A Comparison of Biomass Production and Quality of Congo and Rhodes Grasses in Nigeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Grass Establishment
2.3. Biomass Yield Estimation and Morphology Evaluation
2.4. Analyses of Nutrient Concentration
2.5. Statistical Analysis
- Yijkm = observation for species i, harvest j, year k, replicate/subject m;
- μ = overall mean;
- Si = effect of species i;
- Hj = effect of harvest j;
- Yk = effect of year k (repeated);
- (S × H)ij, (S × Y)ik, (H × Y)jk, (S × H × Y)ijk = interaction terms;
- Subjectm = random effect for experimental unit (plot) across years (to model repeated measures);
- εijkm = residual error.
3. Results
4. Discussion
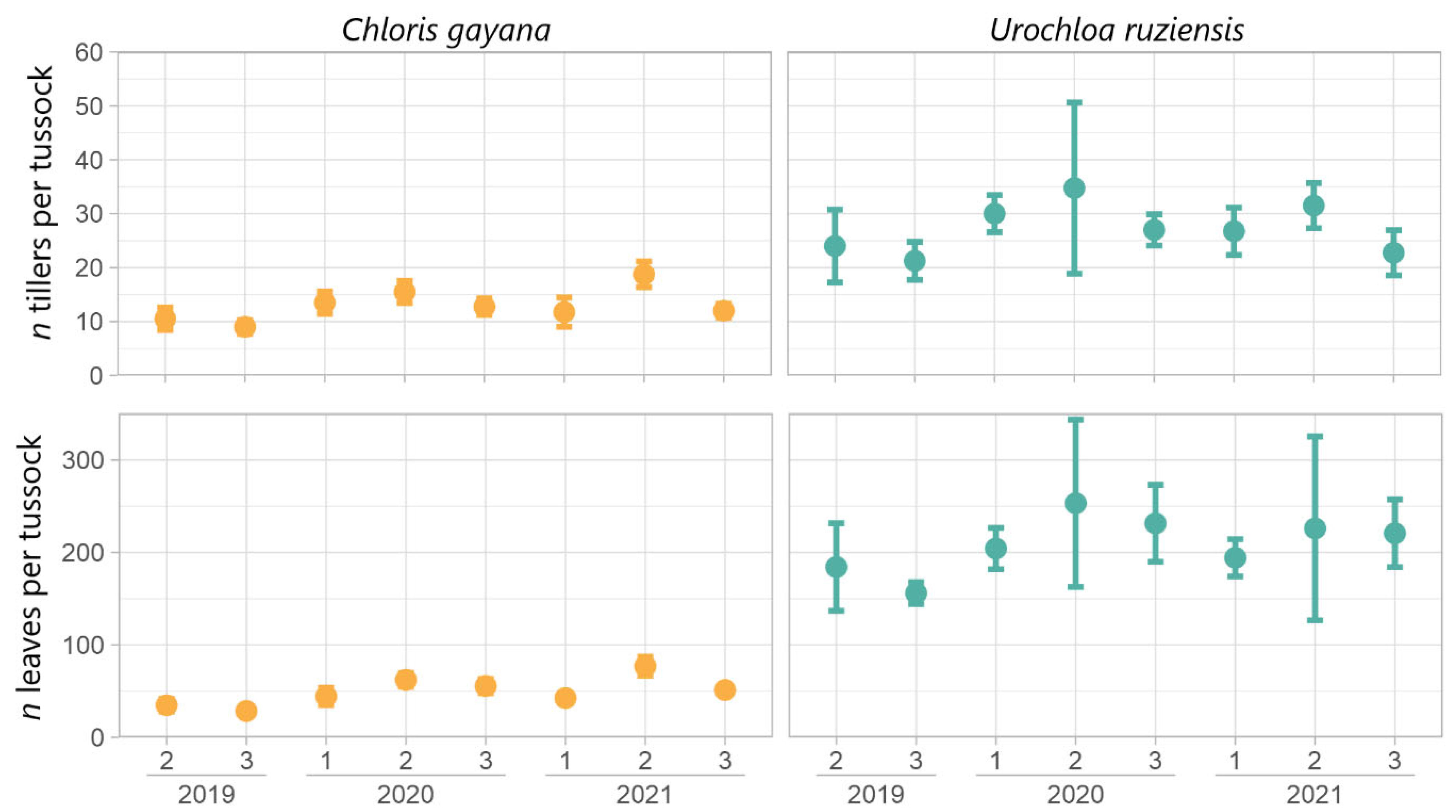
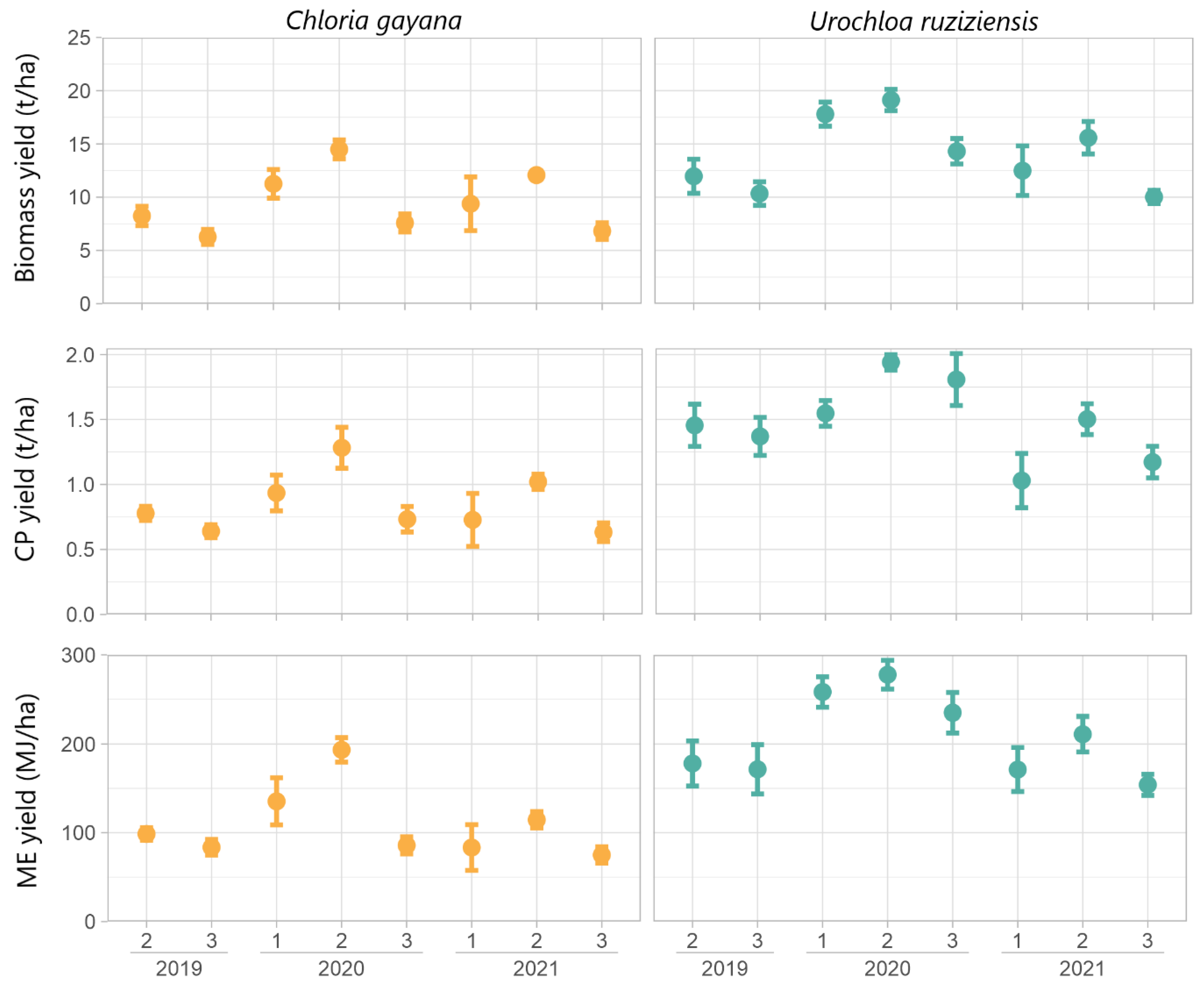
4.1. Morphological Characters and Yield
4.2. Nutrient Concentrations
4.3. Implications for Tropical Ruminant Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CG | Chloris gayana |
| UR | Urochloa ruziziensis |
| CP | crude protein |
| SSA | Sub-Saharan Africa |
| N | nitrogen |
| P | phosphorus |
| Ca | calcium |
| ME | metabolisable energy |
| NTPT | number of tillers per tussock |
| NLPT | number of leaves per tussock |
| NDF | neutral detergent fibre |
| ADF | acid detergent fibre |
| DM | dry matter |
| g | gram |
| kg | kilogramme |
References
- Robinson, T.; Pozzi, F. Mapping Supply and Demand for Animal-Source Foods to 2030; FAO Animal Production and Health Working Paper No. 2; FAO (Food and Agriculture Organization of the United Nations): Rome, Italy, 2011; Available online: https://www.fao.org/4/i2425e/i2425e00.htm (accessed on 12 January 2023).
