity, selectivity, and efficiency of alkaline phosphatase analysis. Capillary electrophoresis offers valuable insights into enzyme behavior, including substrate specificity and reaction kinetics, providing a deeper understanding of alkaline phosphatase activity. By harnessing the potential of capillary electrophoresis in enzyme analysis, researchers can develop and optimize detection methods not only for alkaline phosphatase but also for other enzymes, with broader implications in biotechnology, medicine, and biochemistry. This review paper seeks to shed light on the significance of capillary electrophoresis in analyzing alkaline phosphatase, explore its potential in advancing enzyme analysis, and demonstrate how this research can lead to innovations in the detection and understanding of enzymes in various scientific fields, including PTMs.
2. Background
Electrophoresis, as a basic concept, dates back to approximately 1931, when it was first defined as a separation method for solutes based on their different rates of migration when subjected to an electric field in the presence of an electrolyte (running buffer). However, it was not until 1937 that the technique was developed and utilized by Arne Tiselius, who used filter papers and gels as supporting media [
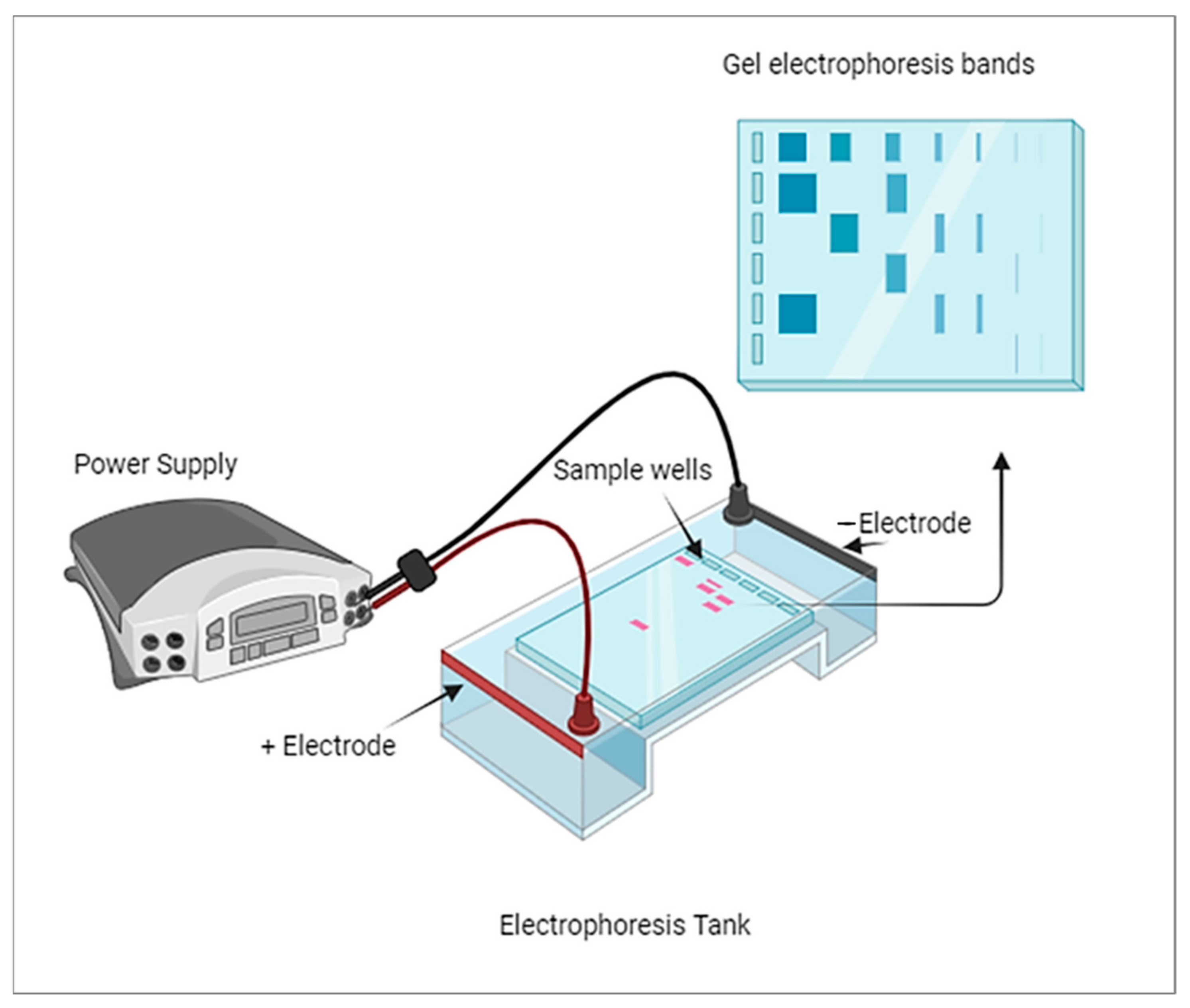
7]. His work laid the foundation for the establishment of gel electrophoresis methodologies. The technique of electrophoresis remained primarily focused on separating chemical and non-biological substances until advancements were made to extend its application to biological molecules. It was around 1960 when electrophoresis started to gain popularity as a known technique for separating biological molecules based on their physical and chemical differences. The development and refinement of electrophoresis techniques have since become instrumental in various fields of science, particularly in molecular biology and biochemistry. Gel electrophoresis, in particular, has played a critical role in analyzing DNA, RNA, and proteins, leading to significant advancements in genetics, proteomics, and other related disciplines. A thick band in gel electrophoresis typically indicates a higher concentration of the molecule being analyzed, whether it is a protein, DNA, or RNA.
Figure 1 shows the typical diagram of gel electrophoresis. The thickness of the band is primarily related to the amount of the molecule present rather than its size. Larger molecules may migrate more slowly through the gel, which can result in broader bands, but the concentration is the primary factor determining band thickness.
