Abstract
This study examines the growth dynamics of Nassella tenuis (Phil.) Barkworth, a palatable perennial tussock grass, abundant in the natural grasslands of Central Argentina. It focuses on the effects of water regimes and grazing history. Plants were collected from sub-humid and semiarid grasslands with contrasting grazing histories (grazed and ungrazed) and cultivated under controlled conditions. Key growth traits, such as leaf elongation, senescence, and net growth rates, as well as tiller production, were assessed across the growth cycle. The results reveal that sub-humid grasslands favor faster growth rates and higher tiller production, while semiarid grasslands exhibit lower growth rates, potentially reflecting adaptive strategies for water-limited environments. Seasonal analysis revealed distinct life cycle patterns: plants from sub-humid grasslands exhibited higher elongation rates during autumn and spring, whereas growth in semiarid plants remained consistently low across seasons. Grazing history significantly influenced growth patterns, with grazed plants showing reduced tiller numbers and growth rates but lower senescence rates, particularly in semiarid grasslands. These findings underscore the importance of aligning grazing management practices with the growth dynamics of N. tenuis and the water regime of the site to optimize forage production while maintaining grassland resilience.
1. Introduction
Understanding the impact of grazing history on plant functional responses is crucial in grassland ecology, as these responses are influenced by the plant’s origin, prior growth conditions, climate, and soil variables [1]. Key factors such as phenotypic plasticity, local adaptation, genetic variability, and plant–herbivore interactions play a significant role in determining these responses. They also influence a plant’s ability to survive in diverse environments. For example, preemptive acclimation enables plants to anticipate environmental changes and adapt to them [2] by modifying their morphology, physiology, and behavior in response to environmental stimuli across different timescales [3]. These adaptations often result in changes to metabolic pathways and physiological functions [4,5]. Perennial grasses have longer growing cycles, which makes them more resilient to stress than annual herbaceous species. As a matter of fact, they have developed ways to reduce damage from environmental stresses like grazing, drought, and fire [6,7]. Growth conditions—such as light, temperature, humidity, nutrient availability, herbivory, and the presence of pathogens—strongly affect growth performance [8,9]. Water availability is particularly crucial, as it influences regrowth potential, resource allocation, and overall plant survival under grazing pressure.
In natural grasslands, forage production and loss occur simultaneously through growth and senescence [10]. Leaf morphogenesis—including emergence, expansion, and senescence—is driven by key environmental factors, such as water availability, temperature, grazing intensity, and grazing history [11]. Understanding these dynamics, particularly the interplay between water availability and grazing, is essential for optimizing livestock production systems. For instance, in semiarid environments, forage grasses generally exhibit lower leaf growth compared to sub-humid grasslands, due to differences in water regimes and rainfall distribution [12]. To maximize forage utilization in both sub-humid and semiarid grasslands, it is important to consider the specific characteristics of forage species under diverse environmental conditions. Species with a faster leaf turnover rate benefit from more frequent defoliation. Such a characteristic reduces losses due to senescence and promotes rapid recovery of leaf area and tillering potential. Aligning grazing frequency with the foliar turnover dynamics of palatable tussock grasses can enhance forage accumulation, reduce senescence losses, and support the development of more productive and persistent pastures [10].
The majority of land in Central Argentina is dedicated to agricultural activities, particularly cattle ranching, with natural grasslands serving as the primary source of forage. However, the stocking rate in these environments often exceeds the carrying capacity, which is critical for preventing ecosystem degradation [13]. Precipitation plays a fundamental role in these systems, with livestock productivity closely linked to both rainfall and land use history—key factors that determine plant community condition and forage production. The livestock-rearing region of southern Central Argentina is concentrated in two well-defined areas: Pampa Austral District and Caldenal District. The Pampa Austral District is located to the south of the Pampas Phytogeographical Region [14], encompassing the Tandil and Ventania hills along with an inter-hill area characterized by gentle slopes. This temperate and sub-humid zone has annual precipitation ranging from 800 to 900 mm, decreasing from east to west [15]. Livestock raising is concentrated in approximately 180,000 hectares of marginal grasslands unsuitable for cultivation due to topographical or soil depth limitations [16,17]. The Caldenal District is part of the Phytogeographic Region of the Espinal [14], characterized by small patches of woody vegetation interspersed within a matrix of grasslands predominantly composed of perennial tussock grasses. This semiarid area has distinctly marked dry summer periods, with annual precipitation ranging from 500 to 600 mm [15]. Livestock activities here are also primarily cattle-based, with a high stocking density on approximately 350,000 hectares [18]. The persistence of perennial grasses is closely associated with their growth strategy, reproductive potential, and adaptability to environmental stress [19]. The growth patterns of perennial forage grass species can be monitored through regular measurements of leaf length during their developmental cycle. Key parameters such as leaf elongation rate (LER) and leaf senescence rate (LSR) play a crucial role in determining both individual and net leaf growth rate (LGR). These parameters provide insights into variations in forage production in response to environmental conditions [20]. Moreover, the productive capacity of natural grasslands relies on the renewal of plant individuals over time, which depends on the number of flowering tillers. Their ability to produce forage for defoliation events is determined by the number of vegetative tillers [21]. Despite numerous studies examining the effects of environmental conditions, grazing frequency, and intensity on plant community traits [22,23,24], our understanding of how these factors specifically influence the growth and reproductive potential of key forage species remains limited. This study aims to address this gap by characterizing the growth traits of Nassella tenuis (Phil.) Barkworth, a cool-season palatable perennial tussock grass, abundant in the natural grasslands of Central Argentina [25,26,27]. Recognized for its high nutritional value as forage [28,29], N. tenuis exhibits functional responses to limited water availability and high grazing pressure, such as precipitation-use efficiency strategies and a strong regrowth capacity [30,31]. These responses underscore the importance of implementing defoliation strategies that align with the species’ morphogenetic and growth traits to optimize plant production and preserve forage structure under varying conditions.