- Cooke, A.S.; Machekano, H.; Gwiriri, L.C.; Tinsley, J.H.I.; Silva, G.M.; Nyamukondiwa, C.; Safalaoh, A.; Morgan, E.R.; Lee, M.R.F. The Nutritional Feed Gap: A Review of Seasonal Variation in Ruminant Forage Availability and Quality in Southern Africa. SSRN 2024. [Google Scholar] [CrossRef]
- Tolera, A.; Abebe, A. Livestock production in pastoral and agro–pastoral production systems of Southern Ethiopia. Livest. Res. Rural Develop. 2007, 19, 177. Available online: http://www.lrrd.org/lrrd19/12/tole19177.htm (accessed on 23 October 2024).
- Jimoh, S.O.; Muraina, T.O.; Bello, S.K.; Eldeen, N.N. Emerging issues in grassland ecology research: Perspectives for advancing grassland studies in Nigeria. Acta Oecol. 2020, 106, 103548. [Google Scholar] [CrossRef]
- Chapman, D.F.; Mackay, A.D.; Caradus, J.R.; Clark, D.A.; Goldson, S.A. Pasture productivity in New Zealand 1990–2020: Trends, expectations, and key factors. N. Z. J. Agric. Res. 2025, 68, 1221–1264. [Google Scholar] [CrossRef]
- Valle, C.B.; Jank, L.; Resende, R.M.S. Tropical forage breeding in Brazil. Revist. Ceres 2009, 56, 460–472. [Google Scholar] [CrossRef]
- Simeão, R.M.; Resende, M.D.V.; Alves, R.S.; Pessoa-Filho, M.; Azevedo, A.L.S.; Jones, C.S.; Pereira, J.F.; Machado, J.C. Genomic Selection in Tropical Forage Grasses: Current Status and Future Applications. Front. Plant Sci. 2021, 12, 665195. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Boval, M.; Maxin, G.; Lebas, F. Congo Grass (Brachiaria ruziziensis). Feedipedia, a Programme by INRAE, CIRAD and FAO. 2017. Available online: https://www.feedipedia.org/node/484 (accessed on 4 March 2024).
- Schultze-Kraft, R.; Teitzel, J.K. Brachiaria ruziziensis Germain & Evrard. In Record from Proseabase; Mannetje, L.’t, Jones, R.M., Eds.; PROSEA (Plant Resources of South-East Asia) Foundation: Bogor, Indonesia, 1992. [Google Scholar]
- ILRI. Ruzi Grass (Brachiaria ruziziensis) for Livestock Feed on Small-Scale Farms; International Livestock Research Institute: Nairobi, Kenya, 2013; ILRI Forage Facts Sheet; Available online: https://hdl.handle.net/10568/2339 (accessed on 25 August 2024).