Alkaline phosphatase (ALP) is one of the biological molecules that has been separated and studied using various electrophoresis methods. The provided timeline highlights the progress and improvements made in the field of electrophoresis to efficiently separate and identify different isoenzymes of ALP. In 1965, Beckman, Nilson, and Baker utilized starch gel electrophoresis to study ALP [
8]. They observed different bands on the gel, which indicated the presence of three different isoenzymes in the samples that they analyzed. In the following years, researchers continued to investigate ALP isoenzymes using different electrophoresis techniques. In 1967, Yong focused on identifying the isoenzymes of ALP by testing various inhibitors. For this purpose, agar gel electrophoresis was employed. Then, in 1971, Savage et al. used polyacrylamide gel electrophoresis to further study the migration rate of ALP [
9]. This advanced technique allowed them to identify a total of five different isoenzymes of ALP, indicating a greater level of resolution compared to previous methods. Throughout these experiments, researchers applied different types of gels and improved electrophoresis methods to enhance the separation of ALP isoenzymes efficiently. The ability to identify and differentiate various isoenzymes of ALP has been crucial in understanding their roles in different physiological and pathological conditions. Electrophoresis has played a significant role in the field of biochemistry, enabling researchers to study and characterize various biomolecules, including enzymes like alkaline phosphatase.
The traditional electrophoresis principle, although simple, is where separating molecules based on charge and size migrate through a gel matrix under an electric field; smaller, negatively charged molecules move faster, and larger or less negatively charged molecules move slower. However, the principle has limitations. Traditional gel-based electrophoresis has lower resolution and longer separation times compared to capillary electrophoresis. It may require larger sample volumes and could have higher detection limits for some enzymes due to band broadening and dilution effects. Additionally, traditional electrophoresis setups may involve more manual handling and are less suitable for high-throughput analysis. While commonly used for the basic separation and visualization of enzymes and biomolecules in research, it may not be ideal for high-throughput or clinical applications. For the separation and analysis of ALP isoenzymes, conventional electrophoresis techniques such as affinity electrophoresis with wheatgerm lectin [
10,
11] and isoelectric focusing in immobilized pH gradients are commonly employed [
12,
13,
14].