Grasslands are vital for providing forage for herbivores, and understanding the interactions between environmental conditions and growth dynamics of perennial grasses is essential for optimizing resource management. Sustainable livestock production in grasslands under fluctuating water regimes relies on the efficient response of perennial grasses to appropriate management practices that enhance resource use. N. tenuis serves as an ideal model species due to its prevalence in the studied grasslands and its value as a palatable forage resource. Moreover, its resilience to water stress further supports its use as a representative species for evaluating the effects of environmental factors on leaf growth and reproductive tiller production. Therefore, the objective of this study is to investigate the relationship between environmental variables—specifically grazing history and water regime—and the growth patterns of N. tenuis. The aim is to generate valuable insights for the efficient management of forage resources in these ecosystems. Specifically, we assess the impact of previous domestic livestock activities on the morphological and growth traits of N. tenuis, examining its variations throughout the growing season in plants originating from both semi-humid and semiarid grasslands. We hypothesize that if these traits are not adaptive, there will be no significant differences between individuals from grasslands with different water regimes or grazing histories when grown under similar conditions, since their traits would not be influenced by the plants’ prior status. By investigating the growth patterns of N. tenuis, this research aims to extend the findings to other forage species with similar characteristics. This study will contribute to management practices that enhance the resilience and productivity of grasslands affected by variable water availability.
2. Materials and Methods
2.1. Study Site
The study was conducted in two natural sub-humid grasslands and two semiarid grasslands. Within each type of environment, two sampling areas with contrasting grazing histories were selected: one had been excluded from domestic livestock grazing for the past 10 years, using a seven-wire fence and electric wire; the other had been subjected to continuous grazing pressure (0.4 UG ha−1, primarily by cattle and horses) [32].
The sub-humid grasslands of Austral Pampas District are located in a mountain range (38°10′ S, 61°14′ W) (Figure 1A), in piedmont environments among gentle slopes ranging from 150 to 250 m.a.s.l. The region has a temperate climate, with an average annual temperature of 14 °C [33], and receives approximately 800 mm of rainfall per year, mainly during spring and autumn, with occasional snowfall in winter. This area forms part of the Austral District of the Pampas Phytogeographic Region, characterized by grass steppes [26]. Dominant perennial tussock grasses include species of Nassella and Piptochaetium, while common shrubs include Discaria americana and Acanthostyles buniifolium [34].
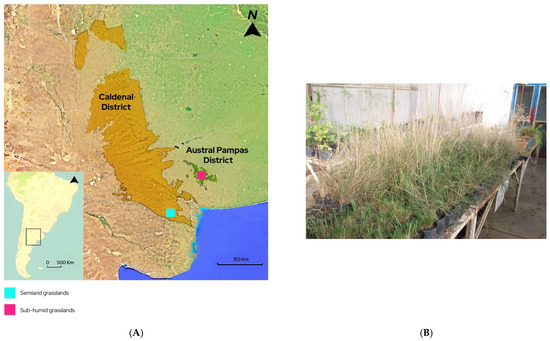
Figure 1.
(A) Study sites with the location of the sub-humid and semiarid grasslands in the Austral Pampas District and Caldenal District, respectively. (B) Plants of Nasella tenuis from sub-humid and semiarid grasslands with contrasting grazing histories, cultivated under greenhouse conditions.
The semiarid grasslands are located at the southeastern limit of the Caldenal District (38°45′ S, 63°45′ W) (Figure 1), in the southern region of the Espinal. They are characterized by an herbaceous matrix composed of perennial tussock grasses and a woody component. The herbaceous layer is dominated by perennial tussock grasses such as Nassella, Jarava, and Piptochaetium, while the dominant woody species belong to the genus Neltuma [26]. The climate is classified as dry temperate, with an average annual temperature of 15.3 °C. The region receives an average of 344 mm of rainfall per year, primarily during autumn and spring, with an annual water deficit of approximately 400 mm [35].
Historically, both grassland types were inhabited by native herbivores such as Pampas deer (Ozotoceros bezoarticus) and guanacos (Lama guanicoe), although these species are now either extinct or present in very low numbers. Since the 19th century, domestic livestock—including cattle, horses, sheep, and goats—have been prevalent [13]. The studied grasslands are predominantly grazed by cattle and horses and are characterized by high animal stocking rates and continuous grazing. Poor livestock management has triggered processes like shrub encroachment, the replacement of palatable species with unpalatable ones, and the spread of invasive plants [36,37].
2.2. Field Survey and Greenhouse Cultivation
Ten Nassella tenuis plants were collected from sub-humid and semiarid grasslands with contrasting grazing histories. Each area, approximately 4 hectares in size, was divided into 10 grids (0.4 hectares each), and from the center of each grid, one adult N. tenuis plant was randomly collected. Then, the plants were transferred to a greenhouse, where they were irrigated regularly to maintain the soil at field capacity. They were grown in 5 L blow-molded plastic pots filled with fertile soil enriched with vermiculite and organic matter (Figure 1B). After a 30-day acclimatization period, plant height, diameter, and the number of tillers were recorded. Three tillers per plant were marked, and leaf growth and senescence were monitored weekly over a 9-month period (March to December 2020). The number of flowering tillers was also recorded at the end of the growth cycle (late December 2020 to early January 2021).
2.3. Growth Rate Calculations and Data Analysis
At the end of the growth cycle (early summer 2020), the leaf elongation rate per tiller (LER; mm tiller−1 d−1) was calculated for growing leaves as the difference between the sum of final (LFf) and initial (LFi) leaf lengths, divided by the number of days between successive measurements. The leaf senescence rate per tiller (LSR; mm tiller−1 d−1) was determined as the decrease in green leaf length between consecutive measurement intervals, divided by the number of days in the interval [38]. The net leaf growth rate per tiller (LGR; mm tiller−1 d−1), representing the rate at which green leaves are maintained on the plant, was calculated as the difference between the leaf elongation rate (LER) and the leaf senescence rate (LSR) [38].
To evaluate the effects of grazing history (with and without grazing) and water regime (sub-humid and semiarid) on the growth of N. tenuis, for each growth trait measured and calculated, a factorial ANOVA (Grazing History × Water Regime) was performed. Significant interactions were further analyzed using simple ANOVA based on the CMD from the factorial ANOVAs. Mean separation was conducted using Tukey’s Test. A Pearson correlation analysis was performed between parameters measured at the beginning and at the end of the growth cycle. Prior to all analyses, data were transformed using the square root function to meet the assumptions for parametric analysis [39].