- Cook, B.G.; Pengelly, B.C.; Brown, S.D.; Donnelly, J.L.; Eagles, D.A.; Franco, M.A.; Hanson, J.; Mullen, B.F.; Partridge, I.J.; Peters, M.; et al. Tropical Forages: An Interaction Selection Tool. Web Tool. CSIRO, DPI&F(Qld), CIAT, ILRI, Brisbane, QLD, Australia. 2005. Available online: https://hdl.handle.net/10568/49072 (accessed on 19 December 2023).
- Husson, O.; Charpentier, H.; Razanamparany, C.; Moussa, N.; Michellon, R.; Naudin, K.; Razafintsalama, H.; Rakotoarinivo, C.; Rakotondramanana; Séguy, L. Brachiaria sp., B. ruziziensis, B. brizantha, B. decumbens, B. humidicola. In Manuel pratique du semis direct à Madagascar; CIRAD: Paris, France, 2008; Volume III, Chapter 3, Part 4.1; Available online: https://agris.fao.org/search/en/providers/122653/records/6473697c53aa8c89630dafd7 (accessed on 9 October 2025).
- Santana, J.C.S.; Ítavo, L.C.V.; Ítavo, C.C.B.F.; Dias, A.M.; Niwa, M.V.G.; Moraes, G.J.; Arcanjo, A.H.M.; Gurgel, A.L.M.; Borges, A.D.; Formigoni, G.M.; et al. Productive characteristics, chemical composition, in vitro digestibility, and degradation kinetics of two Brachiaria grasses at different regrowth ages. Trop. Anim. Health Prod. 2022, 54, 342. [Google Scholar] [CrossRef]
- Ponsens, J.; Hanson, J.; Schellberg, J.; Moeseler, B.M. Characterisation of phenotypic diversity, yield and response to drought stress in a collection of Rhodes grass (Chloris gayana Kunth) accessions. Field Crops Res. 2010, 118, 57–72. [Google Scholar] [CrossRef]
- Imaz, J.A.; Giménez, D.O.; Grimoldi, A.A.; Striker, G.G. Ability to recover overrides the negative effects of flooding on growth of tropical grasses Chloris gayana and Panicum Coloratum. Crop Pasture Sci. 2015, 66, 100–106. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Boudon, A.; Lebas, F. Rhodes Grass (Chloris gayana). Feedipedia Programme by INRAE, CIRAD, AFZ and FAO. 2016. Available online: https://www.feedipedia.org/node/480 (accessed on 5 July 2024).
- Murphy, S. Tropical Perennial Grasses—Root Depths, Growth and Water Use Efficiency. NSW Industry and Investment, Primefacts N° 1027; 2008. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/343695/Tropical-perennial-grasses-root-depths-growth-and-water-use-efficiency.pdf (accessed on 17 April 2024).
- Jayasinghe, P.; Donaghy, D.J.; Barber, D.G.; Pembleton, K.G.; Ramilan, T. Suitability evaluation of three tropical pasture species (Mulato II, Gatton Panic, and Rhodes Grass) for Cultivation under a subtropical Climate of Australia. Agronomy 2022, 12, 2032. [Google Scholar] [CrossRef]
- Daba, A.W.; Qureshi, A.S.; Nisaren, B.K. Evaluation of Some Rhodes Grass (Chloris gayana) Genotypes for Their Salt Tolerance, Biomass Yield and Nutrient Composition. Appl. Sci. 2019, 9, 143. [Google Scholar] [CrossRef]
- Kawamura, K.; Watanabe, N.; Sakanoue, S.; Inoue, Y. Estimating forage biomass and quality in a mixed sown pasture based on partial least squares regression with waveband selection. Grassl. Sci. 2008, 54, 131–145. [Google Scholar] [CrossRef]
- Akpensuen, T.T. Defoliation frequencies of forage legumes: Effects on yield and nutritive value for beef cattle production. Sumerianz J. Agric. Vet. 2022, 5, 6–13. [Google Scholar] [CrossRef]
- FAO. The Future of Livestock in Nigeria. Opportunities and Challenges in the Face of Uncertainty. Rome. 2019. Available online: https://www.fao.org/documents/card/ru/c/ca5464en/ (accessed on 8 June 2023).