3. Capillary Electrophoresis (CE)
The history of CE is relatively short compared to other separation techniques, but it has seen rapid advancements and improvements. In 1983, Lukacs and his professor, Professor Jorgenson, published a seminal paper that demonstrated the potential of using capillaries to overcome the limitations of traditional gel electrophoresis methods. They built upon the earlier work of Hjerten and Catsimpoolas, who had developed capillary zone electrophoresis, and identified ways to reduce interferences between bands, thus enhancing the resolution and efficiency of separations [
15,
16,
17,
18]. This breakthrough opened the door for further research and development in the field of capillary electrophoresis. Over the years, CE has become a complementary method to other modern separation instruments and has found applications in various scientific fields, including biochemistry, pharmaceuticals, environmental analysis, and more.
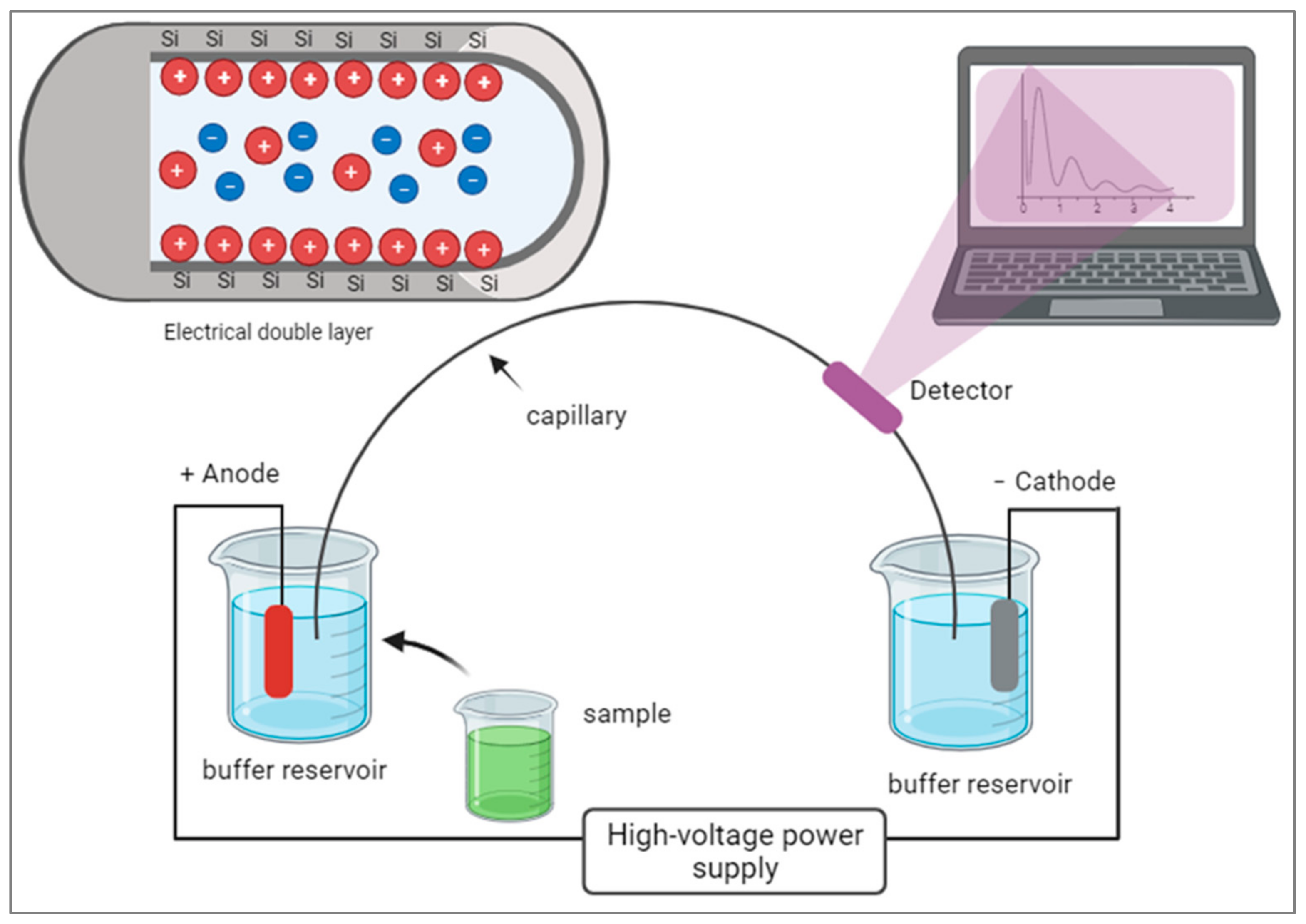
Capillary electrophoresis (CE) is an advanced form of electrophoresis that uses narrow capillaries filled with a conductive buffer to separate molecules under an electric field. CE offers higher separation efficiency and speed due to the small capillary size and the ability to generate higher electric fields. It requires smaller sample volumes, leading to enhanced sensitivity and lower detection limits. CE systems can be automated and integrated with other techniques, like mass spectrometry [
19], for comprehensive enzyme analysis. CE has broad applications in high-throughput analysis, protein characterization, and monitoring enzyme activity in various fields, including pharmaceutical research, clinical diagnostics, and biotechnology. CE can utilize different modes of separation, such as capillary zone electrophoresis (CZE), capillary isoelectric focusing (CIEF), or capillary gel electrophoresis (CGE), depending on the specific application. In CE, the movement of charged molecules through a narrow capillary tube occurs under the influence of an electric field. The electrical double layer forms at the capillary wall, consisting of an inner layer of counterions and an outer layer of co-ions. As analytes migrate through the capillary, they experience electroosmotic flow driven by this double layer, allowing for the separation of molecules based on their charge and size.