To assess the variation in elongation, senescence, and leaf growth rates of N. tenuis plants, a one-way ANOVA was performed for each season (autumn, winter, and spring). These analyses compared the growth cycle among grasslands with different combinations of water regimes and grazing histories. Data were arcsine square root transformed, and means were compared using Tukey’s Test [39].
3. Results
The water regime and grazing history of the studied grasslands differentially affected plants’ diameter and height, as well as the net leaf growth rate. However, no interactions between these factors were observed for vegetative tillers, flowering tillers, leaf elongation rate, and leaf senescence rate (Table 1 and Table S1).

Table 1.
Results of factorial ANOVA for each variable measured in Nassella tenuis plants from grazed and ungrazed natural grasslands at sub-humid and semiarid sites.
The mean number (±SE) of vegetative and flowering tillers was significantly higher (p < 0.05) in plants from ungrazed (70.7 ± 13.4 and 40.0 ± 5.9) and sub-humid grasslands (60.0 ± 7.0 and 34.7 ± 8.9), compared to those from grazed (40.1 ± 9.3 and 20.0 ± 2.0) and semiarid grasslands (30.1 ± 3.5 and 10.2 ± 1.3). In contrast, the leaf elongation rate showed no significant differences (p ≥ 0.10) between different water regimes (17.7 ± 4.0 for sub-humid, 16.3 ± 2.8 for semiarid) or grazing histories (21.7 ± 4.4 for plants from ungrazed areas, 19.4 ± 4.8 for those from grazed areas). However, the leaf senescence rate was significantly lower (p < 0.05) in plants from grazed sites (1.7 ± 0.4) compared to those from ungrazed sites (3.2 ± 0.8), and in semiarid grasslands (2.1 ± 0.7) compared to sub-humid grasslands (4.1 ± 0.4). For morphological and growth traits with significant interactions, we evaluated site differences within each grazing history, showing distinct responses to the water regime in plants from grazed grasslands (Table 2).

Table 2.
One-way ANOVA results (mean ± SE) of Nassella tenuis plant parameters from grazed and ungrazed natural grasslands in sub-humid and semiarid sites.
The correlations between LER, LSR, LGR, and flowering tillers with morphological variables of N. tenuis recorded at the beginning of the growth cycle varied by grazing history and grassland type (Table 3). In ungrazed sub-humid grasslands, larger plants with more tillers showed a positive correlation with leaf growth, senescence, and flowering rates. In grazed sub-humid grasslands, negative correlations were found between leaf rates and plant size, though flowering capacity remained associated with tiller number. In ungrazed semiarid grasslands, larger plants exhibited higher senescence rates and greater flowering capacity. In grazed semiarid grasslands, leaf growth and elongation rates were positively correlated with plant size, but flowering capacity was not significantly associated with any morphological variable.

Table 3.
Pearson correlations between annual leaf elongation, senescence, growth rates, flowering tiller number, and morphological traits of Nassella tenuis from grazed and ungrazed natural grasslands in sub-humid and semiarid regions.
The growth dynamics of N. tenuis varied significantly across seasons, influenced by the combination of grassland water regime and grazing history (Table 4). In autumn, plants from sub-humid grasslands exhibited higher LER than those from semiarid grasslands (regardless of grazing history), while no significant differences were observed in winter. In spring, plants from grazed sub-humid grasslands showed the highest LER, whereas the lowest rates were recorded in plants from both grazed and ungrazed semiarid grasslands (Figure 2). LSR also varied by season and treatment: ungrazed plants from both sub-humid and semiarid grasslands exhibited the highest rates in autumn and spring, while grazed plants from sub-humid grasslands consistently had the lowest rates across all seasons (Figure 3). LGR followed a similar pattern: no significant differences were found in autumn and winter. However, plants from grazed sub-humid grasslands showed the highest growth in spring, whereas those from ungrazed sub-humid and all semiarid grasslands exhibited lower rates (Figure 4).

Table 4.
Results of one-way ANOVA for leaf elongation rate (LER), leaf senescence rate (LSR), and leaf growth rate (LGR) of N. tenuis from natural grasslands with different combinations of grazing histories and water regimes, for each season of the growth cycle (autumn, winter, and spring).
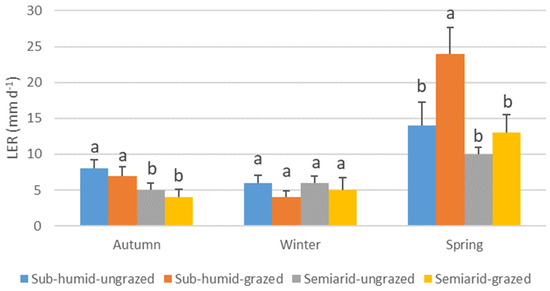
Figure 2.
Average leaf elongation rate (mm d−1) (LER) of Nassella tenuis plants from grasslands with different water regimes and grazing histories, measured in autumn, winter, and spring. Within each season, columns with the same letter do not differ significantly (p < 0.05).
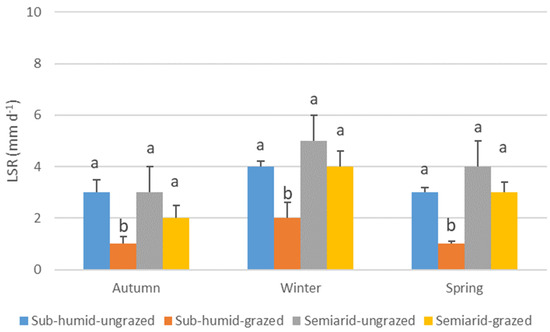
Figure 3.
Average leaf senescence rate (mm d−1) (LSR) of Nassella tenuis plants from grasslands with different water regimes and grazing histories, measured in autumn, winter, and spring. Within each season, columns with the same letter do not differ significantly (p < 0.05).
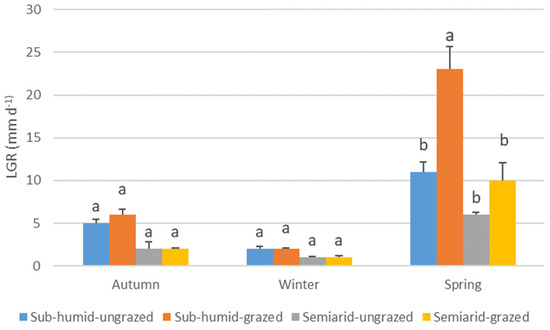
Figure 4.