- UN. World Population Prospects. Key Findings and Advance Tables; United Nations: New York, NY, USA, 2017; Available online: https://population.un.org/wpp/assets/Files/WPP2017_KeyFindings.pdf (accessed on 7 March 2024).
- Olowolafe, E.A.; Dung, J.E. Soils derived from biotite-granites on the Jos Plateau, Nigeria: Their nutrient status and management for sustainable agriculture. Resour. Conserv. Recycl. 2000, 29, 231–244. [Google Scholar] [CrossRef]
- Karki, U. Forage definition and classification. In Sustainable Year-Round Forage Production and Grazing/BrowsingManagement for Goats in the Southern Region. Handbook for Training Field Extension and Technical Assistance Personnal; Karki, U., Ed.; Tuskegee University Extension Programme: Tuskegee, AL, USA, 2013; pp. 3–12. Available online: https://southern.sare.org/resources/sustainable-year-round-forage-production-and-grazing-browsing-management-in-the-southern-region/ (accessed on 2 September 2023).
- Nancy, J.T.; Harold, M.; Shirley, A.; Jan-Åke, P. Determination of Crude Protein in Animal Feed, Forage, Grain, and Oilseeds by Using Block Digestion with a Copper Catalyst and Steam Distillation into Boric Acid. J. AOAC Int. 2002, 85, 309–317. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robert, J.B.; Lewis, B.A. Method for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Nancy, T.; Lawrence, N.; Andy, C. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Intl. 2012, 95, 1392–1397. [Google Scholar] [CrossRef]
- Heckman, M. Minerals in Feeds by Atomic Absorption Spectrophotometry. J. AOAC 1967, 50, 45–50. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, F.; Wu, Q.; Wang, W.; Cui, Z.; Zhang, F.; Wang, Y.; Lv, L.; Liu, Y.; Bo, Y.; et al. Estimation of Energy Value and Digestibility and Prediction Equations for Sheep Fed with Diets Containing Leymus chinensis Hay. Agriculture 2023, 13, 1213. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 17 May 2024).
- Volenec, J.J.; Cherney, J.H.; Johnson, K.D. Yield components, plant morphology, and forage quality of alfalfa as influenced by plant population1. Crop Sci. 1987, 27, 321–326. [Google Scholar] [CrossRef]
- Alzueta, I.; Abeledo, L.G.; Mignone, C.M.; Miralles, D.J. Differences between Wheat and Barley in Leaf and Tillering Coordination under Contrasting Nitrogen and Sulfur Conditions. Eur. J. Agron. 2012, 41, 92–102. [Google Scholar] [CrossRef]
- Li, T.; Peng, L.; Wang, H.; Zhang, Y.Y.; Cheng, Y.; Hou, F. Multi-Cutting Improves Forage Yield and Nutritional Value and Maintains the Soil Nutrient Balance in a Rainfed Agroecosystem. Front. Plant Sci. 2022, 13, 825117. [Google Scholar] [CrossRef] [PubMed]
- Akpensuen, T.T.; Namo, O.A.T. Establishment yield and nutrient composition of four legumes as influenced by age of growth in a cool tropical climate at Jos, Plateau State, Nigeria. Trop. Grassl. 2023, 11, 83–94. [Google Scholar] [CrossRef]
- Ojong, N.; Takor, M.; Egbe, A.; Bechem, E.; Etchu, K.; Mutai, C. The effect of cutting regime and genotype on growth, seed yield, seed quality and herbage yield of seven Urochloa (syn. Brachiaria) grass genotypes in the Adamawa region of Cameroon. Grassl. Sci. 2024, 70, 77–92. [Google Scholar] [CrossRef]
- Wassie, W.A.; Tsegay, B.A.; Wolde, A.T.; Limeneh, B.A. Evaluation of morphological characteristics, yield and nutritive value of Brachiaria grass ecotypes in northwestern Ethiopia. Agric. Food Secur. 2018, 7, 89. [Google Scholar] [CrossRef]
- Oesterheld, M.; Loreti, J.; Semmartin, M.; Sala, O.E. Inter-annual variation in primary production of a semi-arid grassland related to previous-year production. J. Veg. Sci. 2001, 12, 137–142. [Google Scholar] [CrossRef]