Figure 2 shows a diagram illustrating the components of CE.
However, it is acknowledged that CE may be less efficient than some modern separation methods in terms of limit of detection and reproducibility. Researchers continue to investigate and refine capillary electrophoresis to improve its analytical separation capabilities. Efforts are being made to enhance sensitivity, increase throughput, and optimize the reproducibility of results. Additionally, advancements in microfluidics and miniaturization have led to the development of microdevices for CE, which further expand its applications and potential for future research [
20,
21,
22]. Overall, capillary electrophoresis has come a long way since its inception, and ongoing research aims to make it an even more powerful and efficient technique for analytical separations in various scientific disciplines.
The capillary is usually made with glass. The glass is limited by high temperature and relatively low flexibility and robustness, but all of which are improved by using fused silica. The silica’s outer surface is sometimes coated with a polymer. The thinner fused silica capillary allows for small volumes of samples. This enhances the ratio of surface-to-volume. The small size of the capillary tube also reduces the heat and voltage, which reduces the denaturing of the protein during separation [
23].
Alkaline phosphatase is an enzyme that catalyzes the hydrolysis of phosphate esters with an alkaline pH. Fused silica is negatively charged [
24] and the alkaline buffer in the two reservoirs has a negative charge. This means that, combined, they are electrostatic repulsive forces [
25]. In capillary electrophoresis, the electrostatic repulsive forces between the buffer and the capillary wall help to prevent the adsorption of proteins onto the capillary wall, which could lead to loss of the analyte and distorted results. This is particularly important for proteins like alkaline phosphatase, as adsorption could lead to a decrease in the detected enzyme concentration and affect the accuracy of the analysis.