Average leaf growth rate (mm d−1) (LGR) of Nassella tenuis plants from grasslands with different water regimes and grazing histories, measured in autumn, winter, and spring. Within each season, columns with the same letter do not differ significantly (p < 0.05).
4. Discussion
4.1. Effects of Water Regime and Grazing History on Growth and Morphology
Our findings reveal that both water regime and grazing history distinctly affect N. tenuis plant performance. For example, plants from ungrazed and sub-humid grasslands produced more vegetative and flowering tillers compared to those from grazed and semiarid grasslands. This suggests that environmental conditions and grazing pressure shape growth and reproductive structures, even under optimal greenhouse conditions with frequent watering. These differences observed under uniform greenhouse conditions likely reflect adaptations developed under the selective pressures of grazing and water availability in their native environments. Our results align with findings that report reduced size and growth in perennial grasses such as Festuca idahoensis and Elymus lanceolatus in long-term grazed upland grasslands in Montana, North America [40]. In contrast, it was found that Piptochaetium napostaense, another cool-season perennial tussock from Central Argentina, displayed similar responses to varying water and nutrient levels regardless of grazing history when grown in a greenhouse [41]. The authors attributed these patterns to phenotypic plasticity within adapted genotypes. This discrepancy may stem from methodological differences, as P. napostaense was cultivated from seed under controlled conditions, allowing early acclimation, whereas our study involved the transplantation of mature plants [42].
The interaction between grazing history and water availability emphasizes that grazing shapes how N. tenuis responds to environmental conditions. Grazed plants consistently exhibited reduced height, diameter, and foliar growth rates compared to their ungrazed counterparts. The most pronounced reductions occurred in semiarid grasslands. These results underscore the combined effects of grazing pressure and water regime in promoting differentiation in growth traits. Grazed plants were smaller in diameter and height, suggesting that selective pressures from grazing and water limitations stimulate the development of avoidance and tolerance traits, such as reduced size. A similar trend of miniaturization in perennial grasses under intense grazing has been reported as a form of grazing resistance [22]. Basal area and tiller density of palatable grasses were reported to increase as livestock pressure decreased in the arid grasslands of western Argentina [43]. Reductions in the average diameter of grasses in pastures grazed by sheep and kangaroos in the semiarid region of southeastern Australia were also observed. These findings emphasize that grazing effects are modulated by environmental factors such as herbivore type and rainfall records [44]. In resource-limited environments characterized by high grazing pressure and low rainfall, smaller tussocks may serve as a survival strategy, being less attractive to herbivores and better suited for regeneration after grazing. While these traits, such as reduced size and fewer tillers, may enhance survival under intense grazing and water limitations, they often come at the cost of reduced productivity [45].
Extensive research has documented that perennial grasses adapt to grazing through increased growth [46,47] or enhanced leaf replacement rates [48,49]. However, our findings confirm that the combination of water stress and high grazing pressure compromises the productive capacity of N. tenuis in semiarid grasslands, as evidenced by reduced tiller growth. Grasslands excluded from grazing exhibited higher leaf growth rates in N. tenuis, underscoring the influence of grazing pressure on growth dynamics, even under water-limited conditions. These findings are consistent with reports of significantly lower regrowth rates in perennial grasses under high grazing pressure in the Limpopo Valley, South Africa [50]. Responses to grazing pressure, however, can vary depending on species-specific traits and environmental context, such as grazing intensity and water availability. For example, grazing and precipitation were observed to significantly influence functional trait variability in Stipa breviflora in Inner Mongolia [51]. In dry years, S. breviflora showed reduced growth under intense grazing, whereas moderate grazing enhanced its growth during wetter years. It has been further explained that the slow growth and consequent accumulation of dry matter content in perennial grass leaves under water stress in the Central Great Plains, USA, reflect an adaptive strategy [52]. This characteristic enhances resilience by improving resource-use efficiency and tolerance to adverse conditions such as drought.
4.2. Functional Responses and Growth Trade-Offs Under Environmental Stress
The absence of interaction between water regime and grazing history for vegetative and flowering tillers, as well as leaf elongation and senescence rates, suggests that these factors influence these growth traits independently. Notably, plants from grazed grasslands exhibited lower senescence rates than those from ungrazed areas. This pattern may indicate an adaptive response to grazing stress, where reduced leaf senescence helps maintain photosynthesis and nutrient storage during critical periods. Similarly, early leaf senescence was found to play a key role in grass survival during prolonged droughts following grazing in wetter periods, highlighting a comparable mechanism of resource conservation [53]. Our study found no significant differences in leaf elongation rates across varying water regimes and grazing histories. This stability in N. tenuis may reflect a conservative growth strategy that prioritizes maintaining functional leaf area rather than adjusting this trait to fluctuating environmental conditions. It was reported that understory herbaceous species tend to preserve leaf architecture to sustain efficient light interception and photosynthetic rates, even during adverse periods characterized by water scarcity, shading, and low temperatures [54].
The relationships between leaf elongation, senescence, and growth rates, as well as the number of flowering tillers, with morphological traits (diameter, height, and initial tiller number) in N. tenuis varied significantly depending on grazing history and water regime. These patterns provide valuable insights into how environmental conditions shape the performance of perennial grasses. In plants from ungrazed sub-humid grasslands, larger individuals with more tillers exhibited higher leaf growth, elongation, and senescence rates, along with greater flowering potential. This suggests that under favorable conditions, resource allocation supports both vegetative and reproductive development. In contrast, grazing in sub-humid grasslands reversed these patterns, with larger plants showing reduced leaf growth and senescence rates. Nevertheless, flowering capacity remained positively correlated with tiller number, indicating that while grazing constrains vegetative growth in larger plants, reproductive output remains resilient. This may reflect a trade-off prioritizing reproduction under grazing-induced stress. Such trade-offs among plant traits can contribute to maintaining relative fitness under unpredictable conditions and maximizing reproductive success [55].