- Li, F.Y.; Snow, V.O.; Holzworth, D.P. Modelling the seasonal and geographical pattern of pasture production in New Zealand. N. Z. J. Agr. Res. 2011, 54, 331–352. [Google Scholar] [CrossRef]
- Kim, M.; Chemere, B.; Sung, K. Effect of Heavy Rainfall Events on the Dry Matter Yield Trend of Whole Crop Maize (Zea mays L.). Agriculture 2019, 9, 75. [Google Scholar] [CrossRef]
- Waterman, R.C.; Grings, E.E.; Geary, T.W.; MacNeil, M.D. Influence of seasonal forage quality on glucose kinetics of young beef cows. J. Anim. Sci. 2007, 85, 2582–2595. [Google Scholar] [CrossRef]
- Zhang, Q.; Bell, L.W.; Shen, Y.; Whish, J.P.M. Indices of forage nutritional yield and water use efficiency amongst spring-sown annual forage crops in north-west China. Eur. J. Agron. 2018, 93, 1–10. [Google Scholar] [CrossRef]
- Gilo, B.N.; Tolossa, A.R.; Tebeje, B.E.; Liban, J.D. Changes in herbaceous vegetation attributes and nutritional quality as influenced by cutting frequencies in the enclosure of Borana rangelands, southern Ethiopia. Ecol. Indic. 2022, 145, 109672. [Google Scholar] [CrossRef]
- Cop, J.; Vidrih, M.; Hacin, J. Influence of cutting regime and fertilizer application on the botanical composition, yield and nutritive value of herbage of wet grasslands in Central Europe. Grass Forage Sci. 2009, 64, 454–465. [Google Scholar] [CrossRef]
- Inyang, U.; Vendramin, J.M.B.; Sellers, B.; Silveira, M.L.A.; Lunpha, A.; Sollenberger, L.E.; Adesogab, A. Harvest frequency and stubble height affect herbage accumulation, nutritive value, and persistence of “Mulato II,” Brachiariagrass. Forage Graz. Land. 2010, 8, 1–7. [Google Scholar] [CrossRef]
- Yang, T.H.; Cheng, H.; Eun, J.K.; Chang, S.H.; Zhang, Y.; Ben-Tian, M.; Hou, F. Responses of high-sugar ryegrass productive performance to stimulated grazing on the loess plateau. Pral. Sci. 2015, 32, 1473–1481. [Google Scholar] [CrossRef]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2011, 131, 641–654. [Google Scholar] [CrossRef]
- Jayasinghe, P.; Ramilan, T.; Donaghy, D.J.; Pembleton, K.G.; Barber, D.G. Comparison of Nutritive Values of Tropical Pasture Species Grown in Different Environments, and Implications for Livestock Methane Production: A Meta-Analysis. Animals 2022, 12, 1806. [Google Scholar] [CrossRef]
- Boval, M.; Edouard, N.; Sauvant, D. A meta-analysis of nutrient intake, feed efficiency and performance in cattle grazing on tropical grasslands. Anim. Consort. J. 2015, 9, 973–983. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Osuga, I.M.; Abdulrazak, S.A.; Muleke, C.I.; Fujihara, T. Effect of supplementing Rhodesgrass hay (Chloris gayana) with Berchemia discolor or Zizyphusmucronata on the performance of growing goats in Kenya. Anim. Physiol. Anim. Nutr. 2011, 96, 634–639. [Google Scholar] [CrossRef]
- Rotta, P.P.; Menezes, A.C.B.; Costa e Silva, L.F.; Valadares Filho, S.D.C.; Prados, L.F.; Marcondes, M.I.; Gionbelli, M.P.; Chizzotti, M.L. Protein requirements for beef cattle. In BR—Corte: Nutrient Requirements of Zebu and Crossbred Cattle; Filho, S.C.V., Silva, L.F.C., Gionbelli, M.P., Rotta, P.P., Marcondes, M.I., Chizzotti, M.L., Prados, L.F., Eds.; Suprema Gráfica Ltda: Camacan, Brazil, 1994; pp. 185–212. [Google Scholar] [CrossRef]
- Moran, J. Tropical Dairy Farming: Feeding Management for Smallholder Dairy Farmers in the Humid Tropics; Csiro Publishing: Collingwood, VIC, Australia, 2005; pp. 51–59. Available online: www.landlinks.com.au (accessed on 3 October 2024).