Its detection in capillary electrophoresis can be achieved using various methods, including indirect detection and on-column detection [
26]. In indirect detection, a substrate is used to generate a detectable product when it reacts with the enzyme (alkaline phosphatase). The product formed from the enzymatic reaction is then detected in the capillary. One common approach is to use a fluorescent or UV-absorbing substrate that, upon hydrolysis by alkaline phosphatase, produces a fluorescent or UV-absorbing product. The formation of the product is proportional to the enzyme concentration, allowing for quantification. In on-column detection, the analyte itself is directly detected as it migrates through the capillary. For proteins like alkaline phosphatase, the detection is based on their intrinsic properties, such as absorbance, fluorescence, or refractive index. With regard to capillary electrophoresis separation, the prepared protein sample is injected into a narrow capillary filled with an electrolyte solution. An electric field applied across the capillary charges protein molecules to ensure that they can migrate through the capillary; then, based on their charge-to-mass ratio, they separate proteins. Smaller and more highly charged proteins will move faster than larger or less charged ones, leading to the separation of the protein mixture. As the protein molecules pass through the detection point in the capillary, they are exposed to a UV or visible light source, for instance. Proteins, like many other biomolecules, absorb light in the UV-Vis range due to the presence of aromatic amino acids (such as tryptophan, tyrosine, and phenylalanine) and chromophores. The extent of light absorption depends on the concentration of proteins in the capillary. The detected absorbance signals are recorded and analyzed to generate an electropherogram, which is a graph showing the peaks corresponding to different proteins in the sample. The height of each peak represents the relative concentration of the corresponding protein in the mixture. Through the use of appropriate detection methods, the protein’s migration through the capillary can be monitored and quantified without the need for derivatization or reactions with substrates.
4. Indirect Detection of Alkaline Phosphatase
In the case of a UV-Vis detector, p-nitrophenol (pNP) is the product of the enzyme reaction of alkaline phosphatase and the substrate p-nitrophenol phosphate (pNPP). pNP has chromophores that make it sensitive for spectrometric detection and it has two functional groups: one is the OH and the other is NO
2. These occupy opposite ends of the benzene ring. The absorption spectra occur in 405 nm for this compound. By following the steps in
Figure 3, capillary electrophoresis with a UV-Vis detector can be used to detect and quantify ALP. Indirect detection is typically performed using discontinuous assays with pre- or post-reaction sampling, as it involves stopping the enzymatic reaction at specific time points to quench the reaction and prevent further product formation. The reaction mixture is then analyzed at each time point to measure the accumulated product or remaining substrate concentration. So, here, the enzyme reaction is initiated by mixing the enzyme with its substrate and other necessary components. After a specific incubation period, the reaction is stopped (e.g., by changing the pH or adding a quenching reagent). A small volume of the reaction mixture is sampled and analyzed via capillary electrophoresis using indirect detection methods. The concentrations of the product(s) or remaining substrate are determined, and the enzyme activity or concentration is calculated based on these measurements. Discontinuous assays provide information about the extent of the enzymatic reaction at specific time points, allowing for the determination of initial reaction rates and steady-state kinetics. Xu et al. (1998) evaluated the enzymatic reaction of ALP using the substrate pNPP (p-nitrophenyl phosphate) and a UV-Vis absorbance detector [
27]. They determined the Michaelis constant (Km) for pNPP as 4.8 mM and the limit of detection (LOD) for commercial ALP as 4.4 × 10 − 5 IU. The conditions inside the capillary play a crucial role in enzyme detection, and optimizing factors such as buffer concentration, temperature, pH, and components is essential. Iqbal (2011) characterized ALP activities using a running buffer of sodium phosphate at pH 8.5 and detected the substrate pNPP at 322 nm [
28]. Another assay performed by Aptisa et al. modified the capillary with polycationic polybrene to control protein adsorption, which required further optimization of pH [
29].