In N. tenuis plants from semiarid grasslands, the absence of grazing may be associated with higher senescence rates in larger individuals, potentially reflecting a resource-recycling strategy under water-limited conditions [56]. Similarly, early leaf senescence in grasses has been documented to facilitate the redistribution of nutrients, such as nitrogen and phosphorus, to critical parts of the plant, enhancing survival during prolonged drought periods [53]. The flowering potential remained closely linked to plant size and structure, underscoring the pivotal role of morphological traits in reproductive success. However, grazing in this environment altered these dynamics: positive correlations were observed between leaf elongation and growth rates and plant size, while no significant associations were detected between flowering capacity and morphological traits. The decoupling of vegetative growth and reproductive outcomes under grazing stress suggests a shift in resource allocation, whereby plants prioritize maintaining vegetative functioning over reproduction as an adaptive response to harsh environmental conditions. The theory that plants under environmental stress, such as drought or grazing, tend to allocate resources to functions that maximize survival has been supported by previous studies [57]. In this context, investment in vegetative growth allows plants to sustain active photosynthesis and resource acquisition, ensuring their persistence in challenging conditions. This strategy may explain why N. tenuis maintains vegetative performance while reducing reproductive investment in response to a previous history of grazing pressure.
The contrasting patterns observed between plants from grazed and ungrazed grasslands highlight how environmental stressors shape growth and reproductive strategies in N. tenuis. In ungrazed semiarid grasslands, their large plants exhibited higher senescence rates and greater flowering capacity, suggesting a strategy that prioritizes reproduction over longevity to enhance survival under moisture-limited conditions. Conversely, in grazed semiarid grasslands, positive correlations between leaf growth and elongation rates and plant size, alongside the absence of significant associations between flowering capacity and morphological traits, point to a trade-off. Under these conditions, plants seem to prioritize immediate vegetative growth over reproductive investment, reflecting adaptive responses to grazing stress. These findings emphasize the complex interplay between grazing intensity and environmental stressors, which collectively shape resource allocation strategies and influence the health and productivity of grasslands in both semiarid and sub-humid regions.
4.3. Seasonal Growth Patterns and Plant Origin
The seasonal growth dynamics of N. tenuis highlight the influence of the plants’ origin, whether from semiarid or sub-humid grasslands, and their grazing history, rather than immediate environmental conditions, since all plants were cultivated under optimal soil moisture. Compared to plants from semiarid grasslands, the higher leaf elongation rates observed in those from sub-humid grasslands during autumn, regardless of grazing history, reflect intrinsic differences in resource allocation strategies shaped by the environmental conditions of their native ecosystems. Sub-humid environments, with greater water availability, may select for traits that promote rapid early-season growth, aligning with findings from previous studies on how plant origin influences growth traits in perennial grasses [58]. Interestingly, no differences in elongation rates were observed during winter, suggesting that colder temperatures limit growth, irrespective of plants’ origin or grazing history. Enzymatic inhibition caused by low temperatures, coupled with reduced photosynthetic rates, has been identified as a major constraint on leaf growth and elongation in perennial grasses [59,60]. In spring, plants from grazed sub-humid grasslands exhibited the highest elongation rates, in stark contrast to the lowest rates recorded in plants from semiarid grasslands, irrespective of grazing history. This pattern underscores the enduring influence of grassland type, where sub-humid origins favor more robust growth responses to favorable seasonal conditions, while plants from semiarid grasslands exhibit inherently lower growth potential, even under optimal cultivation conditions. Smaller individuals of the C4 perennial forage species Trichloris crinita, native to the Arid Chaco region of Argentina, have been shown to exhibit consistently low growth rates throughout the growth cycle as an adaptive strategy [61]. This functional trait, combined with a reduced shoot-to-root ratio, allows these plants to evade grazing pressure while persisting in water-deficient environments by allocating more resources to reserves stored in the stem base. These findings are consistent with the growth dynamics observed in N. tenuis plants from semiarid grasslands, which also demonstrated lower growth rates, possibly reflecting a similar strategy to cope with resource limitations and grazing history.
Leaf senescence rates followed a different trend, with ungrazed plants from both sub-humid and semiarid origins exhibiting the highest rates in autumn and spring. This pattern suggests that ungrazed plants, often larger due to reduced grazing-induced biomass removal, allocate resources toward nutrient recycling via senescence, particularly in periods of active growth. This is consistent with strategies observed in other perennial grasses, where nutrient mobilization through leaf senescence supports reproductive and vegetative functions under resource-limited scenarios [56,62]. In contrast, the consistently lower senescence rates in plants from grazed sub-humid grasslands may reflect an adaptive strategy to maximize photosynthetic capacity and resource acquisition, likely shaped by grazing history [63]. Leaf growth rates mirrored these trends, with significant differences only observed in spring. Plants from grazed sub-humid grasslands demonstrated the highest growth rates, potentially reflecting the combined effects of grazing-induced compensatory growth and intrinsic traits associated with their origin. In contrast, the lower growth rates in plants from ungrazed sub-humid and all semiarid grasslands suggest that the absence of grazing pressure or semiarid origins may limit the expression of growth-promoting traits under favorable conditions.
4.4. Adaptive Strategies and Resource Allocation in Variable Environments
Depending on previous environmental conditions and the stage of the growth cycle, N. tenuis alternates between fast-growth and low-growth strategies. Fast-growth strategies are characterized by rapid leaf elongation and high growth rates, typically favored in resource-rich or favorable conditions, allowing plants to maximize above-ground biomass and compete effectively. In contrast, low-growth strategies involve slower growth and reduced elongation rates, often associated with resource conservation and persistence under stress, such as limited water availability or grazing pressure [64]. Plants of N. tenuis originating from sub-humid grasslands exhibited higher leaf elongation and growth rates during autumn and spring, regardless of grazing history, reflecting traits aligned with a fast-growing strategy. These findings indicate that sub-humid conditions promote rapid above-ground growth to optimize resource capture during favorable periods [65]. Conversely, plants from semiarid grasslands displayed lower growth and elongation rates across seasons, aligning with a low-growth strategy. This suggests an inherent adaptation to resource-limited environments, where slower growth reduces metabolic demands, potentially increasing survival under prolonged stress [65].