- Meissner, H.H.; Koster, H.H.; Nieuwoudt, S.H.; Coetze, R.J. Effects of energy supplementation on intake and digestion of early and mid-season ryegrass and Panicum/Smuts finger hay, and on in sacco disappearance of various forage species. S. Afr. J. Anim. Sci. 1991, 21, 33–42. [Google Scholar]
- Ravhuhali, K.E.; Mudau, H.S.; Mokoboki, H.K.; Moyo, B.; Motsei, L.E. Effect of harvesting site on mineral concentration of browse species found in semi-arid areas of South Africa. J. Saudi Soc. Agric. Sci. 2023, 22, 165–173. [Google Scholar] [CrossRef]
- Freer, M.; Dove, H.; Nolan, J.V. Nutrient Requirements of Domesticated Ruminants; CSIRO Publishing: Melbourne, VIC, Australia, 2007; Available online: https://www.scribd.com/document/910627702/Nutrient-Requirements-of-Domesticated-Ruminants-Csiro-instant-access-2025 (accessed on 15 October 2023).
- Rashid, M. Goats and Their Nutrition; Manitoba Goat Association: Winnipeg, MB, Canada, 2008; Available online: https://extension.msstate.edu/publications/mineral-requirements-and-impact-dairy-and-meat-goat-production (accessed on 8 June 2024).
- Silva, L.F.C.; Filho, S.C.V.; Rotta, P.P.; Marcondes, M.I.; Zanetti, D.; Gionbelli, M.P.; Engle, T.E.; Paulino, M.F. Mineral requirements for beef cattle. In BR—Corte: Nutrient Requirements of Zebu and Crossbred Cattle; Filho, S.C.V., Silva, L.F.C., Gionbelli, M.P., Rotta, P.P., Marcondes, M.I., Chizzotti, M.L., Prados, L.F., Eds.; Suprema Gráfica Ltda: Camacan, Brazil, 2016; pp. 213–249. [Google Scholar] [CrossRef]


| Fertiliser/Harvests | Quantity/ Number of Harvests | Year | ||
|---|---|---|---|---|
| 2019 | 2020 | 2021 | ||
| Fertiliser | 30 kg P/ha | 10 June | - | - |
| 40 kg N/ha | - | 8 May | 8 May | |
| 40 kg N/ha | 7 July | 7 July | 7 July | |
| 40 kg N/ha | 1 September | 1 September | 1 September | |
| Harvests | 1 | - | 10 June | 10 June |
| 2 | 5 August | 5 August | 5 August | |
| 3 | 29 September | 29 September | 29 September | |
| Variables | Species | Harvest | Year × Species | Year × Harvest | Species × Harvest | Year × Species × Harvest | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | ||
| Morphological characters and yields | NTPT | 171.9 | <0.001 | 4.0 | 0.051 | 0.1 | 0.822 | 1.7 | 0.202 | 1.8 | 0.200 | 0.0 | 0.862 |
| NLPT | 482.8 | <0.001 | 0.6 | 0.436 | 0.6 | 0.456 | 4.4 | 0.040 | 0.1 | 0.825 | 1.6 | 0.208 | |
| Biomass yield | 44.7 | <0.001 | 19.2 | <0.001 | 0.3 | 0.600 | 4.2 | 0.045 | 0.0 | 0.872 | 0.2 | 0.666 | |
| CP yield | 104.9 | <0.001 | 0.7 | 0.403 | 3.4 | 0.068 | 4.7 | 0.034 | 2.8 | 0.099 | 0.2 | 0.650 | |