Another approach was taken by Sun et al. (2006), who used the technique to measure ion-transfer currents, building on the work completed by Bard et al. (1995), which investigated currents generated by redox reactions [
30,
31]. The ALP redox reaction transfers electrons from an organic phosphate substrate to the enzyme, reducing the substrate while oxidizing the enzyme. This removes a phosphate group from the substrate, producing an inorganic phosphate ion. Studies that rely on contactless conductivity, though promising, are not yet considered conclusive, as the process involving the integration of the two systems is still under development [
32,
33]. Conductivity detector integration is particularly suitable for molecules that lack redox groups or display weak redox reactions, but it has received limited attention in the literature in terms of the effects of pH [
34] and conductivity [
35]. Further sophistication and modification strategies are required to improve the interfaces of electrolyte and electrode surfaces. Overall, electrochemical detectors, such as conductivity detectors, hold great potential for cost-effective and sensitive analysis in capillary electrophoresis. However, ongoing research and refinement are necessary to optimize the integration of these systems and improve their performance for a wide range of applications.
5. On-Column Detection of Alkaline Phosphatase
In this detection method, the substance of interest is detected directly as it moves through the capillary and during its migration it can be real-time-monitored during the enzymatic reaction; therefore, it is called a continuous assay.
Figure 4 shows that continuous assays provide kinetic information about the enzymatic reaction and are useful for studying reaction rates, enzyme inhibition, and enzyme kinetics. It involves continuously monitoring the enzymatic reaction as it progresses over time. This approach allows for real-time observation of the reaction kinetics. The enzyme reaction is initiated by mixing the enzyme with its substrate and other necessary components. The reaction mixture is injected into the capillary, and electrophoresis is started. As the reaction progresses, the products of the enzymatic reaction, or any other species involved in the reaction, move through the capillary with different electrophoretic mobilities. These products are detected as they elute from the capillary, and their concentrations are monitored over time, usually through absorbance or fluorescence detection. The rate of product formation or substrate consumption is then used to determine the enzyme’s activity or concentration.
Grodner et al. (2017) evaluated the inhibitory effect of a propylamine group on ALP activities using a running buffer of sodium dihydrogen phosphate with a pH of 2.5 and detected the substrate pNPP at 200 nm [
36]. Their optimization resulted in a Michaelis constant (Km) of 1.5 mM, which showed an improvement compared with Xu et al.’s findings [
27]. However, using a very low pH in their study might not be ideal for ALP separation, as higher pH conditions are commonly considered to be better for ALP analysis. Gattu et al. (2018) introduced a novel approach by identifying a sequence enzyme assay of ALP using capillary electrophoresis coupled to mass spectrometry [
26]. They immobilized ALP in the capillary, enabling the efficient separation of peptides. While their optimization allowed for a Michaelis constant (Km) of 1.100 mM, their findings were limited to peptide determination. Recent studies have further explored various parameters, including migration time, which previous research has not extensively addressed. Takayanagi et al. (2020) discussed a relationship between migration time and the rate of reaction of ALP activities [
37]. They also examined the inhibition analysis of ALP, which was determined by the low signal.
Using a laser-induced fluorescence detector for ALP detection is another powerful approach in capillary electrophoresis. This method is particularly popular in the literature, especially when researchers focus on affinity binding for ALP analysis [
38] and ALP concentration [
39]. In laser-induced fluorescence detection, ALP enzyme reactions using fluorogenic substrates are monitored based on their fluorescence signals. Various aspects need to be considered and troubleshooted when using this technique. Some of the challenges include the adhesion of ALP in the capillary, the activation of ALP, inhibition using different metal chelators, and the selection of suitable labels (e.g., glycosphingolipids) in different buffers [
40,
41,
42,
43,
44]. The integration of laser-induced fluorescence detection with capillary electrophoresis allows for the highly sensitive analysis of enzymes and proteins. However, a major limitation of this method is that most fluorogenic dyes are limited in their compatibility with specific functional groups within compounds. For instance, Fluorescein isothiocyanate may be less reactive at low concentrations of amine groups, whereas rhodamine dyes might be more efficient with succinimidyl ester groups. This selectivity of fluorogenic dyes can limit their universal application. Moreover, the lack of standardization for ALP assays with fluorescence detection can pose challenges. To overcome these limitations, researchers have turned to nanomaterials, such as nanoparticles, to enhance the performance and sensitivity of the technique. Quantum dots [
45] and magnetic nanoparticles [
46] have been reported in ALP enzyme assays with capillary electrophoresis. These nanoparticles possess unique physical properties that can enhance the immunoaffinity within the capillary and improve the accuracy and efficiency of the analysis. By leveraging nanomaterials and addressing the limitations of fluorescence detection, researchers can further enhance the capabilities of capillary electrophoresis in enzymatic and protein analysis, including the detection and characterization of ALP. These advancements contribute to the continuous improvement and expansion of capillary electrophoresis as a powerful tool in various scientific fields.