Interestingly, grazing history further modulated these dynamics, particularly during spring. Grazed plants from sub-humid grasslands exhibited the highest elongation and growth rates, likely reflecting compensatory growth in response to defoliation, as documented in other perennial grasses. In contrast, plants from semiarid grasslands displayed limited growth responses to seasonal changes, regardless of grazing history, suggesting a constrained capacity to capitalize on favorable conditions. Leaf senescence rates were similarly influenced by these interactions, with ungrazed plants from both grassland types showing higher senescence rates in spring, potentially as a nutrient recycling mechanism. These findings highlight the intricate interplay between grassland type, grazing pressure, and seasonal factors in shaping the growth dynamics of N. tenuis, emphasizing its plasticity in adopting contrasting growth strategies. The growth–survival trade-off between fast- and low-growth strategies allows perennial plants to persist in grasslands subjected to recurrent stressors, such as livestock grazing and drought periods [66].
5. Conclusions
The findings of this study highlight the strong influence of grassland type and grazing history on the growth strategies of N. tenuis. While water regime affected overall growth rates, grazing history primarily influenced the balance between vegetative production and senescence, with both factors interacting with the stage of the growth cycle. These results underscore the importance of considering environmental origin, phenological stage, and grazing management practices such as rotational grazing and adjustment of stocking rates, when evaluating the forage potential of this species. N. tenuis exhibits a high degree of plasticity, adjusting its resource allocation between vegetative and reproductive functions in response to environmental conditions. This adaptability supports its persistence and productivity in variable environments, positioning it as a promising species for sustainable rangeland management. However, the observed reduction in tiller number under semiarid and grazed conditions reveals its sensitivity to harsh environments, reinforcing the need to regulate grazing intensity to preserve both its forage value and grassland resilience.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/grasses4030035/s1, Table S1: Effects of Water Regime, Grazing History, and Their Interaction on Morphological and Growth Rate Variables of Nassella tenuis.
Author Contributions
All authors were involved in the study’s conception, design, data collection and analysis, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Scientific and Technical Research Council (CONICET) and General Secretariat of Science and Technology, National University of the South (SGCyT) (PGI 24/B268).
Data Availability Statement
All data are available in CONICET Repository Data http://hdl.handle.net/11336/260998 (accessed on 5 May 2023).
Acknowledgments
We wish to thank the administrations of Ernesto Tornquist Provincial Park, Sierras Grandes Protected Area, and Laguna Chasicó Protected Area (Buenos Aires Province). We are especially grateful to Centro de Recursos Naturales Renovables de la Zona Semiárida-CERZOS (CONICET/UNS) and SGCyT Universidad Nacional del Sur. We would like to express our gratitude to Luciana Caserta for her assistance in editing the figures and to Alexia Insua for reviewing the English in this article. Their contributions were invaluable in improving the quality of this research.
Conflicts of Interest
The authors declare that they have no conflicts of interest to report regarding the present study and have no other ethical statements to declare.
References
- Thomas, T.W.; Davies, K.W.; Mata-Gonzalez, R.; Svejcar, L.N.; Clenet, D. Effects of a decade of grazing exclusion on three Wyoming big sagebrush community types. Glob. Ecol. Conserv. 2022, 40, e02338. [Google Scholar] [CrossRef]
- Aphalo, P.J.; Sadras, V.O. Explaining pre-emptive acclimation by linking information to plant phenotype. J. Exp. Bot. 2022, 73, 5213–5234. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Trewavas, A. What is plant behaviour? Plant Cell Environ. 2009, 32, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Orrock, J.L. A judgment and decision-making model for plant behavior. Ecology 2018, 99, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Malinowski, D.P.; Volaire, F. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agron. Sustain. Dev. 2016, 36, 29. [Google Scholar] [CrossRef]
- Volaire, F.; Morvan-Bertrand, A.; Prud’homme, M.-P.; Benot, M.-L.; Augusti, A.; Zwicke, M.; Roy, J.; Landais, D.; Picon-Cochard, C. The resilience of perennial grasses under two climate scenarios is correlated with carbohydrate metabolism in meristems. J. Exp. Bot. 2020, 71, 370–385. [Google Scholar] [CrossRef]
- Gauthier, M.; Barillot, R.; Andrieu, B. Simulating grass phenotypic plasticity as an emergent property of growth zone responses to carbon and nitrogen metabolites. In Silico Plants 2021, 3, diab034. [Google Scholar] [CrossRef]
- Cavallaro, A.; Carbonell-Silletta, L.; Askenazi, J.O.; Goldstein, G.; Bucci, S.J.; Scholz, F.G. Phenotypic plasticity in leaf traits in response to experimental precipitation increase: Wettability, foliar water uptake, and gas exchange. Ecohydrology 2023, 16, e2573. [Google Scholar] [CrossRef]
- Lemaire, G.; Agnusdei, M. Leaf tissue turnover and efficiency of herbage utilization. In Grassland Ecophysiology and Grazing Ecology; Lemaire, G., Hodgson, J., Moraes, A., Carvalho, P.C.F., Nabinger, C., Eds.; CABI Publishing: London, UK, 2000; pp. 265–288. Available online: https://www.cabidigitallibrary.org/doi/10.1079/9780851994529.0265 (accessed on 2 September 2024).
- Skarpe, C.; Hester, A.J. Plant traits, browsing and grazing herbivores, and vegetation dynamics. In The Ecology of Browsing and Grazing; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Berlin, Germany, 2008; pp. 217–261. [Google Scholar] [CrossRef]
- Hendrickson, J.R.; Moffet, C. Forage systems for the temperate subhumid and semiarid areas. Agriculture 2020, 2, 387–405. [Google Scholar] [CrossRef]
- Bilenca, D.N.; Miñarro, F. Identification of Valuable Grassland Areas (AVPs) in the Pampas and Campos of Argentina, Uruguay, and Southern Brazil; Fundación Vida Silvestre Argentina: Buenos Aires, Argentina, 2004; pp. 1–323. [Google Scholar]
- Arana, M.D.; Natale, E.; Ferretti, N.; Romano, G.; Oggero, A.; Martínez, G.; Posadas, P.; Morrone, J. Biogeographic scheme of the Republic of Argentina. Opera Lilloana 2021, 56, 1–238. (In Spanish) [Google Scholar]
- INTA (Instituto Nacional de Tecnología Agropecuaria). AgroMet and AgroCultivos Reports. Available online: https://www.argentina.gob.ar/inta/informacion-agroclimatica/informes-agromet-y-agrocultivos (accessed on 20 December 2024). (In Spanish).