| ME yield | 98.6 | <0.001 | 7.8 | 0.007 | 0.0 | 0.969 | 5.1 | 0.027 | 0.0 | 0.915 | 0.0 | 0.993 | |
| Nutrient concentrations | CP | 442.6 | <0.001 | 492.6 | <0.001 | 40.8 | <0.001 | 0.1 | 0.819 | 87.7 | <0.001 | 0.0 | 0.914 |
| NDF | 863.2 | <0.001 | 111.6 | <0.001 | 10.9 | 0.002 | 2.1 | 0.156 | 0.6 | 0.432 | 0.0 | 0.947 | |
| ADF | 318.2 | <0.001 | 201.1 | <0.001 | 4.3 | 0.043 | 1.7 | 0.197 | 58.2 | <0.001 | 0.5 | 0.565 | |
| Ash | 421.2 | <0.001 | 55.9 | <0.001 | 1.3 | 0.256 | 0.9 | 0.339 | 4.5 | 0.038 | 1.8 | 0.181 | |
| Ca | 2.1 | 0.157 | 78.0 | <0.001 | 1.9 | 0.169 | 1.0 | 0.334 | 0.7 | 0.393 | 3.6 | 0.063 | |
| P | 144.1 | <0.001 | 29.4 | <0.001 | 10.4 | 0.002 | 0.1 | 0.758 | 20.5 | <0.001 | 0.1 | 0.801 | |
| Variables | Grass Species | Harvest Times | |||
|---|---|---|---|---|---|
| UR | CG | 1 | 2 | 3 | |
| Number of tillers per tussock | 24.2 a | 11.5 b | 20.5 | 22.5 | 17.4 |
| Number of leaves per tussock | 185.2 a | 44.0 b | 121.3 | 139.5 | 123.9 |
| Biomass yield, t/ha DM | 12.4 a | 8.4 b | 12.5 b | 13.9 a | 9.2 c |
| Crude protein yield, t/ha | 1.3 a | 0.8 b | 1.1 | 1.3 | 1.1 |
| Crude protein, g/kg DM | 96.1 a | 77.4 b | 82.4 c | 94.1 b | 111.2 a |
| Metabolisable energy, MJ/kg DM | 13.2 a | 10.1 b | 9.5 c | 13.6 a | 11.9 b |
| Neutral detergent fibre, g/kg DM | 546.8 a | 619.4 b | 678.3 a | 663.8 a | 633.3 b |
| Acid detergent fibre, g/kg DM | 326.0 b | 377.0 a | 422.6 a | 410.3 a | 362.5 b |
| Ash, g/kg DM | 60.2 a | 46.5 b | 56.9 c | 57.4 b | 64.7 a |
| Calcium, g/kg DM | 2.8 | 2.7 | 2.5 c | 3.0 b | 3.5 a |
| Phosphorus, g/kg DM | 1.3 b | 1.8 a | 1.6 b | 1.8 a | 2.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akpensuen, T.T.; Pyalson, N.E.; Cooke, A.S.; Lee, M.R.F.; Rivero, M.J. A Comparison of Biomass Production and Quality of Congo and Rhodes Grasses in Nigeria. Grasses 2025, 4, 45. https://doi.org/10.3390/grasses4040045
Akpensuen TT, Pyalson NE, Cooke AS, Lee MRF, Rivero MJ. A Comparison of Biomass Production and Quality of Congo and Rhodes Grasses in Nigeria. Grasses. 2025; 4(4):45. https://doi.org/10.3390/grasses4040045
Chicago/Turabian StyleAkpensuen, Tersur T., Nenken E. Pyalson, Andrew S. Cooke, Michael R. F. Lee, and M. Jordana Rivero. 2025. "A Comparison of Biomass Production and Quality of Congo and Rhodes Grasses in Nigeria" Grasses 4, no. 4: 45. https://doi.org/10.3390/grasses4040045
APA StyleAkpensuen, T. T., Pyalson, N. E., Cooke, A. S., Lee, M. R. F., & Rivero, M. J. (2025). A Comparison of Biomass Production and Quality of Congo and Rhodes Grasses in Nigeria. Grasses, 4(4), 45. https://doi.org/10.3390/grasses4040045