6. Alkaline Phosphatase Detection from Whole Cells
It is important to note that the successful detection of ALP from whole cells using capillary electrophoresis depends on optimizing the sample preparation, separation conditions, and detection methods. Moreover, the sensitivity of the technique should be considered, as the concentration of ALP in whole cells may be relatively low, requiring careful optimization to achieve reliable results. Additionally, the use of complementary techniques like mass spectrometry [
47] can provide more comprehensive information about PTMs in complex biological samples. Studying PTMs of ALP using capillary electrophoresis can provide mechanistic insights into how specific modifications regulate the enzyme’s activity, substrate specificity, subcellular localization, and protein–protein interactions.
Balbaied et al. showcased the utilization of capillary electrophoresis to assess the release of ALP from viable cells, contrasting it with an absorbance-based assay [
48]. The outcomes displayed a wide-ranging ALP measurement span, spanning from 1.5 to 1500 U/L, with a detection threshold as low as 0.043 U/L. This accomplishment was achieved through the application of a 70 μL sample and a 10 min incubation period, utilizing an optimal substrate concentration of 9.6 mM p-aminophenol phosphate. A noteworthy statistical distinction (
p < 0.05) was identified when compared to the absorbance-based assays. Despite being a discontinuous method, this approach underscored the benefits of identifying ALP release from cells, aligning with contemporary trends in gene expression systems that employ microelectrode array technologies [
49] and tools designed for electrophysiological activity monitoring.
The focus on electrochemical detectors, such as conductivity detectors, in conjunction with capillary electrophoresis has gained attention due to their potential for cost-effective analysis [
50,
51]. Furthermore, these systems can be miniaturized to develop microdevices, making them even more attractive for various applications. In the investigation of ALP, disodium phenol phosphate is used as a substrate, which converts to phenol during the enzyme reaction [
52,
53]. Sun et al. (2004) demonstrated that using disodium phenyl phosphate as a substrate could achieve a lower limit of detection (LOD) of 3.5 × 10 − 21 mol/L [
54]. Their aim was to study ALP from cell lysis, and they successfully separated ALP isoenzymes in mouse bone marrow cell lines using bicarbonate buffer for the lysis solution. However, there has been some disagreement regarding the use of disodium phenyl phosphate, as it leads to the indirect detection of the quantity of phenol formed. To address this, amperometry has been integrated into the system to quantify the amount of phenol formed in each zone.
Capillary electrophoresis enables the identification of protein isoforms with distinct post-translational modifications (PTMs) due to their slightly different migration times, allowing for differentiation. Moreover, the intensity of electropherogram peaks in the capillary electrophoresis reflects the protein’s concentration in the sample, enabling researchers to quantify the abundance of specific PTMs and understand the extent of modification. Coupling capillary electrophoresis with mass spectrometry enables comprehensive PTM profiling, facilitating the identification and characterization of specific PTMs on proteins and shedding light on their regulatory roles in various cellular processes. By correlating PTM patterns with specific cellular conditions or disease states, scientists can gain insights into the functional implications of PTMs on protein activity and cellular signaling pathways.