- Pizarro, J.B. The evolution of agricultural production in the Pampas during the second half of the 20th century. Rev. Interdiscip. Estud. Agrar. 2003, 18, 63–125. Available online: http://bibliotecadigital.econ.uba.ar/download/riea/riea_v18_n1_03.pdf (accessed on 2 September 2024). (In Spanish).
- SENASA. Bovines and Bubalines Primary Sector: Characterization of Cattle Stock. Available online: https://www.argentina.gob.ar/senasa/mercados-y-estadisticas/estadisticas/animalestadisticas/bovinos/bovinos-y-bubalinos-sector-primario (accessed on 2 September 2024).
- Estelrich, H.D.; Castaldo, A. Livestock receptivity and stocking rate in different micro-regions of La Pampa Province (Argentina) and its relation to rainfall. Semiárida. Rev. Fac. Agron. UN La Pampa 2014, 24, 7–19. (In Spanish) [Google Scholar]
- Duchini, P.G.; Guzatti, G.C.; Echeverria, J.R.; Américo, L.F.; Sbrissia, A.F. Experimental evidence that the perennial grass persistence pathway is linked to plant growth strategy. PLoS ONE 2018, 13, e0207360. [Google Scholar] [CrossRef] [PubMed]
- Barre, P.; Turner, L.B.; Escobar-Gutiérrez, A.J. Leaf length variation in perennial forage grasses. Agriculture 2015, 5, 682–696. [Google Scholar] [CrossRef]
- Gibson, D.J. Grasses & Grassland Ecology; Oxford University Press: New York, NY, USA, 2009; pp. 1–305. [Google Scholar]
- Díaz, S.; Lavorel, S.; McIntyre, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I.; et al. Plant trait responses to grazing—A global synthesis. Glob. Change Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- da Silveira Pontes, L.; Maire, V.; Schellberg, J.; Louault, F. Grass strategies and grassland community responses to environmental drivers: A review. Agron. Sustain. Dev. 2015, 35, 1297–1318. [Google Scholar] [CrossRef]
- Joswig, J.S.; Wirth, C.; Schuman, M.C.; Kattge, J.; Reu, B.; Wright, I.J.; Sippel, S.D.; Rüger, N.; Richter, R.; Schaepman, M.E.; et al. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 2022, 6, 36–50. [Google Scholar] [CrossRef]
- Wang, L.; Feng, S.-J.; Sun, D.; Wang, J.; Wang, Y.; Lee, S.-J.; Lu, P.; Gan, Y. Plant biotype interacting grazing activity shapes grassland ecosystem functions. Ecosphere 2023, 14, e4730. [Google Scholar] [CrossRef]
- Cabrera, A.L. Regiones Fitogeográficas Argentinas; Enciclopedia Argentina de Agricultura y Jardinería, Tomo II, Fascículo 1; Acme: Buenos Aires, Argentina, 1976; pp. 1–85. (In Spanish) [Google Scholar]
- Cialdella, A.M.; Muñoz-Schick, M.; Morrone, O. Synopsis of the Austro-American species of the genus Nassella (Poaceae, Pooideae, Stipeae). Darwiniana Nueva Ser. 2013, 1, 76–161. (In Spanish) [Google Scholar] [CrossRef]
- Distel, R.A.; Fernández, O.A. Productivity of Stipa tenuis Phil. and Piptochaetium napostaense (Speg.) Hack in semiarid Argentina. J. Arid Environ. 1986, 11, 93–96. [Google Scholar] [CrossRef]
- Distel, R.A.; Pietragalla, J.; Rodríguez Iglesias, R.M.; Didoné, N.G.; Andrioli, R.J. Restoration of palatable grasses: A study case in degraded rangelands of central Argentina. J. Arid Environ. 2008, 72, 1968–1972. [Google Scholar] [CrossRef]
- Busso, C.A.; Montenegro, O.A.; Torres, Y.A.; Giorgetti, H.D.; Rodriguez, G.D. The influence of disturbance type on precipitation-use efficiency at functional group and species scales in an arid habitat. Pol. J. Ecol. 2016, 64, 143–164. [Google Scholar] [CrossRef]
- Modernel, P.; Rossing, W.A.H.; Corbeels, M.; Dogliotti, S.; Picasso, V.; Tittonell, P. Land use change and ecosystem service provision in Pampas and Campos grasslands of southern South America. Environ. Res. Lett. 2016, 11, 113002. [Google Scholar] [CrossRef]
- Michalijos, M.P. Study of Forest Fire Risk in a Sector of the Sierra de la Ventana Region Using Geotechnologies; EdiUNS: Bahía Blanca, Argentina, 2019; pp. 1–120. (In Spanish) [Google Scholar]
- Long, M.A. Common and Rare Species in the Flora of the Southern Mountains of Buenos Aires: Historical, Ecological and Environmental Causes; EdiUNS: Bahía Blanca, Argentina, 2018; pp. 1–305. (In Spanish) [Google Scholar]
- Matteucci, S. Espinal Ecoregion. In Ecoregions and Ecosystem Complexes of Argentina; Morello, J., Matteucci, S., Rodríguez, A.F., Silva, M.E., Eds.; Orientación Gráfica Editora: Buenos Aires, Argentina, 2012; pp. 349–390. (In Spanish) [Google Scholar]
- de Villalobos, A.E.; Peláez, D.V. Functional responses of woody Prosopis caldenia seedlings to drought and livestock grazing in semiarid rangelands of Argentina. Arid Land Res. Manag. 2015, 29, 487–502. [Google Scholar] [CrossRef]
- de Villalobos, A.E.; Schwerdt, L. Seasonality of feral horse grazing and invasion of Pinus halepensis in grasslands of the Austral Pampean Mountains (Argentina): Management considerations. Biol. Invasions 2020, 22, 2941–2955. Available online: https://link.springer.com/article/10.1007/s10530-020-02300-x (accessed on 2 September 2024). [CrossRef]
- de Villalobos, A.E.; Long, M.A. Grasslands response to livestock grazing intensity in the Austral Pampas (Argentina): Testing the Intermediate Disturbance Hypothesis. Phyton-Int. J. Exp. Bot. 2024, 93, 2037–2050. [Google Scholar] [CrossRef]
- Carvalho, D.D.; Matthew, C.; Hodgson, J. Effect of aging in tillers of Panicum maximum on leaf elongation rate. In Proceedings of the International Grassland Congress; International Grassland Congress Organizing Committee, Ed.; International Grassland Congress: São Pedro, Brazil, 2001; pp. 41–42. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: Oxford, UK, 2009; pp. 1–960. [Google Scholar]