Figure 5 shows detecting post-translational modifications (PTMs) in capillary electrophoresis from whole cells, which involves several steps. After releasing cells’ contents in the appropriate buffer, proteins are extracted from the lysate using appropriate solvents. Proteins are then likely to denature and reduce to break down their tertiary and quaternary parts. For targeting PTM-specific enrichment, proteins are modified using different methods, including immunoprecipitation [
55] or affinity chromatography [
56]. For ALP, PTM-specific detection phosphorylation [
55,
56,
57,
58,
59] or glycosylation [
60,
61,
62] can be used. After injecting the protein mixture, data can be analyzed and compared to the untreated cell and other controls in the test.
7. Research Challenges
Capillary electrophoresis (CE) is a significant development in the field of electrophoresis and has evolved considerably since its inception. The integration of detectors with capillary electrophoresis is a preferred approach for identifying ALP isoenzyme assays. UV-Vis detection has become increasingly important due to its simplicity and linear performance. In this context, the substrate pNPP is commonly used as it is self-indicating and facilitates accurate measurements. Optimizing enzyme reactions in capillary electrophoresis methodology involves several key variables, including the running buffer and its pH, ionic strength [
63], temperature of the capillary, and the relationship between incubation time and the quantity of the product. Previous studies have explored using two different buffer pHs (e.g., 7.4 and 2.5) for discontinuous enzyme assays, which is important for the real-time monitoring of enzyme release in cell culture. However, for ALP determination, using two pH gradients of 7.4 and 9.5 has not been addressed in the literature.
Additionally, dynamic assays and their relationship with time have been investigated to optimize continuous enzyme assays for ALP analysis, aiding in the improvement of ALP assay conditions. Nevertheless, each assay may have its own calibration issues related to the device used, the running buffer, the applied voltage, the components, and the type of assay (continuous or discontinuous). Research on ALP release from living cells using UV-Vis detection is relatively rare, along with using cell lysis dissolved in bicarbonate buffer inside the capillary. The use of buffer with a pKa of 6.1 might limit the assay as it significantly differs from the optimal pH of ALP. Capillary electrophoresis involves multiple steps in experiments to allow validation assays for the isoenzyme release from living cells. Optimizing the components of the cell lysis mixture or medium is crucial for the accurate identification of isoenzymes and selective inhibition assays. Additionally, CE samples may need to be diluted to approximately 100× due to their sensitivity.
With the above suggestions, adapting ALP assays in capillary electrophoresis from whole cell lysis can be advantageous for PTM understanding, as well as for single-cell analysis [
64,
65]. Electrophoresis distinguishes isoforms, aiding quantification, while coupling with mass spectrometry reveals detailed PTM profiles and regulatory roles. Correlating patterns with conditions offers insights into protein activity. These intracellular molecular activities enable the detection of phosphorylation-dependent signaling events at the single-cell level. Additionally, this enzymatic activity can reduce potential biases introduced by phosphorylation variations among individual cells, leading to more reliable single-cell data [
66].
8. Conclusions
This review paper has illuminated the importance of capillary electrophoresis in examining alkaline phosphatase, delving into its potential for advancing enzyme analysis, and showcasing how this research could drive innovations in enzyme detection and comprehension across diverse scientific domains, including PTMs. Recent studies have exemplified continuous efforts in thoroughly exploring the various parameters of enzyme assays within fused capillary electrophoresis. By refining factors like substrate concentration, buffer pH, and incubation time within the capillary, researchers are striving to elevate the efficiency and sensitivity of ALP analysis. The incorporation of mass spectrometry and the consideration of migration time contribute additional depth to characterizing enzymatic reactions through capillary electrophoresis methodologies. Capillary electrophoresis serves as a valuable tool, providing insights into enzyme behavior encompassing substrate specificity and reaction kinetics, thereby fostering a more profound grasp of alkaline phosphatase activity. By harnessing the potential of capillary electrophoresis in enzyme analysis, scientists can devise and optimize detection techniques not only for alkaline phosphatase but also for other enzymes, with broader implications spanning biotechnology, medicine, and biochemistry.