- Fahnestock, J.T.; Detling, J.K. Bison–prairie dog–plant interactions in a North American mixed-grass prairie. Oecologia 2002, 132, 86–95. [Google Scholar] [CrossRef]
- Tomas, M.A.; Carrera, A.D.; Poverene, M. Is there any genetic differentiation among populations of Piptochaetium napostaense (Speg.) Hack (Poaceae) with different grazing histories? Plant Ecol. 2000, 147, 227–235. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef]
- Gonnet, J.M.; Guevara, J.C.; Estevez, O.R. Perennial grass abundance along a grazing gradient in Mendoza, Argentina. J. Range Manag. 2003, 56, 364–369. [Google Scholar] [CrossRef]
- Travers, S.; Berdugo, M. Grazing and productivity alter individual grass size dynamics in semiarid woodlands. Ecography 2020, 43, 1003–1013. [Google Scholar] [CrossRef]
- Utrilla, V.; Andrade, M.; Vargas, P.; Alsina, M.; Aguilar, R.; Galván, J. Pasture and grassland productivity and foliar elongation of wheatgrass in Southern Patagonia. Agrociencia Urug. 2023, 27, e993. [Google Scholar] [CrossRef]
- Noy-Meir, I. Compensating growth of grazed plants and its relevance to the use of rangelands. Ecol. Appl. 1993, 3, 32–34. [Google Scholar] [CrossRef]
- Cingolani, A.M.; Posse, G.; Collantes, M.B. Plant functional traits, herbivore selectivity and response to sheep grazing in Patagonian steppe grasslands. J. Appl. Ecol. 2005, 42, 50–59. [Google Scholar] [CrossRef]
- Zheng, S.; Li, W.; Lan, Z.; Ren, H.; Wang, K. Functional trait responses to grazing are mediated by soil moisture and plant functional group identity. Sci. Rep. 2015, 5, 18163. [Google Scholar] [CrossRef]
- Temu, V.W.; Kering, M.K. Compensatory structural growth responses of early-succession native warm-season grass stands to defoliation management. Agronomy 2023, 13, 1280. [Google Scholar] [CrossRef]
- Twine, W.; Gray, V.; Owen-Smith, N. The effect of prolonged heavy grazing pressure on the regrowth of two perennial grass species in a semiarid communal rangeland. Afr. J. Range Forage Sci. 2002, 19, 129–130. [Google Scholar] [CrossRef]
- Ye, R.; Liu, G.; Chang, H.; Shan, Y.; Mu, L.; Wen, C.; Te, R.; Wu, N.; Shi, L.; Liu, Y.; et al. Response of plant traits of Stipa breviflora to grazing intensity and fluctuation in annual precipitation in a desert steppe, northern China. Glob. Ecol. Conserv. 2020, 24, e01256. [Google Scholar] [CrossRef]
- Blumenthal, D.M.; Kray, J.A.; Mueller, K.E. Coordination of leaf, root, and seed traits shows substantial niche differentiation among herbaceous species in semiarid grasslands. New Phytol. 2024, 241, 2410–2422. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, L.; Wang, P.; Zhong, T. Stability in the leaf functional traits of understory herbaceous species after 12 years of nitrogen addition in temperate larch plantations. Front. Plant Sci. 2023, 14, 1282884. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Reynolds, M.P.; Ortiz, R. Mitigating tradeoffs in plant breeding. iScience 2021, 24, 102965. [Google Scholar] [CrossRef]
- Philippe, E.; Diquelou, S.; Prudent, M.; Salon, C.; Maillard, A.; Ourry, A. Macro and micronutrient storage in plants and their remobilization when facing scarcity: The case of drought. Agriculture 2018, 8, 14. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Rouet, S.; Barillot, R.; Leclercq, D.; Bernicot, M.-H.; Combes, D.; Escobar-Gutiérrez, A.; Durand, J.-L. Interactions between environment and genetic diversity in perennial grass phenology: A review of processes at plant scale and modeling. Front. Plant Sci. 2021, 12, 672156. [Google Scholar] [CrossRef]
- Woledge, J.; Parsons, A.J. The effect of temperature on the photosynthesis of ryegrass canopies. Ann. Bot. 1986, 57, 487–497. [Google Scholar] [CrossRef]
- Förster, L.; Grant, J.; Michel, T.; Ng, C.; Barth, S. Growth under cold conditions in a wide perennial ryegrass panel is under tight physiological control. PeerJ 2018, 6, e5520. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, R.E.; Golluscio, R.A.; Blanco, L.J.; Fernández, R.J. Aridity and grazing as convergent selective forces: An experiment with an Arid Chaco bunchgrass. Ecol. Appl. 2010, 20, 1876–1889. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Udvardi, M. Senescence and nitrogen use efficiency in perennial grasses for forage and biofuel production. J. Exp. Bot. 2018, 69, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xie, H.; Wen, J.; Zhang, J.; Wang, Z.Y.; Chai, M. Leaf senescence in forage and turf grass: Progress and prospects. Grass Res. 2024, 4, e004. [Google Scholar] [CrossRef]
- Craine, J.M. Resource Strategies of Wild Plants; Princeton University Press: Princeton, NJ, USA, 2009; pp. 1–344. [Google Scholar]
- Colesie, C.; Stangl, Z.R.; Hurry, V. Differences in growth-economics of fast vs. slow growing grass species in response to temperature and nitrogen limitation individually, and in combination. BMC Ecol. 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Keep, T.; Sampoux, J.; Barre, P.; Blanco-Pastor, J.; Dehmer, K.J.; Durand, J.; Hegarty, M.; Ledauphin, T.; Muylle, H.; Roldán-Ruiz, I.; et al. To grow or survive: Which are the strategies of a perennial grass to face severe seasonal stress? Funct. Ecol. 2021, 35, 1145–1158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).